Lesson Ninety- two Arrhythmia- induced cardiomyopathy
Tachycardia-induced Cardiomyopathy
Tachycardia-induced cardiomyopathy(T-CM)refers to the presence of a reversible LV dysfunction solely due to increase in ventricular rates, regardless of tachycardia origin. The risk of developing T-CM depends not only on the type, but also the duration and rate of tachycardia. A study reported T-CM in 2.7%patients referred for radiofrequency ablation(RFA);however,it also included patients referred for premature ventricular contractions ablation. T-CM has been reported in 10% of patients with atrial tachycardia(AT), and as high as 37% of patients with incessant AT. Moreover, permanent junctional reciprocating tachycardia appears to have the highest association with T-CM(20% to 50%)as it frequently presents as an incessant supraventricular tachycardia.
Pathophysiology and Mechanism
Animal models have been key to understand the pathophysiology and mechanism of T-CM. Similar to humans,animals exposed to persistent tachycardia using a continuous rapid atrial or ventricular pacing develop heart failure (HF)symptoms, left ventricular(LV)systolic dysfunction and dilatation, decrease in LV dP/dtmax and myocardial blood flow,and increase in LV wall stress and end-diastolic pressure and volume.Dilatation tends to be biventricular with mild thinning or no associated hypertrophy or change in heart mass. The progression of these physiological changes include a decrease in systemic blood pressure and increase in LV and pulmonary artery pressure, which plateaus at 1 week, while cardiac output, ejection fraction, and volumes continue to deteriorate the following 4 weeks with development of symptomatic HF within 2 to 3 weeks.
T-CM is characterized by structural and functional myocardial changes. Similar to human studies, T-CM models have also demonstrated electrical remodeling and abnormal Ca homeostasis thought to be responsible for impaired excitation-contraction coupling and diastolic dysfunction. Only total Ca cycling, Ca channel inhibition,and basal ATPase activity have demonstrated a statistical correlation with decrease in left ventricular ejection fraction(LVEF).
Clinical Presentation, Diagnosisi, and Imaging Features
Clinical studies have found a variable time from onset of arrhythmia symptoms to development of T-CM,ranging from 3 to 120 days with an overall LVEF of 32%. Regardless of tachyarrhythmia, HF symptoms will manifest earlier at higher tachycardia rates, such as patients with persistent atrial flutter or tachycardia with 2:1 AV conduction with rates >150 beats/min.A recent clinical study found a more severe LV dysfunction(LVEF 29.3±6.6%)in T-CM when compared with dilated and inflammatory CM (32.1±10.2% and 41.9±12.9%,respectively;P<0.001)
Major reported symptoms include palpitations(29%), HF class Ⅲto Ⅳ(47%), and syncope/presyncope(12%), while the remaining may have no symptoms. Sudden cardiac death is uncommon but has been reported in up to 8%to 12%despite treatment and resolution of cardiomyopathy.
Echocardiogram or cardiac magnetic resonance can assist in excluding other etiologies. T-CM is characterized by a dilated CM (increased LV end-diastolic dimension and area)with moderate to severe biventricular systolic dysfunction and normal LV septal and posterior wall thickness (lack of hypertrophy). Mitral insufficiency may be present due to LV and mitral annular dilatation with lack of leaflet coaptation.
Treatment
A major feature of T-CM is its reversibility once tachycardia is eliminated. Thus, the mainstay treatment consists of suppression of tachycardia based on the culprit arrhythmia1with antiarrhythmic drugs(AADs)and/or RFA.
Elimination of tachyarrhythmia not only resolves LV function within 4 to 12 weeks, but also improves heart failure symptoms by at least 1 New York Heart Association functional class in most patients.Unfortunately, the recovery of T-CM is not always complete. Histopathological abnormalities, diastolic dysfunction, and ventricular dilatation with a hypertrophic response may persist despite normalization of LVEF.
Prnmature Ventricular Contraction-Cardiomyopathy
Premature ventricular contractions-cardiomyopathy(PVC-CM)is defined as the development of LV dysfunction caused solely by frequent PVCs. A PVC burden≥10% is often considered high and significant enough to trigger PVC-CM.The prevalence of PVC-CM has been reported at 7% among patients with frequent PVC burden >10%. Clinical studies have reported a diagnosis of PVC-CM in 9%to 30%of patients referred for RFA of PVC.
