A high-performance liquid chromatography-tandem mass spectrometry method for the quantitative determination of four genotoxic impurities in gefitinib
SUN Chunyan, JI Yinghe, QIN Kunming, GAO Xun*, ZHAO Longshan
(1. School of Pharmacy, Shenyang Pharmaceutical University, Shenyang 110016, China; 2. Yangtze River Pharmaceutical Group Co., Ltd, Taizhou 225300, China; 3. School of Pharmacy, Jiangsu Ocean University, Lianyungang 222001, China)
Abstract: In this work, a method for the determination of the amounts of the four genotoxic impurities in gefitinib has been developed.A simple and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) approach has been developed, optimized, and validated.The genotoxic impurities were 3-chloro-4-fluoroaniline, 3,4-difluoroaniline, 3-fluoro-4-chloroaniline, and 3,4-dichloroaniline.Separation was achieved on an Inertsil ODS-3 column (100 mm×3.0 mm, 3 μm).The column temperature was 40 ℃, and the running time was 12 min.A triple quadrupole mass detector with positive electrospray ionization in the multiple reaction monitoring (MRM) mode was applied.The method was validated in terms of its specifi-city, linearity, precision, accuracy, stability, and robustness.The correlation coefficient of each impurity was higher than 0.999 in the range of 0.6-96.0 μg/L.The limits of detection (LODs) and limits of quantity (LOQs) were in the ranges of 0.2-2.0 μg/L and 0.6-6.0 μg/L, respectively.The recoveries of all impurities at 6, 30, and 60 μg/L were within 91.0%-98.5%.All impurities were stable within 2 h.After detection, only 3-chloro-4-fluoroaniline was detected in the batch number 16052301 and R16052501-1 gefitinib samples, but its concentration was below the impurity limit (6 mg/L).This method is simple, reliable, and suitable for the determination of four genotoxic impurities in gefitinib.It can be further applied as a reference for quality control.
Key words: high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS); genotoxic impurity; gefitinib
Lung cancer is the leading cause of cancer deaths worldwide.Non-small cell lung cancer (NSCLC) accounts for more than 80% of the total lung cancer cases, and most patients are treated in the terminal stage.The 5-year survival rate is less than 15% [1,2].Gefitinib is a selective epidermal growth factor receptor (EGFR) protein tyrosine kinase (PTK) inhibitor for the treatment of chemotherapy failure or unsuitable chemothe-rapy in locally advanced or metastatic NSCLC [3,4].EGFR-PTK plays an important role in signal transduction pathways [5].Many cancers in humans are due to the overexpression of EGFR and the associated human epidermal growth factor receptor (HER-2).Gefitinib inhibits the kinase activity of EGFR and HER-2 when combined with their cognate ligands [6].Gefitinib, the first targeted drug for lung cancer, has been approved for marketing in several countries [7,8].In recent years, gefitinib has been demonstrated to have a certain effect on brain metastasis in the treatment of recurrent NSCLC [9].
3-Chloro-4-fluoroaniline is a key starting material for gefitinib synthesis, and 3,4-difluoroaniline, 3-fluoro-4-chloroaniline, and 3,4-dichloroaniline may be introduced during the synthesis [10,11].The structures of the impurities are shown in Table 1, and the synthetic route is shown in Fig.1.These are genotoxic impurities, which can induce genetic damage even at low concentrations [12,13], leading to genetic mutations and possibly contributing to tumorigenesis.In recent years, with the gradual improvement of genotoxic impurities regulations, the regulatory requirements for genotoxic impurities have gradually increased in the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).Inadequate control of genotoxic impurities in a drug may cause great harm to human health and affect the market value of the drug.Therefore, the amount of genotoxic impurities should be strictly controlled during the production of gefitinib.
