冬凌草对代谢相关脂肪性肝病脂质代谢的影响
马文骏 史婷婷 白明辉 陈小伟 杨兴鑫


[摘要] 目的 探討冬凌草对大鼠代谢相关脂肪性肝病(MAFLD)脂质代谢相关蛋白表达的影响。 方法 通过高脂饲料喂养大鼠复制代谢相关脂肪性肝病模型,选用30只SD雄性大鼠适应性饲养1 d后,采用随机数字表法分为正常组,模型组,冬凌草低、中、高剂量组,每组6只。正常组以普通饲料喂养,其他组均以高脂饲料喂养。冬凌草低、中、高剂量组每天分别按照50、100 mg/(kg·d)和150 mg/(kg·d)进行灌胃;正常组与模型组每天灌胃与其他实验组等体积的生理盐水。HE染色观察大鼠MAFLD肝组织脂肪变性程度;采用全自动生化仪检测三酰甘油(TG)、总胆固醇(TC)、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)、空腹血糖(FBG)、空腹胰岛素(FINS);采用Western blot方法检测过氧化物酶体增殖物激活受体-α(PPAR-α)、固醇调节元件结合蛋白-1c(SREBP-1c)、脂肪酸合成酶(FAS)、乙酰辅酶A羧化酶(ACC)的蛋白表达。 结果 正常组大鼠肝组织显微镜下提示结构清晰及完整,肝小叶结构正常,肝细胞索排列整齐;模型组大鼠肝细胞损伤,可见弥漫性肝细胞脂肪变,并有肝细胞气球样变,汇管区伴有炎症细胞浸润,部分点状肝细胞坏死并有碎屑样坏死;冬凌草高剂量组大鼠肝脏的损伤程度比模型组明显减轻,肝细胞脂肪变性减少,仅少许出现点状坏死,少量脂肪滴空泡,汇管区无明显炎细胞浸润。各组大鼠TC、TG、HDL-C、LDL-C、FBG、FINS水平比较,差异有统计学意义(P < 0.05)。模型组大鼠血清TC、TG、LDL-C、FBG、FINS含量高于正常组,HDL-C含量低于正常组,差异有统计学意义(P < 0.05)。冬凌草低剂量组TG、FINS含量低于模型组,差异有统计学意义(P < 0.05);冬凌草中剂量组TC、TG、LDL-C、FBG、FINS含量低于模型组,且TG、FBG低于冬凌草低剂量组,差异有统计学意义(P < 0.05)。冬凌草高剂量组TC、TG、LDL-C、FBG、FINS含量低于模型组,HDL-C含量高于模型组,且TC、TG、LDL-C、FBG低于冬凌草低剂量组,差异有统计学意义(P < 0.05)。模型组PPAR-α、ACC蛋白表达低于正常组,SREBP-1c、FAS蛋白表达高于正常组,差异有统计学意义(P < 0.05)。冬凌草低剂量组PPAR-α、ACC蛋白表达高于模型组,FAS蛋白表达低于模型组,差异有统计学意义(P < 0.05)。冬凌草中、高剂量组PPAR-α、ACC蛋白表达高于模型组及冬凌草低剂量组,SREBP-1c、FAS蛋白表达低于模型组及冬凌草低剂量组,差异有统计学意义(P < 0.05)。 结论 冬凌草对大鼠MAFLD脂质代谢及相关蛋白表达具有调控作用,为进一步研究MAFLD的发生发展机制奠定了基础。
[关键词] 冬凌草;代谢相关脂肪性肝病;过氧化物酶体增殖物激活受体-α;固醇调节元件结合蛋白-1c;脂肪酸合成酶;乙酰辅酶A羧化酶
[中图分类号] R28 [文献标识码] A [文章编号] 1673-7210(2020)09(a)-0004-06
[Abstract] Objective To investigate the effect of Rabdosia Rubescens on the expression of lipid metabolism-associated proteins in rats with metabolism-associated fatty liver disease (MAFLD). Methods The rats were fed with high-fat diet to replicate the metabolic-related fatty liver disease model. A total of 36 SD male rats were adaptively fed for one day, and they were divided into normal group, model group, and Rabdosia Rubescens low, medium, and high-dose groups by random number table method, each with six animals. The normal group was fed with ordinary feed, and the other groups were fed with high-fat feed. The low, medium, and high-doses of Rabdosia Rubescens were given 50, 100 mg/(kg·d) and 150 mg/(kg·d) per day, respectively; the normal group and the model group were given a gavage with the same volume of normal saline as the other experimental groups every day. HE staining was used to observe the degree of fatty degeneration of rat MAFLD liver tissue; automatic biochemical analyzer was used to detect triacylglycerol (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL- C), fasting blood glucose (FBG), fasting insulin (FINS); Western blot method was used to detect the expressions of peroxisome proliferator-activated receptor-α (PPAR-α), sterol regulatory element binding protein-1c (SREBP-1c), fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC) protein. Results The liver tissue of rats in the normal group showed clear and complete structure under microscope, the structure of