BmDaxx regulates apoptosis in cells and larval tissues of the silkworm(Bombyx mori)
JIAO Peng,XIAO Ying,LIU Zhen,CHEN Ruiting,LU Yan,XIN Huhu,MIAO Yungen()
Abstract We amplified and characterized the BmDaxx gene of the silkworm(Bombyx mori)by real-time quantitative polymerase chain reaction(RT-qPCR).The expression levels of BmDaxx and its downstream genes BmFadd and BmDredd showed significant increases after overexpression of BmDaxx.However,the expression levels of BmDaxx and BmFadd decreased significantly after siRNA interference of BmDaxx.Overexpression of BmDaxx increased the activity of the cystein-asparate protease-3/7(caspase-3/7),while siRNA interference decreased the activity.When BmN cells were treated by caspase-3/7 inhibitor,the transcriptional levels of BmDaxx,BmFadd and BmDredd decreased as compared with controls.The expression level of BmDaxx was significantly higher in silk glands than in other organs at the silking stage,consistent with the silk gland degeneration at this stage.After injecting of the caspase inhibitor,the expression levels of the apoptosis-related genes BmDaxx,BmFadd and BmDredd showed significant decreases.It is concluded that there are direct relationships between BmDaxx and its predicted downstream genes BmFadd and BmDredd,which play critical roles both in BmN cells and in silk gland apoptosis.
Key words silkworm(Bombyx mori);apoptosis;silk gland;death domain-associated protein gene in Bombyx mori(BmDaxx);cystein-asparate protease
Apoptosis,or programmed cell death,is an internally encoded suicide program that can be triggered by extrinsic(cell surface receptor)and intrinsic(mitochondrial depolarization)pathways[1-2].There is now evidence that the two pathways are connected and that the molecules in one pathway can influence the other[3].The extrinsic and intrinsic pathways converge on the same execution pathway,initiated by the cleavage of cystein-asparate protease-3 (caspase-3), which results in characteristic cytomorphological features,including cell shrinkage,chromatin condensation,membrane blebbing,apoptotic body formation and finally phagocytosis of the apoptotic bodies by adjacentparenchymalcells,neoplastic cells or macrophages[4-5].This programmed cell death allows the elimination of cells that have been produced in excess,sustained genetic damage,or developed improperly.This regulation plays an important role in homeostasis and maintains a balance between cell proliferation and cell death[6].Dysregulation of apoptosis leads to a variety of human pathologies including cancer,autoimmune diseases and neurodegenerative disorders[7-10].Physical factors such as ultraviolet(UV)light,or chemical substances such as actinomycin D(Act D),can induce cell apoptosis[11-13].
FAS(also called CD95)is an apoptosis-signaling receptor molecule on the surface of a number of cell types[14].The death domain-associated protein(Daxx)was originally identified as a protein that specifically binds to the death domain of the transmembrane death receptor FAS,which is normally located in the cytoplasm,and it potentiates FAS-induced apoptosis.The sequence of Daxx contains an open reading frame(ORF)of 1 785 bp,and the deduced protein sequence consisted of 594 amino acid residues.The predicted molecular mass of Daxx in Bombyx mori(BmDaxx)is 67.74 kDa.Daxx interacts with proapoptotic receptors,such as FAS and TGFb receptorⅡ[15-16].Canonical FAS pathway recruitment of the FAS associated death domain(FADD)protein in turn activates caspase-8 and downstream caspases and then causes apoptosis[17].Daxx could be associated with both nuclear and cytoplasmic events during apoptosis[18].
Studies have shown thatDaxx plays an important role in many signaling pathways to regulate apoptosis,which can be triggered by a series of stress signals,such as irradiation,hydrogen peroxide treatment and infection by pathogens[16,19].Daxx is a negative regulator of p53,which itself is a target protein of Daxx that can exert its functions by regulating the expression of pro-apoptosis genes,such as Bax,Puma and Bid,or it can induce apoptosis directly by binding to Bcl-2 family members to trigger cytochrome c release[20-23].In addition,Daxx is also found to modulate NF-κB transcriptional activity[24].
The silkworm(B.mori)is not only an important economic animal,but also a model organism.The apoptotic mechanisms of other model organisms(such as Drosophila and nematode)do not fully reflect the apoptotic mechanisms in silkworms.Due to the establishment of a domesticated B.mori genome database,the apoptotic pathway in silkworms has attracted increasing attention[25].Until now,52 apoptosis-related candidate genes were identified[26].In silkworms,five members of the caspase family have been found,including an apoptosis initiator,BmDredd,which was considered to have a similar function to caspase-8[27].Apoptosis mechanisms have been investigated relatively thoroughly in mammals.However,the role of Daxx in insects has several differences,when compared with mammals.The molecular mechanisms of BmDaxx regulating the death-receptor apoptosis pathway in silkworms remain unclear.How BmDaxx interacts with upstream and downstream genes is also ambiguous.