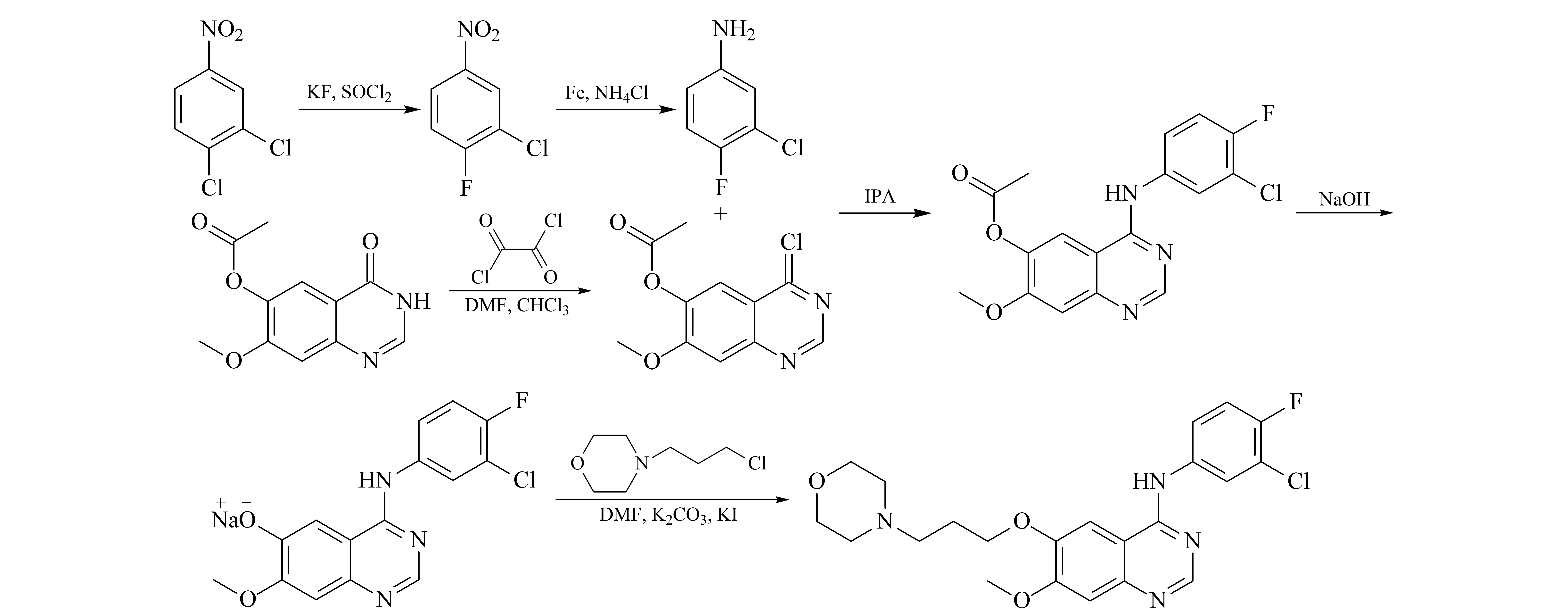
Fig.1 Synthetic route to gefitinib

Table1 Names, structures, and sources of genotoxic impurities
Both EMA and FDA have proposed the use of the threshold of toxicological concern (TTC) as an acceptable limits for genotoxic impurities [14-16].Furthermore, National Medical Products Administration has approved the commercialization of the domestic generic drug gefitinib produced by Qilu Pharmaceutical Co., Ltd (Shandong, China).The abovementioned four impurities were monitored and controlled according to the gefitinib pharmaceutical registration quality standard (YBH02612016) [17].The maximum daily dose of gefitinib tablets is 250 mg.Besides, according to the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) M7 guidelines [18], the limit of genotoxic impurities calculated by the TTC method is 6 mg/L.
In previous studies, the conventional LC or GC method was used to determine the amounts of genotoxic impurities in medicines, but poor sensitivity did not meet the requirements of low impurity content [19-21].In recent years, genotoxic impurities have been determined by GC-MS and LC-MS techniques [22,23], which have better sensitivity and hence can detect even trace amounts of these impurities in drugs.In this study, as MS-based quantitative method for the determination of genotoxic impurities in gefitinib was established and validated.This method is simple and sensitive, and facilitates quality control of the four trace genotoxic impurities in gefitinib.
1 Experimental
1.1 Instruments
The analysis was performed on an Agilent 1200 LC-6460 Triple Quad LC-MS (Agilent Technology, USA) system.
1.2 Chemicals
HPLC-grade acetonitrile and formic acid were purchased from Merck KGaA (Germany) and Fluka (USA), respectively.Potassium hydroxide (purity of 96.0%), hydrochloric acid (ω of 36.0%-38.0%), hydrogen peroxide (purity of 30%) of analytical reagent were obtained from Sinopharm Chemical Reagent Co., Ltd (China).Distilled water from Wahaha Co., Ltd (China) was used throughout the analysis.
