益肝解毒方及其拆方对非酒精性脂肪性肝炎大鼠UCP-2的影响
郑昕 陈骏毅 陆仕洪 周菲 郭兆玮 庄敏之 王亚平
[摘要] 目的 通过高脂饮食建立非酒精性脂肪性肝炎(NASH)大鼠模型,观察益肝解毒方及其拆方对NASH大鼠UCP-2的影响。 方法 将60只Wistar大鼠依据随机数字表法分为正常组、模型组、全方组、化痰组、祛瘀组、解毒组,每组10只,给予高脂饲料喂养16周以复制NASH模型,正常组(生理盐水1.575 mL/100 g灌胃)、模型组(生理盐水1.575 mL/100 g灌胃)、全方组(中药1.730 g/100 g灌胃)、化痰组(中药1.730 g/100 g灌胃)、祛瘀组(中药1.730 g/100 g灌胃)、解毒组(中药1.730 g/100 g灌胃)干预至16周后处死大鼠,收集标本。运用赖式比色法测定各组大鼠血清谷丙转氨酶(ALT)、谷草转氨酶(AST)、谷氨酰转移酶(GGT)、三酰甘油(TG)、总胆固醇(TC)、高密度脂蛋白(HDL)、低密度脂蛋白(LDL)、活性氧(ROS);RT-PCR法检测UCP-2 mRNA;Western blot检测UCP-2蛋白。 结果 大鼠NASH模型成功建立。全方组及各拆方组ALT、AST、GGT、TC、TG、HDL、ROS、UCP-2 mRNA均值较模型组明显降低,LDL较模型组明显升高,差异有统计学意义(P < 0.05)。各拆方组ALT、AST、GGT、TC、TG、HDL、ROS、UCP-2 mRNA均值均高于全方组,差异有统计学意义(P < 0.05),祛瘀组、解毒组ALT、AST、GGT、TC、TG、HDL水平上低于化痰组,差异有统计学意义(P < 0.05),祛瘀组LDL水平均高于其他各组,差异有统计学意义(P < 0.05)。Western blot结果显示,与正常组比较,模型组UCP-2蛋白表达增强;与模型组比較,全方组UCP-2蛋白表达减低;各拆方组UCP-2蛋白表达相近。 结论 全方组效果最好,体现出中药复方是其各组成部分协同作用的结果。益肝解毒方可抑制ROS产生,降低UCP-2高表达和血清中ALT、AST、GGT、TC、TG、HDL水平,提高LDL水平。
[关键词] 非酒精性脂肪性肝炎;拆方研究;益肝解毒方;大鼠
[中图分类号] R575.1 [文献标识码] A [文章编号] 1673-7210(2019)07(b)-0009-05
Effects of Yigan Jiedu Decoction and its preparation on UCP-2 in non-alcoholic steatohepatitis rats
ZHENG Xin1 CHEN Junyi2 LU Shihong3 ZHOU Fei1 GUO Zhaowei1 ZHUANG Minzhi1 WANG Yaping1
1.Department of Traditional Chinese Medicine, Shanghai Fourth People′s Hospital, Shanghai 200081, China; 2.Department of Surgery, Shanghai Fourth People′s Hospital, Shanghai 200081, China; 3.Department of Gynecology, Shanghai Fourth People′s Hospital, Shanghai 200081, China
