圆叶牵牛化学成分研究
李阳 肖朝江 刘健 沈怡 董相 姜北
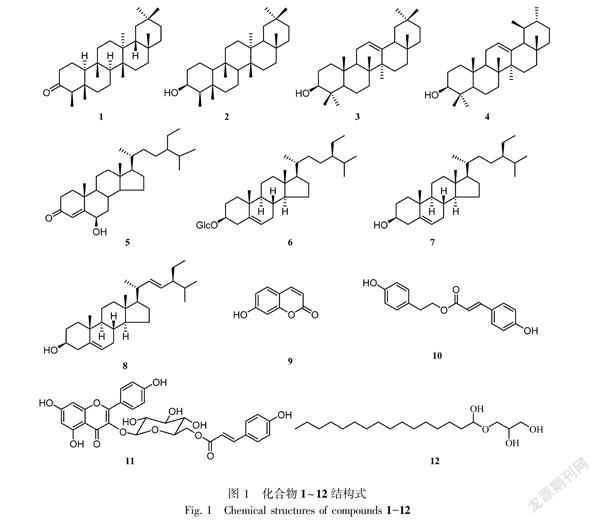
摘 要: 圆叶牵牛植物资源丰富但全草少有研究,对其进行系统的化学成分与生物活性认识很有必要。该文对采自云南大理的圆叶牵牛干燥全草化学成分进行了系统地研究,通过75%乙醇冷浸提取,浸膏以水分散,依次以乙酸乙酯和正丁醇萃取。结果表明:采用硅胶、Sephadex LH-20、RP-18等多种材料柱层析结合重结晶等方法,从圆叶牵牛醇提物乙酸乙酯部分分离得到12个单体化合物,运用现代波谱学技术分别鉴定为木栓酮(1),β-木栓烷醇(2),β-香树脂醇(3),α-香树脂醇(4),6β-羟基豆甾-4-烯-3-酮(5),胡萝卜苷(6),β-谷甾醇(7),豆甾醇(8),7-羟基香豆素(9),对香豆酸-对羟基苯乙醇酯(10),山奈酚香豆酰基葡萄吡喃糖苷(11),单棕榈酸甘油酯(12)。化合物2-5、10-12为首次从该属植物中分离得到。
关键词: 圆叶牵牛, 化学成分, 三萜, 甾体
中图分类号: Q946, R285.5 文献标识码: A 文章编号: 1000-3142(2019)07-0910-07
Abstract: There were abundant resources of Pharbitis purpurea, but lack of the systematic and deep studies about the whole plant. It is necessary to investigate the chemical compositions and biological activities of P. purpurea. For better understanding the chemical profile, the major constituents of the whole herb of P. purpurea collected from Dali, Yunnan, were systematically studied. After the whole herb of P. purpurea (4 kg) were extracted with 75% EtOH-H2O solution, the resulting extract was concentrated and suspended in water, and partitioned successively with EtOAc and n-BuOH. Twelve compounds were isolated from EtOAc-soluble part by various column chromatography methods including silica gel, Sephadex LH-20 and RP18 column chromatography, as well as recrystallization method. Based on the spectral analysis, the compounds were identified as friedelin (1), β-friedelinol (2), β-amyrin (3), α-amyrin (4), 6β-hydroxy-stigmast-4-en-3-one (5), daucosterol (6), β-sitosterol (7), stigmasterol (8), umbelliferone (9), p-hydroxyphenylethanol p-coumarate (10), kaempferol-3-β-D-(6-O-cis-p-coumaroyl)glucopyranoside (11), and glyceroylmonopalmitate (12). Compounds 2-5 and 10-12 were isolated from genus Pharbitis for the first time.