In this work,we investigated the effects of the overexpression and small interference RNA(siRNA)of BmDaxx on transcriptionallevels and the connection with its predicted downstream genes BmFadd and BmDredd in BmN cells.We induced B.mori cell apoptosis by UV irradiation and actinomycin D treatment.The results showed that the expression and transcriptional levels of BmDaxx were closely influenced by related to BmN cell apoptosis,while the transcriptional levels of BmDaxx,BmFadd and BmDredd were significantly decreased in the BmN cells when treated with caspase-3/7 inhibitor[28].We had investigated the relative expression levels of BmDaxx in different organs and at different stages.The results showed that BmDaxx expression was significantly high in silk glands as compared with in other organs at the silking stage,and consistent with silk gland degeneration at this stage.After injection of the caspase inhibitor,the expression levels of the apoptosis-related genes BmDaxx, BmFadd and BmDredd showed significant decreases.These results suggested that BmDaxx,combined with its downstream genes BmFadd and BmDredd,plays a critical role both in BmN cells and in silk gland apoptosis.
1 Materials and methods
1.1 BmN cells and silkworm larvae
The B.mori cell line BmN(originating from the ovary)was maintained in our laboratory and was cultured in a 27℃incubator.The silkworm P50 strain was reared on fresh mulberry leaves under the standard conditions of(25±2)℃,with 75%±5%relative humidity.
1.2 Cloning of the BmDaxx gene from B.mori by polymerase chain reaction
According to the BmDaxx gene sequence(gene ID:100529238)in the GenBank database,a pair of primers used for polymerase chain reaction(PCR)amplification wasdesigned by PrimerPremier analysissoftware,version 5.0 (PremierBiosoft International,Palo Alto,CA,USA)and were synthesized by Sangon Biotech Co.Ltd.,Shanghai,China(Table S1,http://www.zjujournals.com/agr/EN/10.3785/j.issn.1008-9209.2018.09.171).Total RNA was isolated from the tissues of the 5th instar larvae using RNAiso plus,according to the manufacturer’s instructions[TaKaRa Biotechnology (Dalian)Co.Ltd.,China].The concentration of isolated RNA was determined by a NanoDrop 2000 spectrophotometer(Thermo Scientific,Wilmington,NC,USA).Then,for each sample,cDNA was synthesized by using 500 ng of total RNA mixed with the PrimeScript RT master mix[TaKaRaBiotechnology(Dalian)Co.Ltd.,China],according to the manufacturer’s instructions.The resulting cDNAs were used as templates for PCR.PCR was performed with PrimeSTAR DNA polymerase[TaKaRa Biotechnology(Dalian)Co.Ltd.,China]for 32 amplification cycles(denaturing at 98℃for 10 s,annealing at 55℃for 15 s,extension at 72 ℃ for 1 min)in a 20 μL reaction system.The PCR products were recovered using an agarose gel DNA purification recovery kit(Sangon Biotech Co.Ltd.,Shanghai,China)and were cloned into the pMD18-T vector and then sequenced.
1.3 Real-timequantitativepolymerasechain reaction(RT-qPCR)
The primers for RT-qPCR were designed by Primer Premier analysis software,version 5.0(Premier Biosoft International, Palo Alto, CA, USA),according to the NCBI EST database(http://www.ncbi.nlm.nih.gov/),and they were synthesized by Sangon Biotech Co.Ltd.,Shanghai,China(Table S1).The actin 3(A3)was used as a RT-qPCR control gene.RT-qPCR was performed in a total volume of 20 μL on an ABI7300 system(Applied Biosystems,Foster City,CA,USA)using the SYBR®Premix Ex TaqⅡTMfluorescence dye[TaKaRa Biotechnology(Dalian)Co.Ltd.,China]and a two-step amplification protocol consisting of per-denaturing at 95℃for 30 s,followed by 40 cycles of denaturing at 95℃for 5 s and annealing and extension at 60℃for 31 s.The absence of spurious products was confirmed by automated melt curve analysis.CTvalues were usedto calculate the relative gene expression levels.