[Abstract] Objective To establish the rat model of non-alcoholic steatohepatitis (NASH) by high-fat diet, and to observe the effect of Yigan Jiedu Decoction and its preparation on UCP-2 in rats with NASH. Methods According to the random number table method, 60 Wistar rats were divided into normal group, model group, whole formula group, phlegm elimination group, stasis removal group and detoxification group, 10 rats in each group were given high-fat diet for 16 weeks to replicate the NASH model. Rats in the normal group (1.575 mL/100 g normal saline), model group (1.575 ml/100 g normal saline), whole formnla group (1.730 g/100 g of traditional Chinese medicine), phlegm elimination group (1.730 g/100 g of traditional Chinese medicine), stasis removal group (1.730 g/100 g of traditional Chinese medicine), and detoxification group (1.730 g/100 g of traditional Chinese medicine) were put to death after 16 weeks of intervention, and specimens were collected. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamyl transferase (GGT), triglyceride (TG), total cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein (LDL) and reactive oxygen species (ROS) were determined by Reitman colorimetry. UCP-2 mRNA was detected by RT-PCR. UCP-2 protein was detected by Western blot. Results NASH model of rats was successfully established. The mean values of ALT, AST, GGT, TC, TG, HDL, ROS and UCP-2 mRNA in the whole formula group and its preparation groups were significantly lower than those in the model group, while LDL was higher than that in the model group, with statistically significant differences (P < 0.05). The mean values of