Key words: Pharbitis purpurea, chemical constituents, triterpene, steroid
旋花科(Convolvulaceae)牵牛属(Pharbitis或Ipcmoea)植物为一年或多年生缠绕草本,全世界约24种,主要分布于温带及亚热带,我国有3种,大部分地区均有分布(中国科学院中国植物志编辑委员会, 1979)。现代研究表明,牵牛属植物化学成分丰富,主要包括黄酮类、萜类、苯丙素类、挥发油等(梁娜等, 2013; Kim et al., 2009; Lee et al., 2017; Saito et al., 1994);该属部分植物中分离得到的一些单体化合物具有细胞毒活性、抗肿瘤活性、杀虫活性等(李佳桓等, 2014; 马超, 2011; 徐向荣等, 2006; Ju et al., 2011; Kim et al., 2014)。圆叶牵牛(Pharbitis purpurea或Ipcmoea purpurea)原产热带美洲,现已成归化植物分布于中国大部分地区,生于平地以至海拔2 800 m的田边、路边、宅旁或山谷林内,栽培或沦为野生(中国科学院中国植物志编辑委员会, 1979)。文献检索结果显示,该植物种子作为传统中药材(牵牛子来源植物之一)有较多的研究,但该植物全草尚无深入系统的研究。根据本研究组前期开展的大规模药用植物活性筛选实验结果,发现圆叶牵牛全草提取物具有一定的镇痛活性,因此对该植物全草進行系统的化学成分与生物活性研究很有必要。
为深入了解该植物的基本化学品质、为后续开展相关生物活性研究及开发利用工作创造条件,我们对圆叶牵牛全草提取物的化学成分进行了分离鉴定。本研究以75%乙醇作为提取溶剂,采用硅胶、Sephadex LH-20、RP-18等多种材料柱层析结合重结晶等方法,从圆叶牵牛醇提物乙酸乙酯部分分离得到12个单体化合物,结合1H-与13C-NMR技术,对化合物进行了结构鉴定,所分离得到的成分主要为三萜类、甾体类成分。
1 材料与方法
1.1 仪器和材料
Bruker Avance Ⅲ-400核磁共振仪(德国布鲁克公司,TMS为内标);柱层析硅胶和薄层层析硅胶板GF254(青岛海洋化工厂);RE-52AA型旋转蒸发仪(上海亚荣生化仪器厂);AL204 型电子天平(梅特勒-托利多仪器上海有限公司);Sephadex LH-20(瑞典Amersham Biosciences公司);大孔树脂D101(天津波鸿树脂科技有限公司);反相RP-18(40~75 μm)(日本Fuji公司);MCI CHP-20(75~150 μm)(日本三菱公司);石油醚、三氯甲烷、乙酸乙酯、丙酮、甲醇等有机试剂均为工业级溶剂,重蒸后使用;正丁醇、异丙醇为分析纯(上海化工有限公司);10%硫酸烘烤显色或碘蒸气熏蒸显色。
植物样品2017年7月采自云南大理江尾,由大理大学药学与化学学院张德全博士鉴定为圆叶牵牛(Pharbitis purpurea),植物标本(编号:20170709-2)保存于大理大学药物研究所姜北教授研究组。
1.2 提取和分离
干燥的圆叶牵牛全株4 kg,粉碎后以75%乙醇冷浸提取3次,提取液合并浓缩后得到浸膏749 g,浸膏以水分散,依次以乙酸乙酯和正丁醇萃取。取乙酸乙酯部位样品(117 g)以适量粗硅胶(80~100目)拌样,氯仿-丙酮混合溶剂梯度洗脱(1∶0~0∶1)硅胶柱色谱(300~400目),在TLC检测下合并得10个流分(A-J)。
B组分经硅胶柱色谱,以石油醚-乙酸乙酯混合溶剂梯度洗脱(1∶0~0∶1),B2组分以石油醚-乙酸乙酯重结晶得到化合物1(50 mg);B3组分以石油醚重结晶得到化合物2(39 mg);B4-5组分以石油醚重结晶得到化合物3(22 mg)和4(42 mg)。
D组分进行硅胶柱层析(石油醚-丙酮1∶0~0∶1),D2组分经甲醇反复洗涤得到化合物7(106 mg)和8(69 mg)。
E组分经MCI柱色谱(60%~100%甲醇/水),E2组分经硅胶柱层析(氯仿-丙酮 1∶0~0∶1),E2-1以丙酮洗涤得到化合物9(15 mg);E2-6以甲醇洗涤得到化合物10(15 mg);E4组分经甲醇洗涤得化合物12(66 mg);E7组分经硅胶柱层析(氯仿-异丙醇1∶0~0∶1)、RP-18柱色谱(50%~100%甲醇/水)、Sephadex LH-20凝胶柱色谱(氯仿-甲醇 1∶1)等分离得到化合物5(6 mg)。
I组分甲醇不溶物经氯仿-甲醇混合溶剂洗涤后得到化合物6(27.9 mg);该组分其余样品经MCI柱色谱(50%~100%甲醇/水)、硅胶柱层析(氯仿-甲醇 1∶0~0∶1)得到化合物11(779 mg)。
2 结构鉴定