1.4 Construction ofBmDaxxoverexpression plasmids and transfection
BmDaxx was amplified by RT-qPCR using the following primers:F,5´-GGATCCATGATTGGAGA TTTAGCAGA(BamHⅠ site is underlined)and R,5´-TCTAGATTAGTCATCATCGCTATCGG(XbaⅠsite is underlined).BmDaxx was inserted into the pIZ/V5-His and pIZ/V5-EGFP-His vectors via the same enzymes.The resulting transformation vectors were designated as pIZ/V5-Daxx-His and pIZ/V5-EGFP-Daxx-His,respectively(Fig.S1,http://www.zjujournals.com/agr/EN/10.3785/j.issn.1008-9209.2018.09.171).
A volume of 12 μL of FuGENE®6 transfection reagent(Promega,USA)was added to the 100 μLserumfree medium and was incubated at room temperature for 5 min.Two micrograms of plasmid DNA were added to the FuGENE®6 transfection reagent/medium,and mixing was performed immediately.The FuGENE®6 transfection reagent/DNA mixture was incubated at room temperature for 15 min,and then the transfection complex was dropped into a six-well cell culture plate.At 48 h post-transfection,green fluorescence was observed by an inverted fluorescence microscope,and then the BmN cells were collected,and RT-qPCR was performed with an ABI7300 system to detect the gene transcription levels.
1.5 siRNA interference of BmDaxx gene in BmN cells
Three BmDaxx siRNAs(their target sequences were as follows:BmDaxx siRNA-1,CCAGACAAACC UGUGACAUTT;BmDaxx siRNA-2,GCGUUACAU CAGUCAUAUUTT;BmDaxx siRNA-3,GCCAAUGC UACUGAGAGUUTT)and a negative control siRNA(UUCUCCGAACGUGUCACGUTT)were synthesized by GenePharma Co.Ltd.(Shanghai,China)using the primers in Table S2(http://www.zjujournals.com/agr/EN/10.3785/j.issn.1008-9209.2018.09.171).The BmN cells were inoculated onto a six-well cell culture plate and were cultured in TC-100 medium with 10%fetal bovine serum(FBS).The cells in each well were transfected with the three BmDaxx siRNAs and the control siRNA with transfection reagent,according to the instructions(GenePharma Co.Ltd.,Shanghai).The cells were separately collected at 24,36,48 and 72 h after transfection,and RT-qPCR was performed to detect the transcriptional level of BmDaxx.
1.6 UV irradiation and actinomycin D treatment
At 24 h after transfection of siRNA or 36 h after transfection of overexpression plasmids,the culture medium was slightly removed.The BmN cells were exposed to UV irradiation(power of 8 W,wavelength of 245 nm,distance of 40 cm and time of 30 s),and then added with 2 mL of fresh medium to each culture dish,and incubated for 12 h.
At 24 h after transfection of siRNA or 36 h after transfection of overexpression plasmids of another cell group,actinomycin D was added to the culture medium to a final concentration of 150 ng/μL,and incubated under the same conditions.
1.7 Caspase-3/7 activity assay and flow cytometric analysis
At 12 h after UV irradiation or actinomycin D treatment,caspase-3/7 activity was measured using a Caspase-Glo®3/7 assay kit(Promega,Madison,WI,USA),according to the instructions.The Caspase-Glo 3/7 buffer and lyophilized Caspase-Glo®3/7 substrate were equilibrated to room temperature before use.Mix the buffer solution with the substrate.The cells were suspended by pipetting and 100 μL per well were added into a white-walled 96-well plate.In addition,100 μL per well of Caspase-Glo®3/7 reagent were added into a white-walled 96-well plate containing 100 μL of blank,negative control cells or treated cells in culture medium.The plate was shaken at 300-500 r/min for 30 s and incubated at room temperature for 1 h.Finally,the caspase activities were detected by the GloMax-96 luminometer(Promega,USA).Moreover,the number of apoptotic cells was determined by flow cytometry (Beckman Coulter FC-500,Kraemer Boulevard Brea,CA,USA)using an annexin V-PI/FITC apoptosis assay kit(Vazyme Biotech Co.Ltd.,Nanjing,China),according to the protocol.
1.8 Caspase inhibitor treatment of BmN cells
Caspase-3/7 inhibitorⅠ(ApexBio,Houston,TX,USA),which is a specific caspase-3/7 signaling pathway inhibitor,was dissolved in dimethyl sulfoxide(DMSO)at 5 mmol/L,and then was added to the culture medium to the final concentration of 50 μmol/L.At 36 h post-treatment,BmN cells were collected,and RT-qPCR was performed to detect the transcriptional levels of apoptosis-related genes.After treatment,another cell group was detected by flow cytometry.