ALT, AST, GGT, TC, TG, HDL, ROS and UCP-2 mRNA in preparation groups were higher than those in the whole formula group, with statistically significant differences (P < 0.05). The levels of ALT, AST, GGT, TC, TG and HDL in the stasis removal group and the detoxification group were lower than those in the phlegm elimination group, and the differences were statistically significant (P < 0.05). The LDL level of the stasis removal group was higher than that of other groups, and the difference was statistically significant (P < 0.05). The results of Western blot showed that compared with normal group, the expression of UCP-2 protein in the model group was increased; compared with model group, the expression of UCP-2 protein in the whole formula was decreased; while the expression of UCP-2 protein among all preparation groups was similar. Conclusion The whole formula group has the best effect, which shows that the TCM compound is the result of the synergistic effect of its components. Yigan Jiedu Decoction can inhibit the production of ROS, reduce the high expression of UCP-2 and the levels of serum ALT, AST, GGT, TC, TG, HDL, and improve the level of LDL.
[Key words] Non-alcoholic steatohepatitis; Recipe compotion; Yigan Jiedu Decoction; Rat
非酒精性脂肪性肝炎(non-alcoholic steatohepatitis,NASH)是非酒精性脂肪性肝病发展到较为严重的阶段,在此阶段进行干预治疗具有较高的临床价值[1]。益肝解毒方是上海市第四人民医院(以下简称“我院”)治疗NASH的协定处方,具有较好的临床效果[2-3]。该方主要由化痰、祛瘀、解毒三类药物组成,为了研究该方组成药物的配伍作用机制,我们根据不同治法将该方拆分为化痰组、祛瘀组、解毒组。通过NASH的动物模型[4],观察全方及各拆方组对NASH大鼠UCP-2的影响,探讨不同组分对NASH的治疗效果,以指导临床应用中更加灵活地加减配伍。
1 材料与方法
1.1 材料