化合物1 C30H50O,无色针晶(石油醚-乙酸乙酯)。1H-NMR (400 MHz, CDCl3) δ: 1.17 (3H, s, Me-28), 1.04 (3H, s, Me-27), 1.00 (3H, s, Me-29), 0.99 (3H, s, Me-26), 0.94 (3H, s, Me-30), 0.87 (3H, d, J = 6.5 Hz, Me-23), 0.86 (3H, s, Me-25), 0.71 (3H, s, Me-24); 13C-NMR (100 MHz, CDCl3) δ: 22.3 (t, C-1), 41.5 (t, C-2), 213.3 (s, C=O), 58.2 (d, C-4), 42.2 (s, C-5), 41.3 (t, C-6), 18.2 (t, C-7), 53.1 (d, C-8), 37.4 (d, C-9), 59.5 (d, C-10), 35.6 (t, C-11), 30.5 (t, C-12), 39.7 (s, C-13), 38.3 (s, C-14), 32.4 (t, C-15), 36.0 (t, C-16), 30.0 (s, C-17), 42.8 (d, C-18), 35.3 (t, C-19), 28.2 (s, C-20), 32.8 (t, C-21), 39.3 (t, C-22), 6.8 (q, C-23), 14.7 (q, C-24), 18.0 (q, C-25), 20.3 (q, C-26), 18.7 (q, C-27), 32.1 (q, C-28), 31.8 (q, C-29), 35.0 (q, C-30)。上述數据与文献报道基本一致(Klass et al., 1992),故鉴定化合物1为木栓酮。
化合物2 C30H52O,白色无定性粉末。1H-NMR (400 MHz, C5D5N) δ: 3.99 (1H, brs, H-3), 1.32 (3H, s, Me-24), 1.25 (3H, s, Me-28), 1.20 (3H, d, J = 6.5 Hz, Me-23), 1.11 (3H, s, Me-30), 1.10 (3H, s, Me-27), 1.05 (3H, s, Me-29), 1.05 (3H, s, Me-26), 0.99 (3H, s, Me-27); 13C-NMR (100 MHz, C5D5N) δ: 17.1 (t, C-1), 36.9 (t, C-2), 71.9 (d, C-3), 50.5 (d, C-4), 39.1 (s, C-5), 42.8 (t, C-6), 18.5 (t, C-7), 54.0 (d, C-8), 38.0 (d, C-9), 62.5 (d, C-10), 36.5 (t, C-11), 31.4 (t, C-12), 39.0 (s, C-13), 40.4 (s, C-14), 33.1 (t, C-15), 39.9 (t, C-16), 30.7 (s, C-17), 43.7 (d, C-18), 33.7 (t, C-19), 28.8 (s, C-20), 36.1 (t, C-21), 37.0 (t, C-22), 12.9 (q, C-23), 17.4 (q, C-24), 19.0 (q, C-25), 20.7 (q, C-26), 19.3 (q, C-27), 32.7 (q, C-28), 35.5 (q, C-29), 32.4 (q, C-30)。上述数据与文献报道基本一致(Duwiejua et al., 1999),故鉴定化合物2为木栓烷醇。
化合物3 C30H50O,无色针晶(石油醚)。1H-NMR (400 MHz, CDCl3) δ: 5.18 (1H, t, J = 3.7 Hz, H-12), 3.23 (1H, dd, J = 10.8, 4.4 Hz, H-3a), 1.06 (3H, s, Me-27), 0.96 (3H, s, Me-26), 0.95 (3H, s, Me-25), 0.91 (3H, s, Me-30), 0.87 (3H, s, Me-23), 0.80 (3H, s, Me-28), 0.79 (3H, s, Me-24), 0.79 (3H, s, Me-29); 13C-NMR (100 MHz, CDCl3) δ: 38.6 (t, C-1), 27.2 (t, C-2), 79.0 (d, C-3), 38.8 (s, C-4), 55.2 (d, C-5), 18.4 (t, C-6), 32.6 (t, C-7), 38.8 (s, C-8), 47.6 (d, C-9), 36.9 (s, C-10), 23.5 (t, C-11), 121.7 (t, C-12), 145.2 (s, C-13), 41.7 (s, C-14), 26.2 (t, C-15), 26.9 (t, C-16), 32.5 (s, C-17), 47.2 (d, C-18), 46.8 (t, C-19), 31.1 (s, C-20), 34.7 (t, C-21), 37.1 (t, C-22), 28.1 (q, C-23), 15.5 (q, C-24), 15.6 (q, C-25), 16.8 (q, C-26), 26.0 (q, C-27), 28.4 (q, C-28), 33.4 (q, C-29), 23.7 (q, C-30)。上述数据与文献报道基本一致(Fukunaga et al., 2009),故鉴定化合物3为β-香树脂醇。