3,4-Difluoroaniline (purity of 98.9%, UIQ6E-1706), 3-chloro-4-fluoroaniline (purity of 99.7%, 160621-1706), 3-fluoro-4-chloroaniline (purity of 99.8%, 20130426-1706), and 3,4-dichloroaniline (purity of 99.6%, 718876625-1706) were purchased from Yangtze River Pharmaceutical Group Co., Ltd (China).Three batches of gefitinib (16052301, 16041901, and R16052501-1) were obtained from Yangtze River Pharmaceutical Group Jiangsu Haici Biological Pharmaceutical Co., Ltd (China).
1.3 Experimental conditions
Chromatographic separation was achieved on an Inertsil ODS-3 column (100 mm×3.0 mm, 3 μm).The injection volume was 4 μL, and the column temperature was maintained at 40 ℃.The mobile phase was made up of 0.1% (volume percentage, the same below) aqueous formic acid (A) and 0.1% formic acid acetonitrile solution (B) (70∶30, v/v).The flow rate was set at 0.8 mL/min, and the running time was 12 min.
The MS conditions were as follows: electrospray ionization (ESI), positive ion mode acquisition; dry gas temperature: 300 ℃; dry gas flow rate: 10 L/min; spray voltage: 344.75 kPa; sheath gas flow rate: 11 L/min; capillary voltage: 4 000 V; nozzle voltage: 1 000 V.Analysis was carried out in the multiple reaction monitoring (MRM) mode to determine the amounts of the genotoxic impurities.Them/zof the parent and daughter ions were 130 and 83 for 3,4-difluoroaniline; 146 and 111 for 3-chloro-4-fluoroaniline and 3-fluoro-4-chloroaniline; 162 and 127 for 3,4-dichloroaniline.
1.4 Preparation of sample and standard solutions
The sample solution was prepared by dissolving gefitinib in water, acetonitrile, and formic acid (50∶50∶0.1, v/v/v) at a concentration of 5 g/L.
A mixture of the standard solutions of 3-chloro-4-fluoroaniline, 3,4-difluoroaniline, 3-fluoro-4-chloroaniline, and 3,4-dichloroaniline was prepared at a concentration of 0.003 mg/L as reference solution-1.The concentration of each impurity was set as 0.003 mg/L to obtain reference solution-2.A series of standard solutions were ranged from LOQ to 300% of the limit concentration for the linearity assessment.The standard solutions are 6, 15, 31, 46, 61, 92 μg/L 3-chloro-4-fluoroaniline; 0.6, 6, 16, 32, 48, 64, 96 μg/L 3,4-difluoroaniline; 6, 15, 30, 45, 60, 90 μg/L 3-fluoro-4-chloroaniline; and 1.2, 6, 15, 30, 45, 60, 90 μg/L 3,4-dichloroaniline.
2 Results and discussion
2.1 Optimization of chromatography and MS conditions
2.1.1Optimization of chromatography conditions
The flow rate was set at 0.5, 0.6, and 0.8 mL/min.In all the three cases, the impurities could be effectively separated, with the retention time being the longest for 3,4-dichloroaniline (13.73, 11.53, and 8.62 min, respectively).Moreover, the running time was the shortest at the flow rate of 0.8 mL/min, and the impurities did not interfere with one another.Therefore, the optimum flow rate was determined to be 0.8 mL/min.

Table2 Linear equations, correlation coefficients (R), linear ranges, LODs, and LOQs of each impurity
y: peak area;x: mass concentration of each impurity, μg/L.
2.1.2Optimization of MS conditions for 3,4-difluoroaniline
When the collision energy (CE) was 0 V, there was no fragmentation, and the detection ion wasm/z130.0.As the CE increases, the response of small-molecule fragments increases, while that of macromolecular fragments decreases.When the CE was lower than 25 V, the parent ion responded well but the daughter ion response was poor.When the CE was 25 V, both the daughter ion and the parent ion responded well.When the CE was higher than 25 V, the daughter ion response was high but the parent ion response was poor.So the CE was 25 V, and the daughter ion wasm/z83.0.