liver lobules was normal, and the hepatocyte cords were neatly arranged. Hepatocyte damage in the model group showed diffuse hepatocyte steatosis with balloon-like hepatocytes, inflammatory cell infiltration in the portal area, and partial punctate hepatocyte necrosis and detrital necrosis. The degree of liver damage in the Rabdosia Rubescens high-dose group was significantly less than that of the model group. The fatty degeneration of hepatocytes was reduced. There was only a few punctate necrosis, a few fat droplets, and no obvious inflammatory cell infiltration in the portal area. The levels of TC, TG, HDL-C, LDL-C, FBG, and FINS of rats in each group were compared, and the differences were statistically significant (P < 0.05). The levels of serum TC, TG, LDL-C, FBG, and FINS in the model group were higher than those in the normal group, while the level of HDL-C was lower than that in the normal group, and the differences were statistically significant (P < 0.05). The content of TG and FINS in the low-dose Rabdosia Rubescens group were lower than those of the model group, and the differences were statistically significant (P < 0.05); the content of TC, TG, LDL-C, FBG, and FINS in the medium-dose Rabdosia Rubescens group were lower than those of the model group, while TG and FBG were lower than those of the low-dose Rabdosia Rubescens group, and the differences were statistically significant (P < 0.05). The content of TC, TG, LDL-C, FBG, and FINS in the high-dose Rabdosia Rubescens group were lower than those of the model group, while the level of HDL-C was higher than that of the model group, and TC, TG, LDL-C, FBG were lower than those of the low-dose Rabdosia Rubescens group, and the differences were statistically significant (P < 0.05). The expressions of PPAR-α and ACC protein in the model group were lower than those of the normal group, while the expressions of SREBP-1c and FAS protein were higher than those of the normal group, and the differences were statistically significant (P < 0.05). The expressions of PPAR-α and ACC protein in the low dose of Rabdosia Rubescens group were higher than those of the model group, while the expression of FAS protein was lower than that of the model group, and the differences were statistically significant (P < 0.05). The expressions of PPAR-α and ACC protein in the medium and high-doses Rabdosia Rubescens group were higher than those of the model group and the low-dose Rabdosia Rubescens group, while the expressions of SREBP-1c and FAS protein were lower than those of the model group and the low-dose Rabdosia Rubescens group, and the differences were statistically significant (P < 0.05). Conclusion Rabdosia Rubescens can regulate lipid metabolism and associated protein expression in rats with MAFLD, which lays a foundation for further study on the mechanism of the occurrence and development of MAFLD.