化合物4 C30H50O,无色针晶(石油醚)。1H-NMR (400 MHz, CDCl3) δ: 5.12 (1H, t, J = 3.7 Hz, H-12), 3.21 (1H, dd, J = 10.8, 4.4 Hz, H-3β), 1.13 (3H, s, Me-27), 0.99 (3H, s, Me-23), 0.99 (3H, s, Me-26), 0.93 (3H, s, Me-24), 0.87 (3H, overlap, Me-29), 0.86 (3H, overlap, Me-30), 0.83 (3H, s, Me-28), 0.79 (3H, s, Me-25); 13C-NMR (100 MHz, CDCl3) δ: 38.8 (t, C-1), 27.3 (t, C-2), 79.1 (d, C-3), 38.8 (s, C-4), 55.2 (d, C-5), 18.4 (t, C-6), 32.9 (t, C-7), 40.0 (s, C-8), 47.7 (d, C-9), 36.9 (s, C-10), 23.4 (t, C-11), 124.4 (t, C-12), 139.6 (s, C-13), 42.1 (s, C-14), 26.6 (t, C-15), 28.1 (t, C-16), 33.8 (s, C-17), 59.0 (d, C-18), 39.7 (t, C-19), 39.6 (s, C-20), 31.1 (t, C-21), 41.5 (t, C-22), 28.1 (q, C-23), 15.6 (q, C-24), 15.7 (q, C-25), 16.9 (q, C-26), 23.3 (q, C-27), 28.8 (q, C-28), 17.5 (q, C-29), 21.4 (q, C-30)。上述数据与文献报道一致(Lee et al., 2003),故鉴定化合物4为α-香树脂醇。
化合物5 C29H48O2,无色针晶。1H-NMR (400 MHz, CDCl3) δ: 5.81 (1H, s, H-4), 4.34 (1H, brs, H-6), 1.37 (3H, s, Me-19), 0.92 (3H, d, J = 6.3 Hz, Me-21), 0.84, 0.83,0.80 (各3H, overlap, Me-26, 27, 29), 0.74 (3H, s, Me-18); 13C-NMR (100 MHz, CDCl3) δ: 37.2 (t, C-1), 34.4 (t, C-2), 200.7 (s, C-3), 126.4 (d, C-4), 168.7 (s, C-5), 73.4 (d, C-6), 38.7 (t, C-7), 29.8 (d, C-8), 53.7 (d, C-9), 38.1 (s, C-10), 21.1 (t, C-11), 39.7 (t, C-12), 42.6 (s, C-13), 56.0 (d, C-14), 24.3 (t, C-15), 28.3 (t, C-16), 56.2 (d, C-17), 12.1 (q, C-18), 19.7 (q, C-19), 36.3 (d, C-20), 19.2 (q, C-21), 34.0 (t, C-22), 26.2 (t, C-23), 45.9 (d, C-24), 29.2 (d, C-25), 20.0 (q, C-26), 18.9 (q, C-27), 23.2 (t, C-28), 12.2 (q, C-29)。上述數据与文献报道一致(Niu et al., 2001),故鉴定化合物5为6β-羟基豆甾-4-烯-3-酮。
化合物6 C35H60O6,白色无定型粉末。1H-NMR (400 MHz, C5D5N) δ: 5.36 (1H, brs, H-6), 5.10 (1H, d, J = 7.3 Hz, H-1′), 4.56 (1H, dd, J = 11.8, 2.4 Hz, H-6′a), 4.45 (1H, dd, J = 11.8, 5.2 Hz, H-6′b), 4.32 (2H, overlap, H-4′, 5′), 4.10 (1H, m, H-3), 4.01 (2H, overlap, H-2′, 3′), 1.00 (3H, d, J = 6.4 Hz, Me-21), 0.95 (3H, s, Me-19), 0.94 (3H, overlap, Me-26), 0.90 (3H, overlap, Me-27), 0.89 (3H, overlap, Me-29), 0.67 (3H, s, Me-18); 13C-NMR (100 MHz, C5D5N) δ: 37.6 (t, C-1), 30.3 (t, C-2), 78.7 (d, C-3), 40.5 (t, C-4), 141.0 (s, C-5), 122.0 (d, C-6), 34.3 (t, C-7), 32.3 (d, C-8), 50.4 (d, C-9), 37.6 (s, C-10), 21.4 (t, C-11), 39.4 (t, C-12), 42.6 (s, C-13), 57.0 (d, C-14), 24.6 (t, C-15) , 28.7 (t, C-16), 56.3 (d, C-17), 12.3 (q, C-18), 19.5 (q, C-19) , 36.5 (d, C-20), 19.3 (q, C-21), 34.3 (t, C-22), 26.5 (t, C-23), 46.1 (d, C-24), 29.5 (d, C-25), 20.1 (q, C-26), 19.5 (q, C-27), 32.3 (t, C-28), 12.3 (q, C-29), 102.7 (d, C-1′), 75.4 (d, C-2′), 78.2 (d, C-3′), 71.8 (d, C-4′), 78.7 (d, C-5′), 63.0 (t, C-6′)。以上数据与文献报道基本一致(Cho et al., 2012),故鉴定化合物6为胡萝卜苷。