Potential Mechanism(s)of PVC-CM
The primary cause of contractile dysfunction in PVC-CM appears to be disorders of the calcium-induced calcium release mechanism itself,with alterations of dyad(L-type Ca channel and Ryanodine receptor)function proposed as a potential mechanism.Similar to other cardiomyopathies, this PVC-CM model has revealed electrophysiological remodeling.Histopathological abnormalities are distinct without evidence of increased inflammation or apoptosis and minimal or no fibrosis. Mitochondrial studies have demonstrated no changes in oxidative phosphorylation.These findings are supported clinically by the lack of scar on cardiac magnetic resonance imaging of patients with PVC-CM.These findings further confirm a primary functional abnormality as a primary mechanism of this reversible CM. Whether all the cellular and molecular changes are in response to the CM rather than the cause of the CM remains unclear.
Predictors of PVC-CM
PVC burden has been shown to be a major predictor of PVC-CM. Two main studies have shown that PVC burden >16% and 24% best identifies patients with a diagnosis of PVC-CM. Although these and other studies suggest that a PVC burden of at least 10% is required to induce PVC-CM, other studies question this minimal PVC threshold,because they have shown improvement in LV function with PVC burden as low as 6% to 8% . The length of ambulatory ECG monitoring has important implications, because increasing the duration from a 24-h to a 7-day ambulatory Holter monitor can doubled the number of patients who reach the 10%threshold.
Some other PVC features have been found to be independent predictors for PVC-CM such as male sex,lack of symptoms or duration of palpitations >30 months, variability of PVC coupling interval, QRS duration of PVC >150 ms, and epicardial origin.PVC-CM index2, including PVC burden, PVC-QRS width, and epicardial origin, has been developed in an attempt to identify patients with high probability of PVC-CM.
Clinical Presentation, Diagnosis, and Imaging Features
The time course for the development of PVC-CM is unclear, but it is estimated to occur within months up to several years. Although animal studies with persistent high PVC burden(33% to 50%)develop CM within 4 weeks, human studies are not consistent in part due to the unclear onset and variability of PVCs.
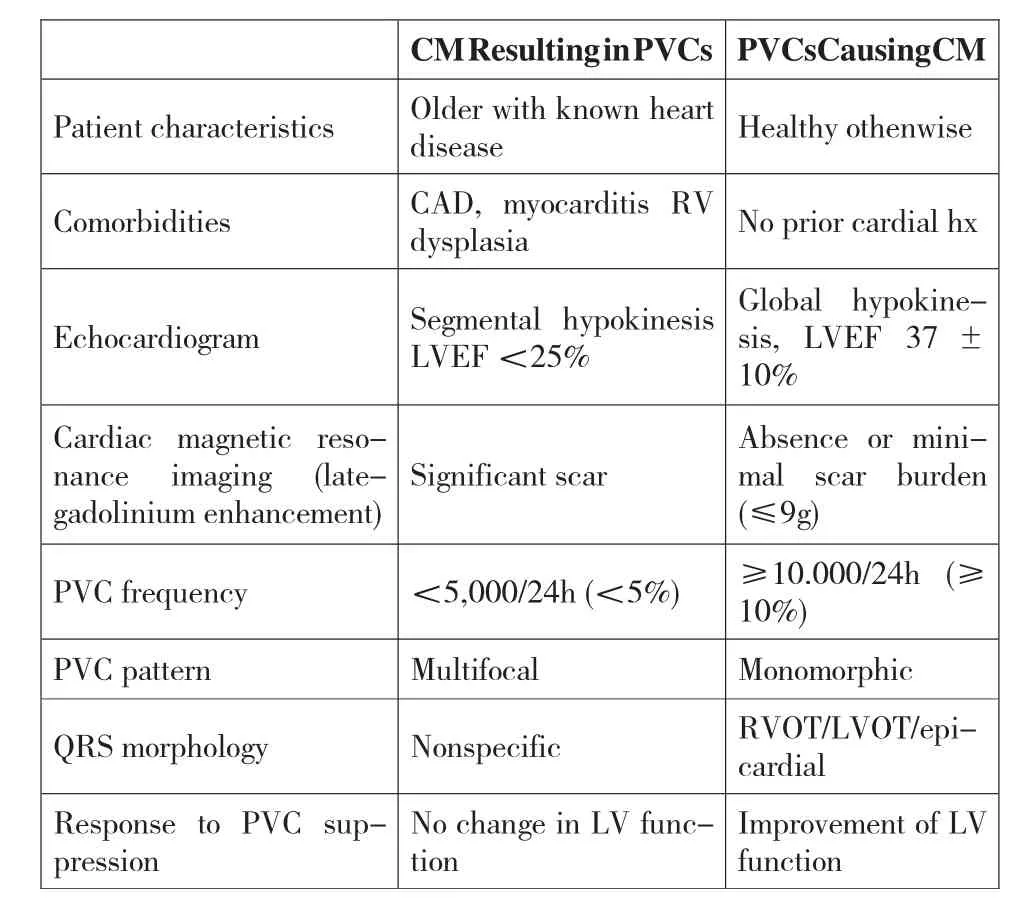
Table 1 Clinical and PVC Features to Identify PVC-CM