2.1.3Effect of injection volume
Since the limit requirement is less than 6 mg/L, the 25% limit concentrationS/Nof each impurity must be greater than 10.TheS/Nof 3,4-dichloroaniline should be greater than 40.When the injection volume was 1 μL, theS/Nof 3,4-dichloroaniline was 30, which did not meet requirements.When the injection volume was 2 μL, theS/Nof 3,4-dichloroaniline was 54.3, which met requirements.However, during the mass spectrometer maintenance, the response of the impurities was diminished, and the injection volume had to be increased.When the injection volume was 4 μL, the response met requirements, and there was no residue.When the injection volume was 5 μL or 10 μL, the response included a residue.According to these observations, the optimal injection volume was determined to be 4 μL.
2.2 Method validation
The determination of the four impurities in gefitinib by HPLC-MS/MS was validated according to the ICH guidelines in terms of system suitability, specificity, LOD, LOQ, linearity, precision, accuracy, stability, and robustness (see Tables 2 and 3).The results indicated that the impurities can be well separated and determined in both the standard solution mixture and gefitinib sample.

Table3 Recoveries and RSDs of each impurity at three spiked levels
2.2.1System suitability
Reference solution-1 was injected in quintuplicate.quintuplicate.The RSD of the peak areas of each impurity was less than 10.0%.The f-values (response factor: C (reference concentration)/A (reference peak area)) of reference solution-1 (the later three injections) and reference solution-2 (in duplicate) were recorded, and the RSD was less than 10.0%, which met requirements.
2.2.2Specificity
The specificity of the method was studied by employing damaging test conditions, including strong-light destruction for 14 day, high-temperature destruction for 14 day, acid destruction for 4.5 h, alkali destruction for 4.5 h, and oxidative destruction for 4.5 h.Under the conditions of acid destruction and oxidative destruction, the peak area of 3-chloro-4-fluoroaniline increased significantly as compared to that under the no-damage conditions, and other genotoxic impurities were not detected.Under the conditions of high-temperature destruction, light destruction, and alkali destruction, the peaks were stable, and the peak area of 3-chloro-4-fluoroaniline did not increase significantly; further, other genotoxic impurities were not detected.These results indicated that the specificity of the developed method was satisfactory.
2.2.3LODs and LOQs
The LODs and LOQs of the four impurities were determined based on theS/Nof about 3.0 and 10.0, respectively.Therefore, the reference stock solution of each impurity was diluted in a stepwise manner until the requiredS/Nwas achieved.The LODs and LOQs are shown in Table 2.xrepresents the mass concentration of each impurity (μg/L) andyrepresents the peak area.Besides, the stability of the LOQs of 3-chloro-4-fluoroaniline, 3,4-dichloroaniline, 3,4-difluoroaniline, and 3-fluoro-4-chloroaniline was investigated, and the RSDs were 1.74%, 4.47%, 7.03%, and 5.20%, respectively.Therefore, the precision RSD of each impurity was less than 10.0%, which met requirements.
2.2.4Linearity and range
The linearity of each impurity was demonstrated by a six- or seven-point calibration curve in the range of 6.0-92.0 μg/L for 3-chloro-4-fluoroaniline, 0.6-96.0 μg/L for 3,4-difluoroaniline, 1.2-90.0 μg/L for 3,4-dichloroaniline, and 6.0-90.0 μg/L for 3-fluoro-4-chloroaniline.The correlation coefficient in each case was higher than 0.999 3.The slope, intercept, and correlation coefficient were derived from the least-squares linear regression analysis of the average peak area versus concentration.The results are listed in Table 2.The results indicated this method has a satisfactory linearity correlation.
2.2.5Precision
The precision of the instrument was determined by analyzing the same test solution six times.The test solutions were prepared at a concentration of about 5.0 g/L.Only 3-chloro-4-fluoroaniline was detected in the six measurements, and the difference was less than 20% of the limit, confirming that the precision of the instrument precision was adequate.
2.2.6Accuracy
The accuracy was evaluated by the recovery test.The test solution was formulated at a concentration of 5.0 g/L, followed by the addition of 6 μg/L mixed standard (low concentration), 30 μg/L mixed standard (medium concentration), and 60 μg/L mixed standard (high concentration), in triplicate.The recoveries and RSDs are shown in Table 3, which demonstrate that the method has good accuracy.