化合物7 C29H50O,无色针晶(石油醚-乙酸乙酯)。1H-NMR (400 MHz, CDCl3) δ: 5.35 (1H, brs,H-6), 3.52 (1H, m, H-3α), 1.01 (3H, s, Me-19), 0.92 (3H, d, J = 6.5 Hz, Me-21), 0.85, 0.82, 0.81 (各3H, overlap, Me-26, 27, 29), 0.68 (3H, s, Me-18); 13C-NMR (100 MHz, CDCl3) δ: 37.3 (t, C-1), 31.6 (t, C-2),71.8 (d, C-3), 42.3 (t, C-4), 140.8 (s, C-5), 121.7 (d, C-6), 31.9 (t, C-7), 31.6 (d, C-8), 50.1 (d, C-9), 37.3 (s, C-10), 21.9 (t, C-11), 39.8 (t, C-12), 42.3 (s, C-13), 56.8 (d, C-14), 24.3 (t, C-15), 29.1 (t, C-16), 56.1 (d, C-17), 12.0 (q, C-18), 19.4 (q, C-19), 36.5 (d, C-20), 19.4 (q, C-21), 33.9 (t, C-22), 26.1 (t, C-23), 45.8 (d, C-24), 29.2 (d, C-25), 19.8 (q, C-26), 18.8 (q, C-27), 24.3 (t, C-28), 11.9 (q, C-29)。上述数据与文献报道基本一致(Nirmal et al., 2012),故鉴定化合物7为β-谷甾醇。
化合物8 C29H48O,无色针晶(石油醚-乙酸乙酯)。1H-NMR (400 MHz, CDCl3), δ: 5.35 (1H, brs, H-6), 5.15 (1H, dd, J = 15.2, 8.5 Hz, H-23), 5.01 (1H, dd, J = 15.2, 8.5 Hz, H-22), 3.52 (1H, m, H-3SymbolaA@ ), 1.01 (3H, s, Me-19), 0.92 (3H, d, J = 6.5 Hz, Me-21), 0.84, 0.83, 0.80 (各3H, overlap, Me-26, 27, 29), 0.70 (3H, s, Me-18); 13C-NMR (100 MHz, CDCl3) δ: 37.4 (t, C-1), 31.5 (t, C-2), 71.8 (d, C-3), 42.6 (t, C-4), 140.8 (s, C-5), 121.7 (d, C-6), 32.4 (t, C-7), 32.1(d, C-8), 50.7 (d, C-9), 36.5 (s, C-10), 21.2 (t, C-11), 40.5 (t, C-12), 42.2 (s, C-13), 56.2 (d, C-14), 23.1 (t, C-15), 29.2 (t, C-16), 56.9 (d, C-17), 12.1 (q, C-18), 19.8 (q, C-19), 40.5 (d, C-20), 21.2 (q, C-21), 138.3 (d, C-22), 129.3 (d, C-23), 51.2 (d, C-24), 31.9 (d, C-25), 19.0 (q, C-26), 21.2 (q, C-27), 25.4 (t, C-28), 12.3 (q, C-29)。上述数据与文献报道基本一致(Tanaka et al., 2013),故鉴定化合物8为豆甾醇。
化合物9 C9H6O3,淡黄色粉末。1H-NMR (400 MHz, CD3OD) δ: 7.84 (1H, d, J = 9.5 Hz, H-4), 7.44 (1H, d, J = 8.5 Hz, H-5), 6.79 (1H, dd, J = 8.5, 2.3 Hz, H-6), 6.70 (1H, d, J = 2.3 Hz, H-8), 6.18 (1H, d, J = 9.5 Hz, H-3); 13C-NMR (100 MHz, CD3OD) δ: 163.7 (s, C-2), 112.3 (d, C-3), 146.0 (d, C-4), 113.1 (s, C-4a), 130.7 (d, C-5), 114.5 (d, C-6), 163.1 (s, C-7), 103.4 (d, C-8), 157.2 (s, C-8a)。上述數据与文献报道基本一致(An et al., 2005),故鉴定化合物9为7-羟基香豆素。