PVC-CM may have a wide range of presentations,from asymptomatic or vague symptomatology to heart failure and even syncope. It is unclear why some patients have symptoms related to PVCs while others do not, but a PVC coupling interval ratio <0.5 (PVC CI ratio: PVC coupling interval/Sinus coupling interval)has been proposed as an important marker of symptoms.
PVC-CM is a diagnosis of exclusion, to be suspected in patients with frequent PVCs >10% ,especially in nonischemic CM.A challenge is to identify when PVCs are the etiology of a CM or just "innocent bystanders" in patients with CM. Even if PVCs are the result of CM, these PVCs, if frequent, may contribute to and further worsen CM and HF symptoms; this is referred to as "superimposed" PVC-CM. In selected cases, echocardiographic and PVC features can help identify these patients (Table 1). PVC-CM is characterized by mild to moderate LV systolic dysfunction,LV dilatation,mild mitral regurgitation,and LA enlargement, which resolved within 2 to 12 weeks after elimination of PVCs. Cardiac imaging is key to identify LV dysfunction and prompt suspicion of PVC-CM in patients with high PVC burden(≥10%)(Table 1). Cardiac magnetic resonance with lategadolinium enhancement has the advantage of identifying scar and quantifying scar burden, which in turn potentially predicts the response to PVC suppression.
Treatment
Currently,a PVC suppression strategy with RFA or AADs is a widely accepted intervention to treat a CM that might be caused or exacerbated by frequent PVCs.However, the treatment of frequent PVCs(≥10%burden)without LV dysfunction (LVEF ≥50%),symptoms, or idiopathic ventricular fibrillation is less clear. PVC suppression is considered successful if burden is decreased by >80% of baseline PVCs, as it likely represents a true effect of treatment rather than spontaneous PVC variability.
PVC suppression in PVC-CM has been shown to improve LV function,LV dilatation,mitral regurgitation,and BNP levels. The mean improvement of LVEF after RFA in most studies is between 10% and 15% even in superimposed PVC-CM. A prospective study demonstrated a significant decrease in BNP levels while primary prophylaxis ICD implantation was avoided in 80% of all patients with PVC burden >13% due to significant improvement of LVEF after successful RFA.Another study found that 81%of patients with abnormal baseline estimated glomerular filtration rate had significant improvement in renal function after RFA of PVCs.