1.9 In vivo caspase inhibitor treatment
Another caspase-3 inhibitor,Z-DEVD-FMK(Selleck Chemicals,Houston,TX,USA),was diluted in phosphate buffer saline(PBS)according to the protocol,and then injected a corresponding quantity into the silkworms at the beginning of the wandering stage based on its mass(22.4 μg/g).Each silkworm injected 6 μL of 10 mmol/L storage solution,and silk glands were dissected at 36 h post-spinning.
1.10 Protein extraction and caspase-3 activity assay in silk glands
The dissected silk glands were ground in 100 μL of tissue lysis buffer(Beyotime,Shanghai,China)per 5-10 mg of tissue.Lysates were then placed in an ice bath for 5 min and were centrifuged at 1.8×104gat 4℃for 12 min.The supernatant was transferred to an ice-cold 1.5 mL centrifuge tube to detect caspase-3 activity immediately.According to the protocol of the caspase-3 activity assay kit(Beyotime,Shanghai,China),50 μL of reaction buffer,10 μL of Ac-DEVD-pNA(2 mmol/L)and the 40 μL sample were mixed.The mixture was incubated for 60-120 min at 37℃,and the absorbance was measured at 405 nm.The total protein concentration was determined by a Bradford protein assay kit[TaKaRa Biotechnology(Dalian)Co.Ltd.,China],according to its protocol.
1.11 Western blotting
The prokaryotic expression vector ofBmDaxxwas named as pET30α(+)-BmDaxx.The above vector was transfected intoE.coliBL21 competent cells.One milliliter of bacteria induced by isopropyl-β-D-thiogalactoside(IPTG)was lysed in 5×sample buffer and boiled at 99 ℃ for 10 min,and 15 μL were analyzed on a 10%sodium dodecyl sulfate(SDS)-polyacrylamide gel.The proteins were separated by electrophoresis and transferred to a nitrocellulose(NC)membrane,and they were subsequently washed with Tris-buffered saline Tween-20(TBST).The membranes were blocked in 5%dried skimmed milk/TBST for 1 h,followed by incubation in 6×His-Tag(1∶5 000)(HuaBio Ltd.,Hangzhou,China)at room temperature for 1.5 h.The membrane was washed 3 times per 10 min and was incubated with 1∶5 000 diluted ImmunoPure goat anti-rabbit IgG second antibody(HuaBio Ltd.,Hangzhou,China)at room temperature for 1.5 h.The specific protein band was visible using FDbio-Dura ECL Western blotting detection reagents(Fdbio Science,Hangzhou,China).
1.12 Statistical analysis
All of the experiments were performed in three independent biological replications,and the reactions of each sample were conducted in triplicate.All of the data were determined by one-way analysis of variance(ANOVA).Significance was set atP<0.05 and extremely significant was set atP<0.01.
2 Results
2.1 Cloning of the BmDaxx gene from B.mori
Thefull-lengthBmDaxxgeneproductwas amplified from the 5th instar larvae by PCR(Fig.1A)and was sequenced.The result of Western blotting analysisshowedthattheBmDaxxproteinwas expressed inE.coliBL21(contained 6×His-Tag)(Fig.1B).
2.2 Overexpression and siRNA interference of BmDaxx in BmN cells

Fig.1 PCR analysis of BmDaxx in B.mori
The overexpression vector,pIZ/V5-Daxx-His,was transfected into BmN cells.After 48 h,the cells were collected,and RT-qPCR was performed to detect relative expression levels ofBmDaxx.Another group transfected by pIZ/V5-EGFP-His control vector was performed to show the transfection efficiency(Fig.2).After overexpression ofBmDaxx,the transcriptional level of theBmDaxxgene was increased by approximately 57-fold,compared with the controls(Fig.2A).
At 36 h post-transfection ofBmDaxx-siRNA and control-negative siRNA,the BmN cells were collected,and RT-qPCR was performed to detect the transcriptional level ofBmDaxx.The expression of theBmDaxxgene was decreased by 57.53%,32.39%and 62.61%after adding siRNA-1,siRNA-2 and siRNA-3,respectively.This finding revealed that siRNA-1 and siRNA-3 had significant interference with theBmDaxxgene(Fig.2B).