化合物10 C17H16O4,白色粉末。1H-NMR (400 MHz, C5D5N) δ: 8.15 (1H, d, J = 15.6 Hz, H-7), 7.56 (2H, d, J = 8.6 Hz, H-2, 6), 7.27 (2H, d, J = 8.5 Hz, H-2′, 6′), 7.16 (2H, d, J = 8.4 Hz, H-3′, 5′), 7.13 (2H, d, J = 8.6 Hz, H-3, 5), 6.90 (1H, d, J = 15.6 Hz, H-8), 3.89 (2H, m, H-8′), 3.03 (2H, t, J = 7.2 Hz, H-7′); 13C-NMR (100 MHz, C5D5N) δ: 127.3 (s, C-1), 130.3 (d, C-2, 6), 117.0 (d, C-3, 5), 160.8 (s, C-4), 140.5 (d, C-7), 119.7 (d, C-8), 167.2 (s, C-9), 130.8 (d, C-1′), 130.7 (d, C-2′, 6′), 116.6 (d, C-3′, 5′), 157.7 (s, C-4′), 36.0 (s, C-7′), 42.3 (s, C-8′)。上述数据与文献报道基本一致(邓京振和赵守训, 1993),故鉴定化合物10为对香豆酸-对羟基苯乙醇酯。
化合物11 C30H26O13,黄色粉末。1H-NMR (400 MHz, CD3OD) δ: 7.96 (2H, d, J = 8.9 Hz, H-2′, 6′), 7.37 (H, d, J = 16.0 Hz, H-3), 7.28 (2H, d, J = 8.5 Hz, H-5, 9), 6.77 ( 4H, overlap, H-3′, 5′, H-6, 8), 6.27 (1H, d, J = 2.0 Hz, H-8), 6.10 (1H, d, J = 2.0 Hz, H-6), 6.04 (1H, d, J = 16.0 Hz, H-2), 5.22 (1H, d, J = 7.5 Hz, H-1″), 4.28 (1H, dd, J = 11.8, 2.3 Hz, H-6″a), 4.17 (1H, dd, J = 11.8, 6.6 Hz, H-6″b), 3.45 (3H, overlap, H-2″, 3″, 5″), 3.32 (1H, overlap, H-4″); 13C-NMR (100 MHz, CD3OD) δ: 159.3 (s, C-2), 135.2 (s, C-3), 179.4 (s, C-4), 162.9 (s, C-5), 100.0 (d, C-6), 166.0 (s, C-7), 94.8 (d, C-8), 158.4 (s, C-9), 105.5 (s, C-10), 122.7 (s, C-1′), 132.2 (d, C-2′, 6′), 116.0 (d, C-3′, 5′), 161.2 (s, C-4′), 104.0 (d, C-1″), 75.8 (d, C-2″), 75.7 (d, C-3″), 71.7 (d, C-4″), 78.0 (d, C-5″), 64.3 (t, C-6″), 168.8 (s, C-1), 114.7 (d, C-2), 146.5 (d, C-3), 127.0 (s, C-4), 131.2 (d, C-5, 9), 116.8 (d, C-6, 8), 161.5 (s, C-7)。以上数据与文献报道基本一致(Tsukamoto et al., 2004),故鉴定化合物11为山奈酚香豆酰基葡萄吡喃糖苷。
化合物12 C19H40O4,白色粉末。1H-NMR (400 MHz, CD3Cl) δ: 4.18 (1H, dd, J = 11.6, 4.8 Hz, H-1a), 4.13 (1H, dd, J = 11.6, 6.1 Hz, H-1b), 3.92 (1H, m, H-2), 3.69 (1H, dd, J = 11.3, 3.9 Hz, H-3a), 3.58 (1H, dd, J = 11.5, 5.9 Hz, H-3b), 2.34 (2H, t, J = 7.6 Hz, H-2′), 1.61 (2H, m, H-3′), 1.33-1.24 (24H, brs, H-4′ to 15′), 0.87 (3H, t, J = 6.8 Hz, H-16′); 13C-NMR (100 MHz, CD3Cl) δ: 65.2 (t, C-1), 74.0 (d, C-2), 63.5 (t, C-3), 174.5 (s, C-1′), 34.2 (t, C-2′), 25.0 (t, C-3′), 29.3-29.8 (t, C-4′ to C-13′), 32.0 (t, C-14′), 22.8 (t, C-15′), 14.3 (q, C-16′)。上述数据与文献报道基本一致(傅宏征等, 1997; 吴少华等, 2005),故鉴定化合物12为单棕榈酸甘油酯。
3 讨论