2.2.7Solution stability
To evaluate the stability, reference solution-1 and the test solution were placed at room temperature for 0, 0.5, 1, 1.5, and 2 h and the peak area of each impurity was recorded.In reference solution-1, the RSDs of 3,4-difluoroaniline, 3-chloro-4-fluoroaniline, 3-fluoro-4-chloroaniline, and 3,4-dichloroaniline were 1.51%, 4.08%, 0.96%, and 2.66%, respectively.Only 3-chloro-4-fluoroaniline was detected in the test solution, and the RSD was 3.68%.These results indicated that the reference solution and test solution were stable within 2 h.
2.2.8Robustness
The robustness of the method was evaluated by deliberately modifying the column temperature, flow rate, and organic comparison of the mobile phase.The effect of the column temperature was studied at 35 and 45 ℃ (altered by 5 ℃).The optimized flow rate was 0.8 mL/min, which was changed to 0.7 and 0.9 mL/min.The starting ratio of mobile phase B was 30%, which was changed to 32% and 28%.Only 3-chloro-4-fluoroaniline was detected under the normal conditions and modified conditions.As opposed to the normal conditions, the absolute change in the 3-chloro-4-fluoroaniline content was no more than 20% of the limit under the modified conditions (Table 4), which demonstrated that the robustness of the method was satisfactory.
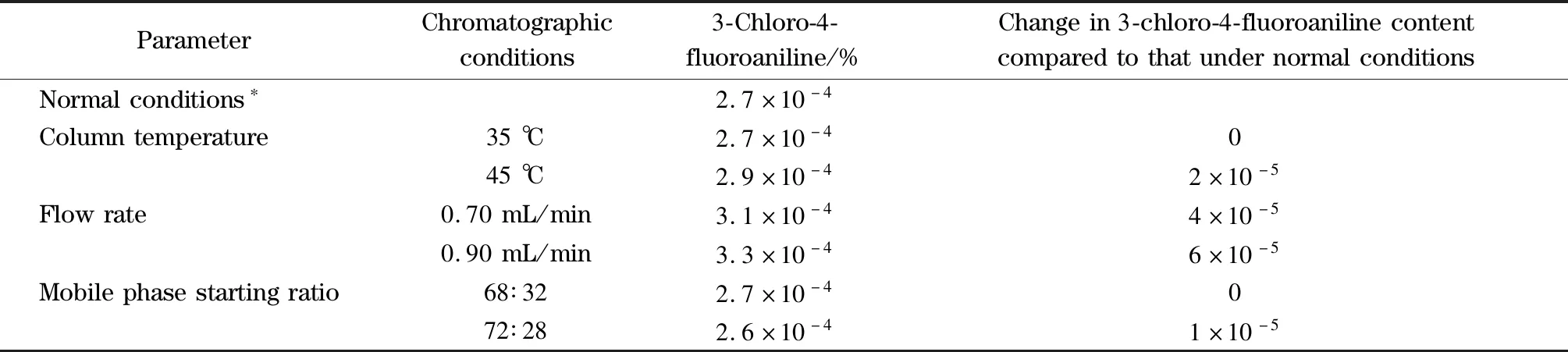
Table4 Changes in the analysis conditions and results of robustness testing
* Column temperature: 40 ℃; flow rate: 0.80 mL/min; mobile phase starting ratio: 70∶30.