词 汇
Pathophysiology n.病理生理学
leaflet n.&v.瓣叶,散页印刷品,传单,小册子;散发传单
coaptation n.对合,接合,适应
histopathological adj.组织病理学的
apoptosis n.细胞凋亡,程序性细胞死亡
mitochondrial adj.线粒体的
oxidative adj.氧化的,具有氧化特性的
phosphorylation n.磷酸化
implication n. 含意,暗指,牵连,牵涉
vague adj.模糊的,微小的,不明确的,不具体的
symptomatology n. 症状学,症候学
innocent adj.无辜的,清白的,无罪的,无辜受害的;n.无辜者,单纯的人
superimpose v.叠加,叠映,使重叠
注 释
1.culprit arrhythmia 本文中指导致心肌病的心律失常,culprit在冠心病心肌梗死介入治疗中常译作“罪犯”,在此可译作“致病”,即致病心律失常。
2.PVC-CM index 指PVC-CM 指数,由PVC 负荷(0-1)×PVC-QRS 宽度(毫秒)×常数C(结构性心脏疾病为1.28,心电图提示心外膜起源为2)计算所得,≥39 分预测发生心肌病的风险高。
参考译文
第92 课 心律失常所致心肌病心动过速所致心肌病
心动过速所致心肌病(T-CM)是指因心室率加快而引起的可逆性左心室功能不全,不考虑心动过速的起源。发生T-CM 的风险不但取决于心动过速的类型,也取决于心动过速的频率和持续时间。有报道T-CM 占射频消融患者的2.7%。不过,这包含行室性期前收缩消融的患者。据报道10%的房性心动过速患者发生T-CM,这在顽固性房性心动过速患者高达37%。此外,持续性结性折返型心动过速因其常为顽固性室上性心动过速,T-CM 的发生率最高(20%~50%)。
病理生理与机制
动物模型是了解T-CM 病理生理和机制的关键。与人类相似,连续心房或心室起搏引起的持续性心动过速可诱发动物心力衰竭症状、左心室收缩功能不全和扩张,降低左心室dP/dtmax 和心肌血流,增加左心室室壁张力、舒张末压力和容量。心脏扩张涉及双心室,伴轻微室壁变薄或不伴肥厚或心脏质量变化。这些生理学变化的进展包括动脉血压降低、左心室和肺动脉压增加,一周时这些变化达到稳定,而心搏出量、左心室射血分数(LVEF)和容量在随后的4 周继续恶化,在2~3 周内即出现心力衰竭症状。
T-CM 特征表现为心肌结构和功能变化。与人类相似,T-CM 模型也证实电重构和钙稳态异常,认为这导致了兴奋-收缩偶联障碍和舒张功能异常。已证实只有总钙循环、钙通道抑制和基础ATP 酶活性与LVEF 降低存在统计学意义的关联。
临床表现、诊断和图像特征
临床研究发现从心律失常症状到发生T-CM 的时间变化不一,从3d 到120d,总体上LVEF 为32%。除了快速型心律失常,较快速率的心动过速如持续性心房扑动或2:1 顺传室率>150 次/min 的心动过速较早出现心力衰竭症状。近期临床研究发现,与扩张型心肌病或炎症性心肌病[分别为(32.1±10.2)%和(41.9±12.9)%]相比,左心室功能不全更为严重[LVEF(29.3±6.6)%]。
主要症状包括心悸(29%),心功能Ⅲ到Ⅳ级(47%),晕厥或先兆晕厥(12%),其余为无症状。猝死不常见,但有报道尽管心肌病得到治疗并缓解,仍然达到8%~12%。
心脏超声或心脏磁共振检查有助于排除其他病因。T-CM 特征表现为扩张性心肌病(左心室舒张末径和面积增大)、中到重度双心室收缩功能障碍、左心室间隔和后壁厚度正常(无肥厚)。由于左心室和二尖瓣环增大瓣尖闭合不佳而出现二尖瓣关闭不全。
治疗
T-CM 的主要特征随心动过速消除而逆转。因此,治疗重点是基于致病心律失常用抗心律失常药物或射频消融手术抑制心动过速。
消除快速心律失常不但可以在4~12 周内恢复左心室功能,而且多数患者至少可改善心力衰竭症状达NYHA 1级。遗憾的是T-CM 不总能完全恢复。尽管LVEF 正常了,组织病理异常、舒张功能不全及伴随肥大反应的心室扩张会持续存在。