本研究共由圆叶牵牛乙醇提取物乙酸乙酯部位分离鉴定了12个化学成分,包括4个三萜类化合物、4个甾体类化合物、2个苯丙素类化合物、1个黄酮类化合物及1个脂肪酸类化合物,其中有7个化合物(2~5、10~12)为首次从该属植物中分离得到;通过查阅文献发现部分化合物有不同程度的镇痛活性。Da et al. (2011)研究发现化合物3和4通过激活大麻素受体,抑制细胞因子的产生,抑制NF-κB、CREB和环氧合酶2的表达,在持续伤害感觉模型中具有持久的镇痛作用和抗炎作用,指出化合物3和4可能是一种潜在的止痛分子。吴龙火等(2011)发现化合物9在小鼠二甲苯致耳朵腫胀实验中能较强抑制小鼠耳肿胀。本实验组前期研究也发现,化合物7在醋酸致小鼠扭体实验中能减少小鼠扭体次数,具有一定镇痛活性(单华, 2017)。由此初步推断,前期研究中发现的圆叶牵牛具有镇痛活性应该具有一定的合理性。然而,鉴于本研究所分离得到的化合物质量很少,加之没有对这些成分在样品中的总体含量进行分析测定,因此,上述化合物是否就是圆叶牵牛中的镇痛活性成分尚无法明确,相关活性成分及镇痛机制仍有待进一步研究。
参考文献:
AN RB, PARK BY, KIM JH, et al., 2005. Coumarins and chromones from Angelica genuflexa [J]. Nat Prod Sci, 11(2): 79-84.
CHO EJ, JI YC, LEE KH, et al., 2012. Isolation of antibacterial compounds from Parasenecio pseudotaimingasa [J]. Hortic Environ Biotechnol, 53(6): 561-564.
DA SK, PASZCUK AF, PASSOS GF, et al., 2011. Activation of cannabinoid receptors by the pentacyclic triterpene α, β-amyrin inhibits inflammatory and neuropathic persistent pain in mice [J]. Pain, 152(8): 1872-1887.
DENG JZ, ZHAO SX, 1993. Isolation and identification of non-alkaloidal constituents of aerial parts of Stephania longa [J]. J Chin Pharm Univ, (2): 73-75. [邓京振, 赵守训, 1993. 粪箕笃地上部分非碱性成分的分离和鉴定 [J]. 中国药科大学学报, (2): 73-75.]
DUWIEJUA M, ZEITLIN IJ, GRAY AI, et al., 1999. The anti-inflammatory compounds of Polygonum bistorta: Isolation and characterisation [J]. Planta Med, 65(4): 371-374.
Chinese Botanical Society Editorial Board of Chinese Academy of Sciences, 1979. Flora Reipublicae Popularis Sinicae [M]. Beijing: Science Press, 64(1): 103-104. [中国科学院中国植物志编辑委员会, 1979. 中国植物志 [M]. 北京: 科学出版社, 64(1): 103-104.]
FU HZ, LOU ZC, YANG XW, et al., 1997. Studies on the chemical constituents of Glandularstalk St. Paulswort (Siegesbeckia pubescens) (I) [J]. Chin Trad Herb Drugs, 28(5): 259-262. [傅宏征, 楼之岑, 杨秀伟, 等, 1997. 腺梗豨莶的化学成分研究(I) [J]. 中草药, 28(5): 259-262.]
FUKUNAGA T, NISHIYA K, TAKEYA K, et al., 2009. Studies on the constituents of goat’s rue: Galega officinalis L. [J]. Chem Pharm Bull, 35(4): 1610-1614.
JU JH, JEON MJ, YANG W, et al., 2011. Induction of apoptotic cell death by Pharbitis nil extract in HER2-overexpressing MCF-7 cells [J]. J Ethnopharmacol, 133(1): 126-131.
KIM KH, CHOI SU, LEE KR, 2009. Diterpene glycosides from the seeds of Pharbitis nil [J]. J Nat Prod, 72(6): 1121-1127.