1.1.1 动物 选用雄性清洁级Wistar大鼠60只,5~6周龄,体重(140±20)g,购自上海斯莱克实验动物有限公司,合格证号SCXK(沪)2016-0002。大鼠在相同条件下分笼饲养,每笼10只,常规喂养标准颗粒饲料,自由饮用水。
1.1.2 药物 全方组:海藻30 g、广郁金15 g、决明子30 g、广姜黄15 g、垂盆草30 g、龙胆草10 g、人参叶15 g、地鳖虫10 g、柴胡10 g;化痰组:海藻30 g、决明子30 g、柴胡10 g;祛瘀组:广郁金15 g、广姜黄15 g、地鳖虫10 g;解毒组:垂盆草30 g、龙胆草10 g、人参叶15 g。以上药物均按照统一产地、品种、供货商的药源,配制处方,由上海雷允上饮片厂完成制剂。浓缩至200 mL,折合成大鼠临床剂量为1.575 mL/100 g,生药量1.730 g/100 g。
1.1.3 主要试剂 血清谷丙转氨酶(ALT)试剂盒(货号C009-1)、谷草转氨酶(AST)试剂盒(货号C010-1)、谷氨酰转移酶(GGT)试剂盒(货号C017)、三酰甘油(TG)试剂盒(货号F001-1)、总胆固醇(TC)试剂盒(货号F0021-1)、高密度脂蛋白(HDL)试剂盒(货号F003-2)、低密度脂蛋白(LDL)试剂盒(货号F004-2)、活性氧(ROS)试剂盒(货号E004)均购自南京建成生物工程研究所;UCP2抗体购自Abcam(货号:Ab67241)。
1.1.4 主要仪器 低温冷冻离心机(上海卢湘仪离心机仪器有限公司TG-16M);酶标仪(芬兰雷勃酶标仪MK3)。
1.2 分组
雄性Wistar大鼠60只,适应性饲养1周后均存活,依据随机数字表法分为正常组、模型组、全方组、化痰组、祛瘀组、解毒组,每组10只。选用高脂饲料造模法[3-4],即给予模型组、全方组、化痰组、祛瘀组、解毒组高脂饲料(88%普通饲料+10%猪油+2%胆固醇),并予药物灌胃至16周处死。正常组予普通饲料喂养并予生理盐水灌胃至16周后处死。中药汤剂灌胃量、生理盐水剂量均为1.730 g/100 g(折算生药量)。
1.3 样本采集
16周后大鼠禁食24 h,称重后以2%戊巴比妥钠腹腔注射麻醉(剂量为5.5 mL/kg),腹主动脉采血,室温静置2 h,3000 r/min,离心15 min,离心半径为5 cm,分离血清并分装于冻存管中,并置于-80℃冰箱备用。迅速摘取肝脏称重后,于肝右叶切取1.0 cm×1.0 cm×0.2 cm大小肝组织2块,4%中性甲醛溶液固定。剩余肝组织分装后于液氮中速冻,-80℃冰箱保存[5]。实验重复3次。
1.4 Western blot的实验步骤
①组织块称重;②利用液氮、研钵粉碎组织块;③加入RIPA缓冲液(每克组织3 mL RIPA),PMSF(30 μL/g,10 mg/mL PMSF),利用Polytron进一步匀浆(15 000 r/min,1 min)维持4℃;④加入PMSF(30 μL/g,10 mg/mL PMSF),冰上孵育30 min;⑤移入离心管4℃ 约20 000 g,15 min;⑥上清液为细胞裂解液可分装-20℃保存;⑦Bradford比色法测定蛋白质浓度;⑧取相同质量的细胞裂解液(体积×蛋白质浓度),并加等体积的2×电泳加样缓冲液;⑨沸水浴中3 min;⑩上样;电泳(浓缩胶20 mA,分离胶35 mA);电转膜仪转膜(100 mA 40 min);膜用丽春红染色,胶用考马斯亮蓝染色;Western blot 试剂盒显色;分析比较记录[6]。
1.5 观察指标
运用赖式比色法测定ALT、AST、GGT、TC、TG、LDL、HDL、ROS、UCP-2,RT-PCR法检测UCP-2 mRNA,Western blot检测UCP-2蛋白[7]。