[Key words] Rabdosia Rubescens; Metabolism-associated fatty liver disease; Peroxisome proliferator-activated receptor-α; Sterol regulatory element binding protein-1c; Fatty acid synthase; Acetyl-CoA carboxylase
代谢相关脂肪性肝病(metabolic associated fatty liver disease,MAFLD)是一种与胰岛素抵抗(insulin resistance,IR)和遗传易感密切相关的代谢应激性肝损伤[1]。随着生活方式的改变和病毒性肝病的控制,MAFLD发病逐年增加,全球成人患病率约25%,已成为我国第一大慢性肝病(占慢性肝病的49.3%),人群危害日益嚴重[2-3]。因此,深入对MAFLD的发病和发展机理研究,找出安全、有效的阻断药,已经迫在眉睫。冬凌草为唇形科植物碎米桠的干燥地上部分—唇形科小灌木,作为清热解毒中草药广泛用于上呼吸道感染和恶性肿瘤辅助治疗。研究还发现冬凌草具有降血脂、降血压和调节糖代谢等功效[4-5]。本研究为探讨冬凌草治疗大鼠MAFLD抗脂质代谢的作用进行实验。
1 材料与方法
1.1 实验材料
动物:SD雄性大鼠30只,SPF级,2月龄,体重180~220 g,购买于上海西普尔必凯实验动物有限公司[动物许可证号:SCXK(沪)-2018-0006],动物实验在浙江中医药大学动物实验研究中心[动物使用许可证:SYXK(浙)2018-0012]。室温22~23℃,空气流通,相对湿度55%~60%,12 h光照维持,昼夜循环。实验期间,动物自由摄食和饮水。动物饲养及动物实验方案均遵循国家《实验动物管理条例》执行,并获得杭州市西溪医院医学伦理委员会的批准。
饲料:普通饲料由浙江中医药大学动物实验研究中心提供;高脂饲料配方:基础饲料69%、猪油10%、胆固醇2%、蔗糖5%、胆盐0.5%、蛋黄粉10%、复合维生素0.5%、酵母粉3%。
1.2 仪器与试剂
紫外分光光度计(美国Beckman,型号:DU-800);低温高速离心机(德国Eppendorf,型号:5920R);Mini-PROTEAN电泳系统以及Mini Trans-Blot转印系统(美国Bio-Rad,型号:1658033);水平脱色摇床(国产华利达,型号:ZD-9556)。
冬凌草(贵阳市药材公司,经鉴定为唇型科茶菜属植物);30% 丙烯酰胺溶液(Bio-Rad公司,批号:161-0156);T-PER Tissue Protein Extraction Reagent(Thermo Pierce公司,批号:78510);BCA蛋白浓度测定试剂盒(增强型)(碧云天生物技术,批号:P0010);PVDF转印膜(Millipore公司,批号:IPVH00010);SuperSignal West Dura Extended Duration Substrate(Thermo