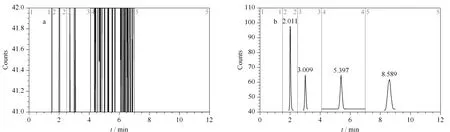
Fig.2 Chromatograms of (a) blank solution and (b) reference solution
2.3 Sample detection

Fig.3 Chromatograms of test solution 16041901
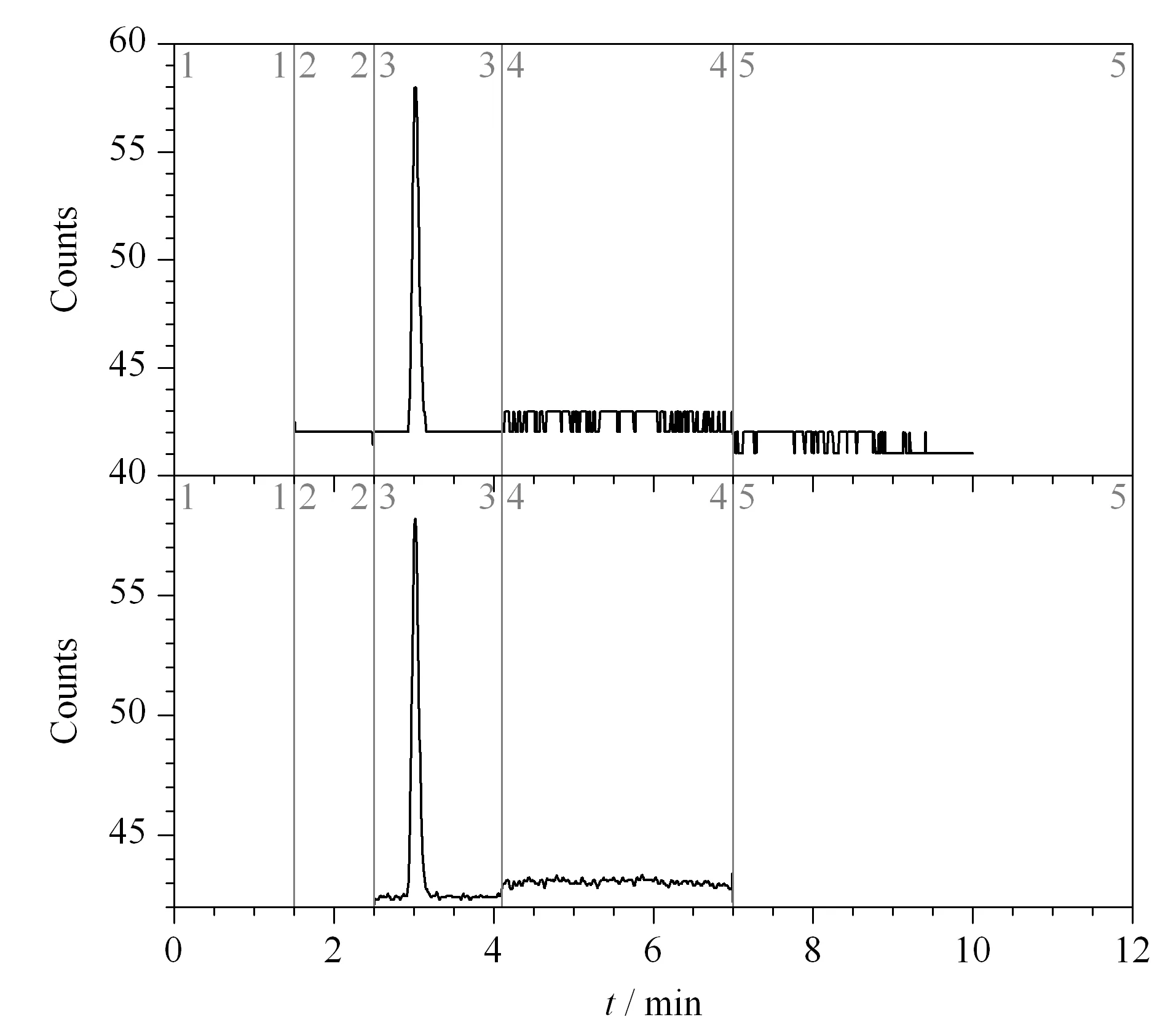
Fig.4 Chromatograms of test solution 16052301
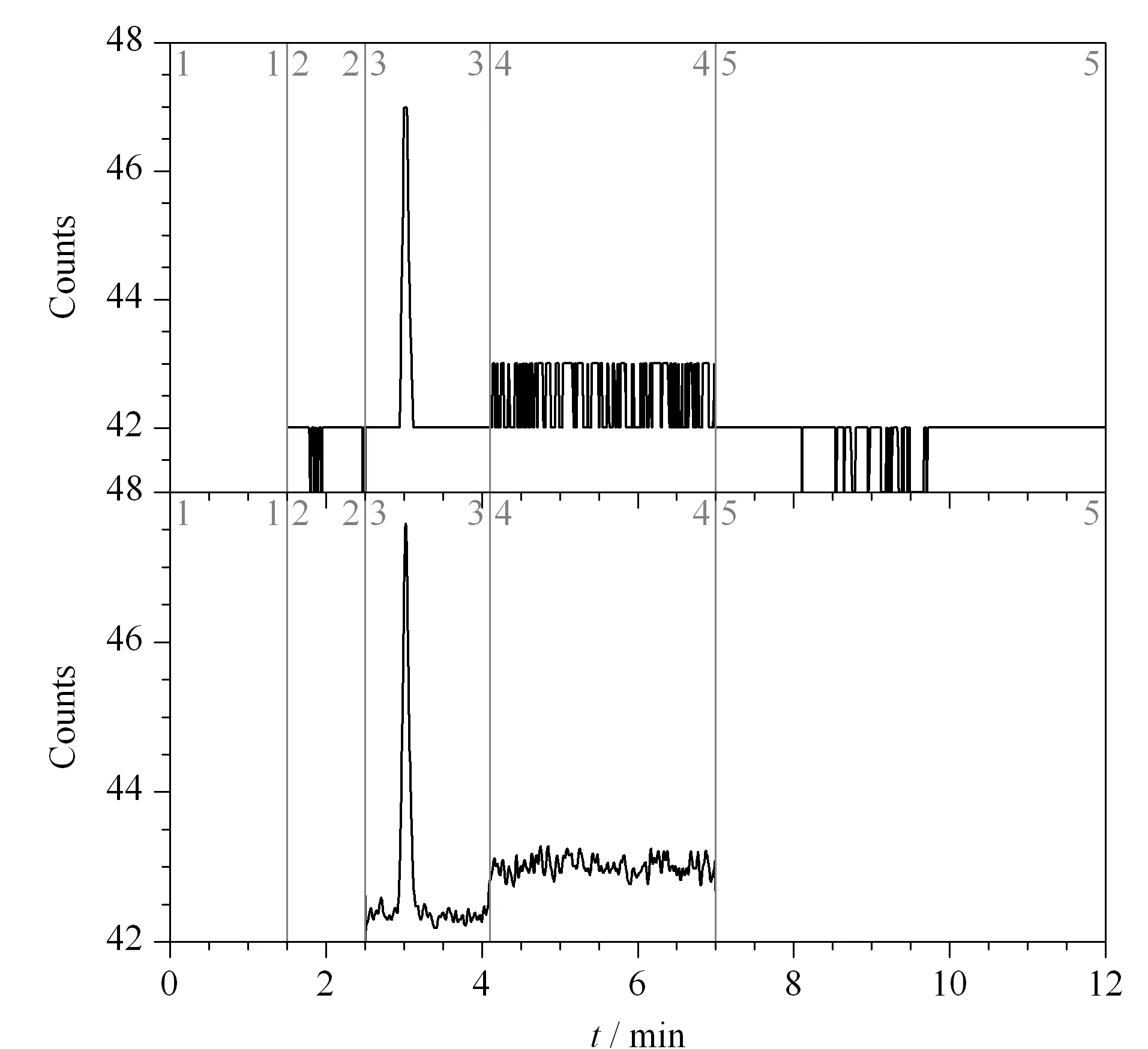
Fig.5 Chromatograms of test solution R16052501-1
Three batches of gefitinib samples were configured as test solutions, in triplicate.After the analysis, 3,4-dichloroaniline, 3,4-difluoroaniline, and 3-fluoro-4-chloroaniline were not detected.In the gefitinib samples with batch number 16052301 and R16052501-1, the 3-chloro-4-fluoroaniline content was detected to be 3 μg/mL, which was less than the limit value of 6 μg/mL.Figs.2 to 5 show the test results for the blank solution, reference solution, and test solutions 16041901, 16052301, and R16052501-1, respectively (time periods 2, 3, 4, and 5 correspond to 3,4-difluoroaniline, 3-chloro-4-fluoroaniline, 3-fluoro-4-chloroaniline, and 3,4-dichloroaniline, respectively.).
3 Conclusion
By methodological tests, the MS-based method has been demonstrated to accurately determine trace amounts of the genetic impurities (3-chloro-4-fluoroaniline, 3,4-difluoroaniline, 3,4-dichloroaniline, and 3-fluoro-4-chloroaniline) in gefitinib.This method is specific and sensitive, and it meets the requirements for the quantitative detection of impurities.The method can be used as quality control for gefitinib genotoxic impurities.The amounts of the genetic impurities are lower than the limit, thus meeting the requirements for content determination.