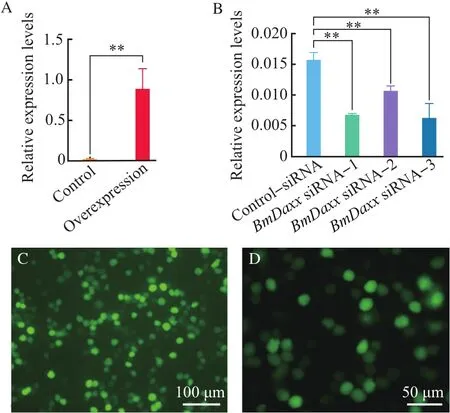
Fig. 2 Relative expression levels of BmDaxx in BmN cells with overexpression and siRNA interference
2.3 BmDaxx activated caspase-3/7 activity
As important effector caspases,caspase-3/7 activities are vital indices for detecting the degree of apoptosis.After overexpression ofBmDaxx,the caspase-3/7-like activity was increased by 41.35%compared with the controls(Fig.3A),but the level ofcaspase-3/7-like activity did not change significantly after transfection byBmDaxx-siRNA in BmN cells,compared with the control-negative siRNA group(Fig.3B).This result showed that overexpression ofBmDaxxincreased the caspase-3/7 activity.

Fig.3 Caspase--3/7 activities in BmN cells with overexpression and siRNAinterference
2.4 Effects of overexpression and siRNA interference of BmDaxx on UV irradiation and actinomycin D-induced apoptosis
To identify the function ofBmDaxx,we employed overexpression and siRNA interference by flow cytometry to detect the apoptosis rate(Fig.4).We chose UV irradiation and actinomycin D treatment as apoptosis-induced conditions.The BmN cells were divided into four cell groups representing the treatments with control vector pIZ/V5-His(control group),overexpression vector pIZ/V5-Daxx-His(overexpression group),control-negative siRNA(control-siRNA group)andBmDaxxsiRNA-3(BmDaxxsiRNAgroup).
At 36 h post-transfection of the expression vector or at 24 h post-transfection of siRNA,the BmN cells were irradiated with UV,or actinomycin D was added to the culture medium to the final concentration of 150 ng/μL.At 12 h post-treatment,the cell apoptosis rate was determined by flow cytometry.
After UV irradiation,the number of early apoptotic BmN cells was increased by approximately 94.26% in theBmDaxxoverexpression group compared with the control vector group,while the number of normal cells was decreased by approximately 20.46% (Fig.4A).Our data also showed that,after UV irradiation,the percentage of early apoptotic cells in theBmDaxxsiRNA interference group was reduced by 5.57%compared with the control-negative siRNA group,while the number of normal cells was increased by approximately 7.39%(Fig.4A).
As shown in Fig.4B,after actinomycin D treatment,the percentage of early apoptotic cells was increased by approximately 76.61%in theBmDaxxoverexpression group compared with the control vector group,while the number of normal cells was decreased by approximately 26.31%(Fig.4B).Our data also demonstrated that,after actinomycin D treatment,the percentage of early apoptotic cells in theBmDaxxsiRNA interference group was increased by approximately 26.84%compared with the controlnegative siRNA group,while the number of normal cells was also increased by approximately 6.57%(Fig.4B).The results showed that the cell apoptosis rate by the flow cytometry detection was increased accordingly when theBmDaxxgene was overexpressed.When the expression ofBmDaxxgene was interfered by three different target siRNAs,the apoptosis rate of UV irradiation and actinomycin D-induced cells was not significantly different.

Fig.4 Effects of overexpression and siRNA interference ofBmDaxx on UV irradiation and actinomycin Dinduced apoptosis
2.5 Relationship among apoptosis-related genes BmDaxx,BmFadd and BmDredd in BmN cells
BmN cells were divided into six cell groups representing the treatments with control vector pIZ/V5-His,overexpression vector pIZ/V5-Daxx-His,control-negativesiRNA andBmDaxxsiRNA-1,BmDaxxsiRNA-2 andBmDaxxsiRNA-3.The cells were collected at 48 h post-transfection with the expression vector or at 24 h post-transfection with siRNA.Expression levels ofBmDaxx,BmFaddandBmDreddwere measured by RT-qPCR.The result showed that,after overexpression ofBmDaxx,the transcriptional levels ofBmDaxx,BmFaddandBmDreddgenes were increased by approximately 57-fold,42.85%and 4.73%,respectively,compared with the control vector group(Fig.5A).
Compared with thecontrol-negativesiRNA group,after treatment withBmDaxxsiRNA-1,the expression levels of theBmDaxx,BmFaddandBmDreddgenes were decreased by 57.63%,30.01%,and 21.32%,respectively.After treatment withBmDaxxsiRNA-2,the expression levels of theBmDaxx,BmFaddandBmDreddgenes were decreased by 32.41%,17.41%,and 5.74%,respectively.The expression levels of theBmDaxxandBmFaddgenes were separately decreased by 60.76%and 33.65%,while theBmDreddgene was increased by 3.23%after treatment withBmDaxxsiRNA-3(Fig.5B).So we speculate that theBmDaxxandBmFaddgenes participate in the same apoptotic pathway and have positive relationships.