Pierce公司,批号:34075);过氧化物酶体增殖物激活受体α(PPAR-α)一抗(Abcam公司,批号:ab126285,稀释度1∶500);乙酰辅酶A羧化酶(ACC)一抗(CST公司,批号:3662,稀释度1∶1000);固醇调节元件结合蛋白-1c(SREBP-1c)一抗(Abcam公司,批号:ab191857,稀释度1∶500);脂肪酸合成酶(FAS)一抗(Abcam公司,批号:ab22759,稀释度1∶1000);GAPDH(Abcam公司,批号:ab181602,稀释度1∶10000);Goat anti-Mouse IgG(H+L)Secondary antibody(Thermo Pierce公司,批号:31160,稀释度1∶5000);Goat anti-Rabbit IgG(H+L)Secondary antibody(Thermo Pierce公司,批号:31210,稀释度1∶5000);HRP conjugated Rabbit ANTI-Goat(Pierce公司,批号:31402,稀释度1∶5000)。
1.3 模型制备及给药方法
取清洁级SD雄性大鼠30只,适应性饲养1 d后随机数字表法分为5组,每组6只:正常对照组,模型组,冬凌草低、中、高剂量组。正常组以普通饲料喂养,其他实验组均以高脂饲料喂养,连续喂养16周。冬凌草低、中、高剂量组每天分别按照50、100 mg/(kg·d)和150 mg/(kg·d)进行灌胃;正常组与模型组每天灌胃与其他实验组等体积的生理盐水。实验大鼠均自由进食和饮水并分笼饲养,明暗各12 h。饲料喂养至第16周末,前天晚上开始禁食不禁水16 h,第2天早上以3%的戊巴比妥钠(0.2 mL/100 g)腹腔注射麻醉后取血,离心转速2500 r/min,离心半径为10 cm,离心15 min后保存于-20℃冰箱中随后进行相关指标检测;同时取肝组织保存后续进行病理及蛋白检测。参照美国非酒精性脂肪性肝炎临床研究协作网推荐的诊断方式,对肝的脂肪变性、小叶炎症和肝细胞气球样变3项组织学特征进行评分,进而评估脂肪肝受损的严重程度[6],作为判断造模成功的标准。
1.3 HE染色观察大鼠肝组织脂肪变性程度
取各组肝组织用10%中性甲醛溶液固定,石蜡包埋,切片,脱蜡至水,4℃保存,然后进行苏木精染细胞核和伊红染细胞质,最后脱水封片,用显微镜观察各组肝组织结构及病理变化情况。
1.4 血清学指标检测方法
按照试剂盒中说明书方法测定血清总胆固醇(TG)、三酰甘油(TC)、高密度脂蛋白胆固醇(HDL-C)、低密度脂蛋白胆固醇(LDL-C)、空腹血糖(FBG)、空腹胰岛素(FINS)的含量。
1.5 采用Western blot方法检测PPAR-α、SREBP-1c、FAS、ACC蛋白的表达
采用总蛋白提取试剂盒进行总蛋白的提取,采用二喹啉甲酸(BCA)定量试剂盒进行总蛋白定量;配制8%~12%分离胶和5%浓缩胶,进行十二烷基硫酸钠聚丙烯酰胺凝胶(SDS-PAGE)电泳分析;蛋白质转模;转印膜封闭;一抗以一定比例溶于T-TBS,4℃孵育过夜,采用T-TBS漂洗5 min×4;二抗以一定比例溶于T-TBS(含2%脱脂奶粉),室温1 h,然后T-TBS漂洗5 min×5,每个目的蛋白独立做3次重复试验;最后采用Image J软件分析条带的光密度值,目的蛋白相对表达量=目的蛋白(光密度值)/内参(光密度值)×10n。