1.6 Western blot法检测相关蛋白
RT-PCR实验引物CUP-2正向序列为5′-GCATTTCGGGCAACATTGGG-3′,反向序列5′-TCCCATTCTCAGCCTTGAC-3′,大小為147 bp。
1.7 统计学方法
采用SPSS 13.0统计学软件进行单因素分析。采用半定量法,计量资料采用均数±标准差(x±s)表示,各组间实验数据的比较采用方差分析,两两比较用LSD-t检验。以P < 0.05为差异有统计学意义。
2 结果
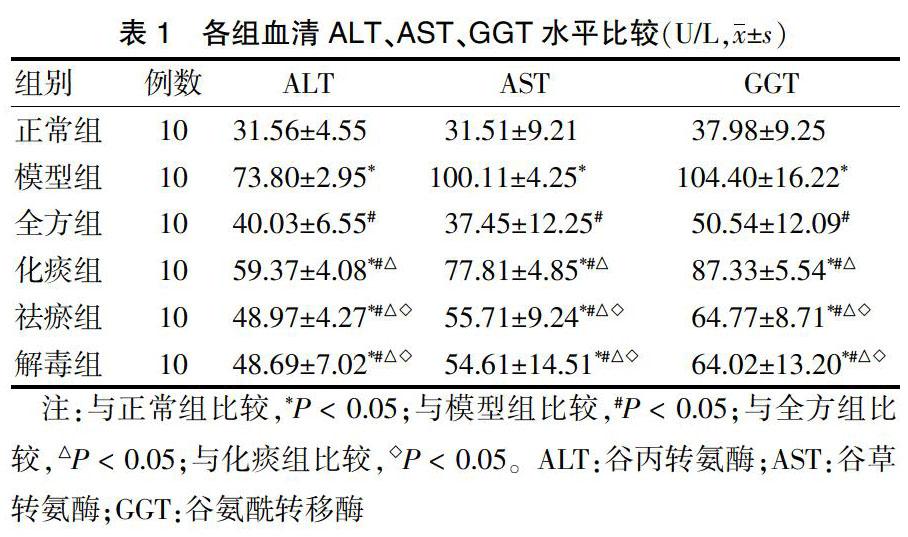
2.1 各组血清ALT、AST、GGT水平比较
模型组ALT、AST、GGT水平较正常组明显升高,差异有统计学意义(均P < 0.05)。全方组化痰组、祛瘀组、解毒组ALT、AST、GGT水平与模型组比较均明显降低,差异有统计学意义(均P < 0.05)。各治疗组之间比较,各拆方组ALT、AST、GGT水平均高于全方组,差异均有统计学意义(均P < 0.05);祛瘀组、解毒组ALT、AST、GGT水平均显著低于化痰组,差异均有统计学意义(P < 0.05);祛瘀组和解毒组ALT、AST、GGT水平比较,差异均无统计学意义(P > 0.05)。见表1。
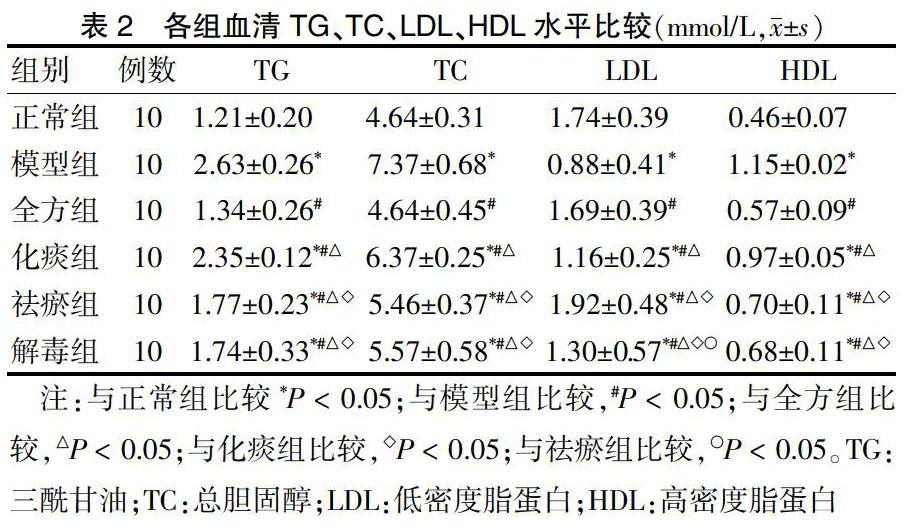
2.2 各组血清TG、TC、LDL、HDL水平比较
模型组TG、TC、HDL水平均显著高于正常组,LDL显著低于正常组,差异均有统计学意义(均P < 0.05)。全方组、化痰组、祛瘀组、解毒组TG、TC、HDL水平较模型组明显降低,LDL较模型组明显升高,差异均有统计学意义(均P < 0.05)。各治疗组之间比较,与全方组比较,化痰组、祛瘀组、解毒组TG、TC、HDL水平均显著升高,化痰组、解毒组LDL显著降低,祛瘀组LDL显著升高,差异均有统计学意义(均P < 0.05);与化痰组比较,祛瘀组、解毒组TG、TC、HDL水平均显著降低,LDL显著升高,差异均有统计学意义(均P < 0.05);与祛瘀组比较,解毒组LDL显著降低(P < 0.05);祛瘀组和解毒组TG、TC、HDL水平比较,差异均无统计学意义(均P > 0.05)。见表2。
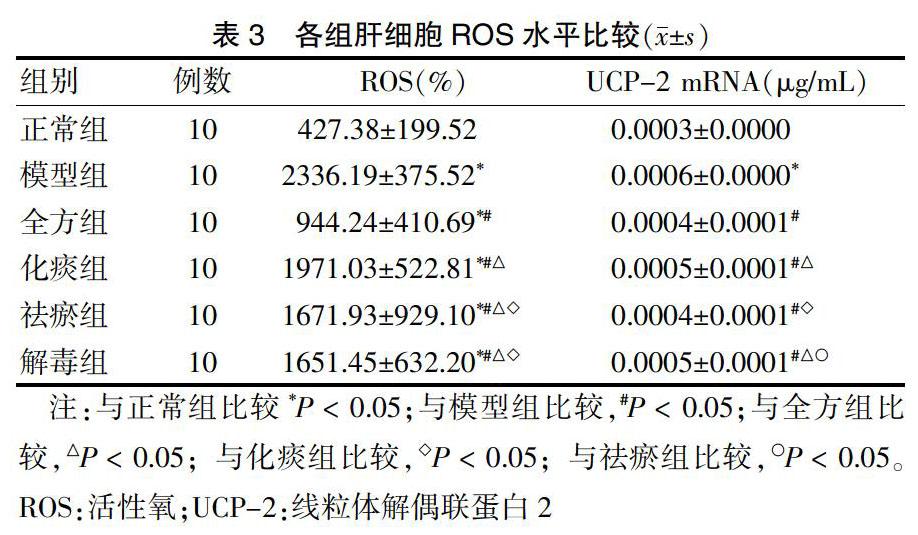
2.3 各组肝细胞ROS水平比较
与正常组比较,模型组ROS明显升高,差异有统计学意义(P < 0.05);与模型组比较,全方组、化痰组、祛瘀组、解毒组ROS水平明显下降,差异有统计学意义(P < 0.05);与全方组比较,各拆方组ROS水平显著升高,差异有统计学意义(P < 0.05);与化痰组比较,祛瘀组、解毒组ROS水平明显下降,差异有统计学意义(P < 0.05)。肝细胞UCP-2 mRNA实验结果显示,与正常组比较,模型组UCP-2 mRNA水平显著升高,差异有统计学意义(P < 0.05);与模型组比较,全方组、化痰组、祛瘀组、解毒组UCP-2 mRNA水平明显下降,差异有统计学意义(P < 0.05);与全方组比较,化痰组、解毒组UCP-2 mRNA水平明显升高(P < 0.05);与化痰组比较,祛瘀组UCP-2 mRNA水平明显下降,差异有统计学意义(P < 0.05);与祛瘀组比较,解毒组UCP-2 mRNA水平明显升高,差异有统计学意义(P < 0.05)。见表3。
表3 各组肝细胞ROS水平比较(x±s)
注:与正常组比较*P < 0.05;与模型组比较,#P < 0.05;与全方组比较,△P < 0.05;与化痰组比较,◇P < 0.05;与祛瘀组比较,○P < 0.05。ROS:活性氧;UCP-2:线粒体解偶联蛋白2