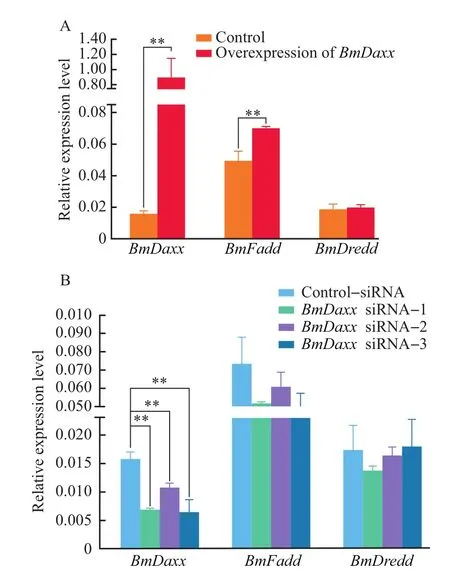
Fig.5 Relationships among B.mori apoptosis-related genes
2.6 Effects of caspase inhibitor on BmN cells
After treatment with inhibitor,the caspase-3/7-like activity was decreased by 22.69%,compared with the control(Fig.6A).At 36 h after caspase-3/7 inhibitorⅠtreatment,the cells were collected,and the transcriptional levels ofBmDaxx,BmFaddandBmDreddwere determined by RT-qPCR.The result showed that,after caspase-3/7 inhibitorⅠtreatment,the transcriptional levels ofBmDaxx,BmFaddandBmDreddgene were decreased by 23.19%,22.48%and 28.19%,respectively,compared with the control group(Fig.6B).To test further the effects of inhibitorⅠon BmN cells,we employed inhibitor cells by flow cytometry to detect the apoptosis rate.Our data showed that,after caspase-3/7 inhibitorⅠtreatment,the number of early apoptotic BmN cells was decreased by approximately 29.00%compared with the control,while the number of normal cells was increased by 33.85%(Fig.6C).Therefore,we speculate that these three genes interact with each other in BmN cells and participate in the same apoptotic pathway.
2.7 Relative expression levels of BmDaxx in different tissues at different stages
Because the 5th instar larvae undergo dramatic changes in the silk glands for pupal development and adult emergence(i.e.,cocoon spinning),to provide insight into the function ofBmDaxxin silkworms,we extracted RNA from different tissues (blood,epidermis,middle silk gland,posterior silk gland,midgut,fat body)and different stages of silkworm larvae,and used RT-qPCR to examineBmDaxxexpression levels.
The experimental data(Fig.7)showed that,during the spinning stage,the transcriptional level ofBmDaxxwas the highest in the middle silk gland and posterior silk gland,especially in the posterior silk gland compared with the other four tissues(blood,epidermis,midgut and fat body).In addition,at the early developmental stage of the silk gland,the transcriptional level ofBmDaxxremained at a low level,but it reached a peak during the spinning period and then decreased gradually during the pupal period.This trend was consistent with the changes in silk glands.These results indicated that theBmDaxxgene is involved in the process of silk gland degradation.