1.6 统计学方法
采用SPSS 19.0软件对所得数据进行统计分析。计量资料以均数±标准差(x±s)表示,两组比较采用t检验,多組比较采用单因素方差分析,进一步两两比较,采用LSD-t检验。计数资料以例数表示。以P < 0.05为差异有统计学意义。
2 结果
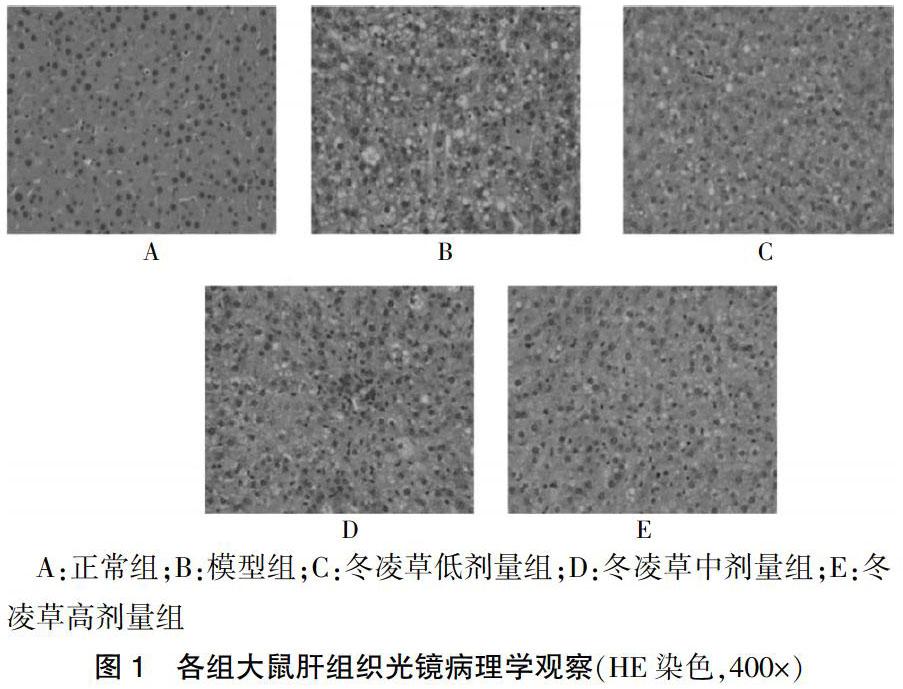
2.1 各组大鼠肝脏组织HE染色结果显示
正常组大鼠肝组织显微镜下提示结构清晰及完整,肝小叶结构正常,肝细胞索排列整齐;模型组大鼠肝细胞损伤,可见弥漫性肝细胞脂肪变,并有肝细胞气球样变,汇管区伴有炎症细胞浸润,部分点状肝细胞坏死并有碎屑样坏死;冬凌草高剂量组大鼠肝脏的损伤程度比模型组明显减轻,肝细胞脂肪变性减少,仅少许出现点状坏死,少量脂肪滴空泡,汇管区无明显炎细胞浸润。见图1。
2.2 各组大鼠血清TC、TG、LDL-C、HDL-C、FBG、FINS含量比较
各组大鼠血清TC、TG、HDL-C、LDL-C、FBG、FINS水平比较,差异有高度统计学意义(P < 0.01)。模型组大鼠血清TC、TG、LDL-C、FBG、FINS含量高于正常组,HDL-C含量低于正常组,差异有统计学意义(P < 0.05)。经药物治疗后,冬凌草低剂量组TG、FINS含量低于模型组,差异有统计学意义(P < 0.05);冬凌草中剂量组TC、TG、LDL-C、FBG、FINS含量低于模型组,且TG、FBG低于冬凌草低剂量组,差异有统计学意义(P < 0.05)。冬凌草高剂量组TC、TG、LDL-C、FBG、FINS含量低于模型组,HDL-C含量高于模型组,且TC、TG、LDL-C、FBG低于冬凌草低剂量组,差异有统计学意义(P < 0.05)。见表1。
2.3 各组大鼠肝组织PPAR-α、SREBP-1c、FAS、ACC蛋白表达水平比较
模型组PPAR-α、ACC蛋白表达低于正常组,SREBP-1c、FAS蛋白表达高于正常组,差异有统计学意义(P < 0.05)。冬凌草低剂量组PPAR-α、ACC蛋白表达高于模型组,FAS蛋白表达低于模型组,差异有统计学意义(P < 0.05)。冬凌草中、高剂量组PPAR-α、ACC蛋白表达高于模型组及冬凌草低剂量组,SREBP-1c、FAS蛋白表达低于模型组及冬凌草低剂量组,差异有统计学意义(P < 0.05)。见图2。
3 讨论
代谢相关脂质性肝病(metabolic associated fatty liver disease,MAFLD),是指肝组织的细胞内脂肪蓄积超过肝湿重的5%或者组织学上每个单位面积有1/3及以上肝细胞发生脂变性,并且无过量饮酒史,是遗传、环境和代谢应激相关因素所导致的以肝细胞内脂肪性堆积为主要的临床病理综合征[7-8]。MAFLD的病理变化随着病程的进展而表现有单纯性脂肪肝、脂肪性肝炎、脂肪性肝纤维化和肝硬化[9-12]。