2.4 Western blot法检测相关蛋白
蛋白电泳显示,与正常组比较,模型组UCP-2蛋白表达增强;与模型组比较,全方组UCP-2蛋白表达减低;各拆方组UCP-2蛋白表达相近。见图1。
3 讨论
中医药以其多靶点的作用机制,在NASH的治疗中独具优势[8]。根据本病临床表现归属于中医“痰浊”“肥胖”“积聚”“胁痛”“瘀血”等范畴[9],起因多为过食肥甘厚味、嗜酒过度,或七情内伤、湿热之邪壅滞肝脾,以致肝失疏泄、肝血瘀滞、脾失健运、聚湿成痰、痰阻气机、痰瘀互结而发病[10]。益肝解毒方是我们针对主要病机[11-12],经多年临床研究证实治疗该病有效的中药方剂。根据组方的不同功效,可将本方拆分成化痰、祛瘀、解毒三个组分[13],通过实验研究,分析不同组分在治疗中的不同作用[14]。UCP-2是肝细胞线粒体内膜上的载体膜蛋白[15],肝脏中通常只有Kupffer细胞中有UCP-2 mRNA表达[16-19],NASH时肝细胞UCP-2表达增强[20]。
本研究通过高脂饮食建立NASH大鼠模型,观察益肝解毒方及其拆方NASH大鼠UCP-2的影响,结果显示,全方组及各拆方组ALT、AST、GGT、TC、TG、HDL、ROS、UCP-2 mRNA水平较模型组明显降低,LDL较模型组明显升高,差异有统计学意义(P < 0.05)。各拆方组ALT、AST、GGT、TC、TG、HDL、ROS、UCP-2 mRNA水平较全方组降低,差异有统计学意义(P < 0.05),祛瘀组、解毒组在ALT、AST、GGT、TC、TG、HDL水平低于化痰组,差异有统计学意义(P < 0.05),祛瘀组LDL水平高于其他各组,差异有统计学意义(P < 0.05),结果显示,益肝解毒方可抑制ROS产生,降低UCP-2高表达。各拆方组实验结果提示,益肝解毒方对NASH大鼠的治疗作用是化痰、祛瘀、解毒三类药物协同作用的结果,各拆方组均不如全方组效果好,祛瘀与解毒组相近,略好于化痰。祛瘀组在改善LDL方面优于其他各组。
总之,全方组疗效最好,体现出中药复方是其各组成部分协同作用的结果。益肝解毒方可抑制ROS产生,降低UCP-2高表达和血清中ALT、AST、GGT、TC、TG、HDL水平,提高LDL水平。
[参考文献]
[1] 王亞平,郑昕,张志银.益肝解毒方治疗痰瘀蕴毒型非酒精性脂肪性肝炎临床观察[J].上海中医药杂志,2013,46(515):25-27.
[2] Arab JP,Barrera F,Arrese M. The Evolving Role of Liver Biopsy in Non-alcoholic Fatty Liver Disease [J]. Ann Hepatol,2018,17(6):899-902.
[3] 郑昕,王亚平,张志银.基于“体质可调”理论治疗非酒精性脂肪性脂肪性肝炎临床研究[J].世界中医药,2014,9(3):308-310.
[4] Golabi P,Fazel S,Otgonsuren M,et al. Association of Parity in Patients with Chronic Liver Disease [J]. Ann Hepatol,2018,17(6):1035-1041.
[5] Liang Q,Chen H,Xu X,et al. miR-182-5p Attenuates High-Fat -Diet-Induced Nonalcoholic Steatohepatitis in Mice [J]. Ann Hepatol,2018,18(1):116-125.