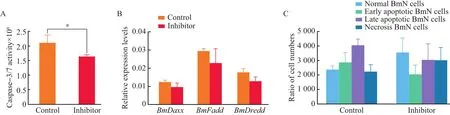
Fig.6 Effects of caspase inhibitorⅠon BmN cells
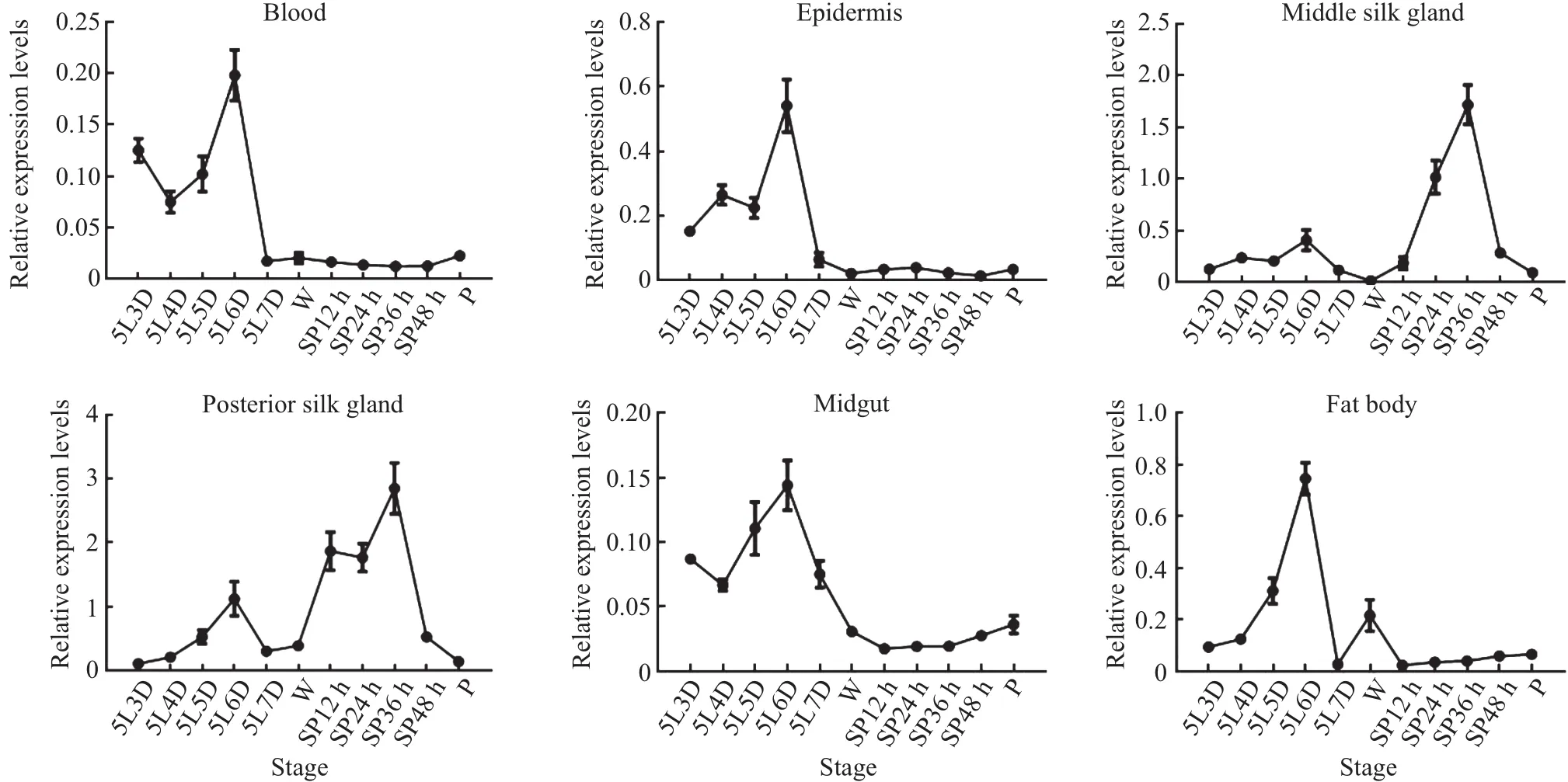
Fig.7 Relative expression levels of BmDaxx in different tissues at different stages
2.8 Effects of caspase inhibitor on silk glands
Silkworms presented drastic changes during the transition from the spinning stage to the pupal stage.During this period,the silk glands undergo rapid degeneration,forming fat.The expression level ofBmDaxxwas consistent with silk gland degeneration(Fig.7),and this degeneration involved apoptosis,also increased caspase-3-like activity.Therefore,we injected caspase inhibitorZ-DEVD-FMK into silkworms at the beginning of the wandering stage.At 36 h after injection,we extracted RNA from silk glands and used RT-qPCR to examine the expression levels of the apoptosis-related genesBmDaxx,BmFaddandBmDredd.The result(Fig.8)showed that, after injection of caspase inhibitor, the transcriptional levels of theBmDaxx,BmFaddandBmDreddgenes were decreased by approximately 66.09%,71.52%and 54.80%,respectively.So we speculate that these three genes participate in the same apoptotic pathway on individual levels.

Fig.8 Relative expression levels of BmDaxx,BmFadd and BmDredd in silk glands after caspase inhibitor Z--DEVD--FMK treatment
3 Discussion
Asa highly conserved nuclearprotein in mammals,FAS death domain-associated protein(Daxx)can activatetheJun-N-terminalkinase(JNK)pathway by binding to FAS death domain and accelerate the apopotosis[16,29-30].In some cases,Daxx also has anti-apoptosis capacity[31-33].But the function of BmDaxx in insect(e.g.domestic silkworm)apoptotic pathway maybe is different from that in mammals.We conducted the overexpression and siRNA interference by flow cytometry to detect the apoptosis rate underUV irradiation and actinomycin D treatment as apoptosis-induced conditions.The results showed the cells were sensitive to cell death following its up-regulation byBmDaxxoverexpression.It implied Daxx promoted apoptosis in cultured insect cells,at least under the conditions used.But the siRNA interference did not show significant effect.We speculated thatBmDaxxmay be interference,but theBmDaxxgene has a competitive inhibition on the apoptotic pathway.In addition,the expression levels of those apoptosis-related genes increased,and they also increased the caspase activities and the number of cell apoptosis,which decreased the interference effect of siRNA.