冬凌草是一种二萜类化合物,其生物学活性主要包括抗炎、抗癌、抗氧化、免疫调节和调控细胞自噬等作用[13-18]。值得注意的是,冬凌草能够减轻四氯化碳、酒精所造成的肝损伤,具体表现为稳定肝细胞的细胞膜结构、减轻炎症反应和过氧化反应所造成的肝细胞损伤[14,19]。提示冬凌草可能对MAFLD具有治疗作用。
已有研究显示[20-21],MAFLD的发病与脂质过氧化、脂质代谢异常、氧化应激过高及IR等因素有关。而肝脏脂肪酸代谢过程涉及到多种关键酶(如:乙酰辅酶A、ACC、FAS)和转录因子(如:肝X受体、法尼酯衍生物X受体、SREBP-1c、PPAR-α)。
本研究通过对高脂饲料喂养的大鼠灌以冬凌草低、中、高剂量进行实验,发现在肝脏组织学上,冬凌草高剂量组大鼠肝脏的损伤程度比模型组明显减轻,肝细胞脂肪变性减少;在生化指标上,冬凌草高剂量组大鼠血清TC、TG、LDL-C、FBG、FINS含量低于模型组,HDL-C含量高于模型组;在肝脏部分关键酶和转录因子上,冬凌草中、高剂量组SREBP-1c、FAS蛋白表达低于模型组,PPAR-α、ACC蛋白表达高于模型组。
因此,推断较高剂量的冬凌草能升高PPAR-α、ACC蛋白表达,降低SREBP-1c、FAS的蛋白表达和FBG、FINS含量,从而达到提高HDL-C,降低TC、TG、LDL-C,改善大鼠肝脏细胞脂肪变性和炎症损伤的效果。探讨其作用机制,可能有以下几点:①激活PPAR-α这个调节肝脏脂质代谢的关键因子,促进极低密度脂蛋白胆固醇(VLDL-C)和乳糜微粒(CM)的分解;促进肝脏的脂肪酸的氧化,降低肝细胞的脂肪酸的合成;增加肝组织载脂蛋白A Ⅰ(ApoA Ⅰ)的表达;促进HDL-C的成熟和代谢;促进胆固醇的逆向转运等多个水平参与脂质代谢的调节[22],从而降低血浆TG 水平,升高HDL-C水平,改善脂质代谢水平[23]。②有研究显示[24],SREBP-1c在肝脏组织中过度表达引起糖脂代谢紊乱,导致肝脏等非脂肪组织的脂质大量堆积,而冬凌草可抑制SREBP-1c的过度表达,通过调节下游一系列脂肪酸合成和糖代谢靶基因包括ACC、FAS、硬脂酰辅酶A去饱和酶1(SCD1)、磷酸烯醇式丙酮酸羧化酶(PEPCK)、葡萄糖激酶(GK)等来参与脂质自稳态的多个方面调节,进而抑制肝脏脂质堆积。③FAS在合成脂肪酸方面发挥着关键的作用,与体内的脂质代谢密切相关[25],冬凌草可能通过抑制降低FAS的表达,抑制脂肪酸合成,减少其在肝细胞中异常沉积。
综上所述,冬凌草可通过降低大鼠肝脏SREBP-1c、FAS蛋白表达和血清TC、TG、LDL-C、FBG、FINS的含量,抑制肝脏脂肪酸吸收和脂肪合成;上调肝脏PPAR-α、ACC蛋白表达和血清HDL-C含量,促进脂肪酸氧化、肝内脂肪分解等,改善MAFLD大鼠肝脏脂肪变性,对MAFLD的治疗可能具有较大的实用价值,也为进一步研究MAFLD的发生发展机制奠定了基础。
[参考文献]
[1] 中华医学会肝病学分会脂肪肝和酒精性肝病学组,中国医师协会脂肪性肝病专家委员会.非酒精性脂肪性肝病防治指南(2018年版)[J].临床肝胆病杂志,2018,34(5):947-957.