[6] Kuftinec GN,Levy R,Kieffer DA,et al. Hepatocellular Carcinoma and Associated Clinical Features in Latino and Caucasian Patients from a Single Center [J]. Ann Hepatol,2018,18(1):177-186.
[7] Uzlová N,Mejzlíková N,Fraňková S,et al. Transient elastography-role in the assessment of the liver disease development [J]. Vnitr Lek,2018,64(10):916-922.
[8] Lakhani HV,Sharma D,Dodrill MW,et al. Phenotypic Alteration of Hepatocytes in Non-Alcoholic Fatty Liver Disease [J]. Int J Med Sci,2018,15(14):1591-1599.
[9] Aliasghari F,Izadi A,Jabbari M,et al. Are Vaspin and Omentin-1 Related to Insulin Resistance,Blood Pressure and Inflammation in NAFLD Patients? [J]. J Med Biochem,2018,37(4):470-475.
[10] Tsuma Y,Mori J,Ota T,et al. Erythropoietin and long-acting erythropoiesis stimulating agent ameliorate non-alcoholic fatty liver disease by increasing lipolysis and decreasing lipogenesis via EPOR/STAT pathway [J]. Biochem Biophys Res Commun,2019,509(1):306-313.
[11] Nielsen J,Christensen VB,Borgwardt L,et al. Prognostic molecular markers in pediatric liver disease - Are there any? [J] Biochim Biophys Acta Mol Basis Dis,2018, 1865(3):577-586.
[12] Patoulias D,Kalogirou M. Empagliflozin promises to bridge the gap between non-alcoholic fatty liver disease,type 2 diabetes,and cardiovascular disease [J]. Prz Gastroenterol,2018,13(4):337-339.
[13] Yuting Y,Lifeng F,Qiwei H. Secreted modular calcium-binding protein 2 promotes high fat diet (HFD)-induced hepatic steatosis through enhancing lipid deposition,fibrosis and inflammation via targeting TGF-β1 [J]. Biochem Biophys Res Commun,2019,509(1):48-55.
[14] Janovsky CCPS,Cesena FH,Valente VAT,et al. Association between Thyroid-Stimulating Hormone Levels and Non-Alcoholic Fatty Liver Disease Is Not Independent from Metabolic Syndrome Criteria [J]. Eur Thyroid J,2018,7(6):302-307.
[15] Méndez-Sánchez N,Zamarripa-Dorsey F,Panduro A,et al. Current trends of liver cirrhosis in Mexico:Similitudes and differences with other world regions [J]. World J Clin Cases,2018,6(15):922-930.
[16] Jackson K,Dressler N,Ben-Shushan RS,et al. Effects of alkaline-electrolyzed and hydrogen-rich water,in a high-fat-diet nonalcoholic fatty liver disease mouse model [J]. World J Gastroenterol,2018,24(45):5095-5108.
[17] Wan S,Zhang L,Quan Y,et al. Resveratrol-loaded PLGA nanoparticles:enhanced stability,solubility and bioactivity of resveratrol for non-alcoholic fatty liver disease therapy [J]. R Soc Open Sci,2018,5(11):181457.
[18] Pattnaik K,Bhuyan P,Singh A,et al. Biopsy Proven Fibrosis in Non-Alcoholic Fatty Liver Disease:An Analysis of Risk Factors [J]. J Clin Exp Hepatol,2018,8(4):367-374.
[19] Pan Q,Qin T,Gao Y,et al. Hepatic mTOR-AKT2-Insig2 signaling pathway contributes to the improvement of hepatic steatosis after Roux-en-Y Gastric Bypass in mice [J]. Biochim Biophys Acta Mol Basis Dis,2018,1865(3):525-534.
[20] Weta IW,Mahadewa TGB,Sutirtayasa WP,et al. The Comparison of Simple Anthropometric and Biochemical Parameters for Predicting Liver Steatosis in Obese Balinese Young Women [J]. Open Access Maced J Med Sci,2018,6(11):2062-2066.