室性期前收缩所致心肌病
室性期前收缩所致心肌病(PVC-CM)是指频发PVC 引起的左心室功能不全。通常认为PVC 负荷≥10%时足以引起PVC-CM。有报道PVC 负荷>10%的患者PVC-CM 的发生率为7%。临床研究发现行PVC 射频消融的患者中9%到30%诊断为PVC-CM。
PVC-CM 的潜在机制
PVC-CM 收缩功能不全的主要原因是钙诱导的钙释放机制的自身异常,潜在的机制是dyad(L 型钙通道和雷诺定受体)功能改变。类似于其他心肌病,PVC-CM 模型揭示电生理重构。组织病理学异常确无炎症或凋亡增加的依据,轻微或无纤维化。 线粒体研究证实无氧化磷酸化变化。临床PVC-CM 患者磁共振心脏显像缺乏疤痕支持这些发现。这些发现进一步证实这种可逆性心肌病的主要发病机制为原发心功能异常。是否所有细胞和分子变化是对心肌病的反应抑或是心肌病的原因尚不清楚。
PVC-CM 的预测指标
PVC 负荷已成为预测PVC-CM 的主要指标。两大研究显示PVC 负荷>16%和>24%能很好的鉴别出PVC-CM 的患者。尽管这些及其他研究提示要诱发PVC-CM,PVC 负荷至少达10%,其他研究对这最小的PVC 负荷提出质疑,因为他们证实PVC 负荷低至6%到8%的患者处理后左心室功能得到改善。动态心电图监测时长具有重要价值,因为将监测时间从24h 延长至7d,达到10%阈值(即负荷值达10%)的患者数量倍增。
发现能独立预测PVC-CM 的一些其他PVC 特征有男性、缺乏症状或心悸超过30 个月、PVC 联律间期多变、PVC QRS 间期>150 ms 及心外膜起源。 PVC-CM 指数,包括PVC 负荷、PVC-QRS 宽度和心外膜起源,已形成以期鉴别PVC-CM 高发可能性的患者。
临床表现、诊断和影响特征
尚不清楚发生PVC-CM 需多长时间。估计发生在数月至数年间。虽然动物研究持续高负荷(33%至50%)PVC 4 周内诱发心肌病,人类研究并不一致,部分原因为不知PVC 何时开始以及它的可变性。
PVC-CM 表现形式宽泛,从无症状到或轻微症状到心力衰竭甚至晕厥。尚不清楚为什么有些患者出现PVC 相关症状而另外的不出现,不过,已认为PVC 联律间期比<0.5(PVC 联律间期比:PVC 联律间期/窦律联律间期)是症状的重要标志。
PVC-CM 是一排他性诊断,对于PVC>10%的患者要考虑,特别是非缺血性心肌病。面临的挑战是对于心肌病患者,PVC 是心肌病的病因还是“无辜的旁观者”。即使PVC 是心肌病所致,这些PVC 如果频发,可促进或进一步恶化心肌病和心力衰竭症状,这称作“叠加型”PVC-CM。在选择性病例,心超和PVC 特征有助于鉴别这些患者(表1)。PVC-CM 特征为轻中度左心室收缩功能异常、左心室扩大、轻度二尖瓣反流、和左心房扩大,这些会在消除PVC 后2 至12 周内得到缓解。心脏影像在鉴别高负荷PVC(≥10%)患者左心室功能不全及考虑PVC-CM 中起到关键作用(表1)。心脏磁共振延迟钆强化有益于识别疤痕并定量分析疤痕负荷,这反过来可预测对PVC 抑制的反应。
治疗
当前,采用射频消融术或抗心律失常药物抑制PVC 的方案已成为治疗PVC 诱发或加重心肌病的广为接受的干预方法。不过,对于不伴左心室功能不全、症状、或特发性心室颤动的频发PVC(负荷≥10%)治疗尚不清晰。认为当PVC 较基础状态下降80%以上时治疗是成功的,这反映治疗的正真作用而非PVC 自发变异。
PVC-CM 抑制PVC 能改善左心室功能、左心室扩张、二尖瓣反流和BNP 浓度。多数研究表明射频消融后LVEF 的改善平均达10%~15%,即使叠加的PVC-CM 也如此。一项前瞻性研究证实所有PVC 负荷>13%的患者在成功射频消融后BNP 水平显著下降,由于LVEF 明显改善而使80%患者免于预防性植入ICD。另一研究发现81%有基础估测肾小球滤过率异常的患者,在PVC 射频消融后肾功能得以明显改善。
表1 鉴别PVC-CM 的临床和PVC 特征