KIM KH, WOO KW, MOON E, et al., 2014. Identification of antitumor lignans from the seeds of morning glory (Pharbitis nil) [J]. J Agric Food Chem, 62(31): 7746-7752.
KLASS J, TINTO WF, MCLEAN S, et al., 1992. Friedeland triterpenoids from Peritassa compta: Complete 1H and 13C assignments by 2D NMR spectroscopy [J]. J Nat Prod, 55(11): 1626-1630.
LEE S, KIM KS, SHIM SH, et al., 2003. Constituents from the non-polar fraction of Artemisia apiacea [J]. Arch Pharmacal Res, 26(11): 902-905.
LEE SR, MOON E, KIM KH, 2017. Neolignan and monoterpene glycoside from the seeds of Pharbitis nil [J]. Phytochem Lett, 20: 98-101.
LI JH, DU GJ, LIU WJ, et al., 2014. Study on anti-tumor and anti-metastasis mechanism of alcohol extracts from Pharbitidis Semen against Lewis lung cancer [J]. Chin J Chin Mat Med, 39(5): 879-884. [李佳桓, 杜鋼军, 刘伟杰, 等, 2014. 牵牛子酒提取物对Lewis肺癌的抗肿瘤和抗转移机制研究 [J]. 中国中药杂志, 39(5): 879-884.]
LIANG N, YANG SJ, ZHAO HJ, et al., 2013. Chemical components and biological activity of volatile oil from aboveground of Pharbitis nil (L.) Choisy [J]. Chin Trad Pat Med, 35(5): 1023-1026. [梁娜, 杨胜杰, 赵洪菊, 等, 2013. 裂叶牵牛地上部分挥发油化学成分与生物活性研究 [J]. 中成药, 35(5): 1023-1026.]
MA C, 2011. Metabonomic study of morning glory seed induced nephrotoxity [D]. Shenyang: Shenyang Pharmaceutical University. [馬超, 2011. 中药牵牛子肾毒性代谢组学研究 [D]. 沈阳:沈阳药科大学.]
NIRMAL SA, PAL SC, MANDAL SC, et al., 2012. Analgesic and anti-inflammatory activity of β-sitosterol isolated from Nyctanthes arbortristis leaves [J]. Inflammopharmacology, 20(4): 219-224.
NIU XM, LI SH, PENG LY, et al., 2001. Constituents from Limonia crenulata [J]. J Asian Nat Prod Res, 3(4): 299-311.
SAITO N, CHENG J, ICHIMURA M, et al., 1994. Flavonoids in the acyanic flowers of Pharbitis nil [J]. Phytochemistry, 35(3): 687-691.
SHAN H, 2017. Anti-nociceptive activity screening of the plants from west Yunnan and analgesic activity of Bauhinia brachycarpa Benth [D]. Dali: Dali University. [单华, 2017. 滇西药用植物镇痛活性筛选及鞍叶羊蹄甲镇痛活性研究 [D]. 大理: 大理大学.]
TANAKA A, SHIMIZU K, KONDO R, 2013. Antibacterial compounds from shoot skins of moso bamboo (Phyllostachys pubescens) [J]. J Wood Sci, 59(2): 155-159.
TSUKAMOTO S, TOMISE K, ABURATANI M, et al., 2004. Isolation of cytochrome P450 inhibitors from strawberry fruit, Fragaria ananassa [J]. J Nat Prod, 67(11): 1839-1841.
WU LH, LIU ZW, ZENG J, et al., 2011. Anti-inflammation and analgesic activities of coumarins compounds from the leaves of Murraya exotica (L.) [J]. Chin J Spectrosc Lab, 28(6): 2999-3003. [吴龙火, 刘昭文, 曾靖, 等, 2011. 九里香叶中香豆素类化合物的抗炎镇痛活性 [J]. 光谱实验室, 28(6): 2999-3003.]
WU SH, WU DG, CHEN YW, et al., 2005. Chemical constituents of Paeonia delavayi [J]. Chin Trad Herb Drugs, 36(5): 648-651. [吴少华, 吴大刚, 陈有为, 等, 2005. 紫牡丹的化学成分研究 [J]. 中草药, 36(5): 648-651.]
XU XR, JIANG HY, ZHANG YN, et al., 2006. Bioactivity of Pharbitis purpurea extracts against Plutella xylostella [J]. Agrochemicals, 45(2): 125-126. [徐向荣, 蒋红云, 张燕宁, 等, 2006. 牵牛子提取物对小菜蛾的生物活性 [J]. 农药, 45(2): 125-126.]