Prior studies have shown thatBmDaxx,BmFaddandBmDreddare important apoptosis-related genes[34].The interactions between these upstream and downstream genes are important for their function.We investigated their expression levels in BmN cells when there wasBmDaxxoverexpression and interference.The data showed that,after overexpression ofBmDaxx,the transcriptional levels ofBmFaddandBmDreddwere increased.In addition,the expression level ofBmDaxxgene was decreased by siRNA interference;meanwhile,the expression level ofBmFaddgene was decreased.These data showed a positive correlation betweenBmDaxxandBmFadd.According to those results,we speculate thatBmDaxxandBmFaddmight interact with each other in the same apoptosis pathway,and theBmDaxxgene might be a direct upstream gene ofBmFadd.However,the connection betweenBmDreddandBmDaxxwas not so clear.We thought the apoptosis pathway was regulated by many genes and the caspase pathway was also regulated by many different interactive proteins.BmDreddexpression patterns differ from those ofBmDaxxandBmFaddmaybe thatBmDreddis not the direct interaction gene,and the interaction betweenBmDreddandBmDaxxstill needs further validation.
Caspase-3(CPP32/apopain)plays an important role in the initiation of apoptosis.It has a specific recognition sequence of substrate polypeptide,namely aspartate-glutamate-valine-aspartate(DEVD).Z-DEVD-Rh 110-DVED-Z,as a fluorescent indicator of caspase-3 activity,breaks Rh 110 polypeptide by caspase-3 action to produce Rh 110 with strong green fluorescence,and its fluorescence intensity is detected at 520-530 nm.Therefore,cell apoptosis can be detected by measuring the activity of caspase 3/7.We used caspase-3/7 inhibitor to treat BmN cells,and the results showed that the transcriptional levels of theBmDaxx,BmFaddandBmDreddgenes were decreased compared with the controls.Due to the caspase inhibitor acting on caspase protein,when the expressed protein was reduced,the expression level of caspase gene would increase.In this study,we detected that the transcriptional levels ofBmDaxxwere decreased,so predicted that the three apoptosisrelated genes,BmDaxx,BmFaddandBmDreddmight be located in the upstream of the apoptosis pathway.
Further,flow cytometry was used to detect the number of early apoptotic BmN cells.These data indicated that the three genes were involved in the cell apoptosis pathway.We injected the caspase inhibitor into silkworm larvae for further testing of the relationship between these apoptosis-related genes.The result showed that,after caspase inhibitor treatment,the transcriptional levels of theBmDaxx,BmFaddandBmDreddgenes were decreased.This finding indicatedBmDaxxmight be located in the upstream of the apoptosis pathway;when apoptosis signals activatingBmDaxx,its direct downstream geneBmFaddis activated,thus activating a series of downstream genes (includingBmDredd) and inducing apoptosis.
Silkworm(B.mori)is an important economic insect and model organism which has been used effectively in various biological researches[35].Silkworm is an insect of complete metamorphosis.During the metamorphosis stage from the late of 5th instar larva through pupa to eclosion,the silk glands had a dramatic change.Generally from the third day to the end of the fifth instar,the silk gland developed rapidly and synthesized a large number of silk proteins in a short time.The silk glands can grow for 1.6×105times in mass compared with its embryonic stage,and become the largest organ in the abdominal cavity of silkworm,accounting for 40%-50%of body mass[36].However,in the short time after the end of cocooning to the pupation,under the action of hormones,the silk glands undergo drastic changes.Finally,the silk glands degenerated and disappeared in the early of pupa[37].We investigated whetherBmDaxxacted as a promoter of the silkworm apoptosispathway.The resultshowed thatthe expression level ofBmDaxxreached a peak at the spinning stage and then decreased gradually during the pupal period.It shared the same trend as the process of silk gland apoptosis.These results indicated that theBmDaxxgene was involved in the process of silk gland degradation.
Overall,BmDaxxhas the function of promoting apoptosis,and with its predicted downstream genesBmFaddandBmDredd,it plays a critical role in both BmN cells and silk gland apoptosis.