[2] Wang FS,Fan JG,Zhang Z,et al. The global burden of liver disease:the major impact of China [J]. Hepatology,2014, 60(6):2099-2108.
[3] Cotter TG,Rinella M. Nonalcoholic Fatty Liver Disease 2020:The State of the Disease[J]. Gastroenterology,2020,158(7):1851-1864.
[4] Miao M,Yan X,Guo L,et al. Effects of the Rabdosia rubescens total flavonoids on focal cerebral ischemia reperfusion model in rats [J]. Saudi Pharm J,2017,25(4):606-614.
[5] 吳岚,包丽萍,李菊霜,等.冬凌草甲素通过调节氧化应激和脂代谢减轻糖尿病大鼠肾损伤[J].临床肾脏病杂志,2017,17(11):692-696.
[6] Bedossa P. Utility and appropriateness of the fatty liver inhibition of progression(FLIP)algorithm and steatosis,activity,and fibrosis(SAF)score in the evaluation of biopsies of nonalcoholic fatty liver disease [J]. Hepatology,2014,60(2):565-575.
[7] Eslam M,Sanyal AJ,George J,et al. MAFLD:A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease [J]. Gastroenterology,2020,158(7):1999-2014.
[8] Shi TT,Wu L,Ma WJ,et al. Nonalcoholic Fatty Liver Disease:Pathogenesis and Treatment in Traditional Chinese Medicine and Western Medicine [J]. Evid Based Complement Alternat Med,2020,2020:8749564.
[9] Alwahsh SM,Gebhardt R. Dietary fructose as a risk factor for non-alcoholic fatty liver disease(NAFLD)[J]. Arch Toxicol,2017,91(4):1545-1563.
[10] European Association for the Study of the Liver(EASL),European Association for the Study of Diabetes(EASD),European Association for the Study of Obesity(EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease [J]. Diabetologia,2016,59(6):1121-1140.
[11] Shi TT,Yang XX,Zhou HP,et al. Activated Carbon N-acetylcysteine Microcapsule Protects Against Nonalcoholic Fatty Liver Disease in Young Rats via Activating Telomerase and Inhibiting Apoptosis [J]. PLoS One,2018,13(1):e0189856.
[12] Lau LHS,Wong SH. Microbiota,Obesity and NAFLD [J]. Adv Exp Med Biol,2018,1061:111-125.
[13] Liermann J,Naumann P,Fortunato F,et al. Phytotherapeutics oridonin and ponicidin show additive effects combined with irradiation in pancreatic cancer in vitro [J]. Radiol Oncol,2017,51(4):407-414.
[14] Bae S,Lee EJ,Lee JH,et al. Oridonin protects HaCaT keratinocytes against hydrogen peroxide-induced oxidative stress by altering microRNA expression [J]. Int J Mol Med,2014,33(1):185-193.
[15] Li Y,Wang Y,Wang S,et al. Oridonin phosphate-induced autophagy effectively enhances cell apoptosis of human breast cancer cells [J]. Med Oncol,2015,32(1):365.
[16] Ding Y,Ding C,Ye N,et al. Discovery and development of natural product oridonin-inspired anticance agents [J]. Eur J Med Chem,2016,122(10):102-117.
[17] 成伯寧,刘殿雷,史婷婷,等.冬凌草对非酒精性脂肪肝大鼠氧化应激及脂肪酸代谢的影响[J].中国临床药理学杂志,2020,36(10):1252-1255.
[18] He HB,Jiang H,Chen Y,et al. Oridonin Is a Covalent NLRP3 Inhibitor With Strong Anti-Inflammasome Activity [J]. Nat Commun,2018,9(1):2550.
[19] 宋琪雯,刘智,孙为民,等.冬凌草对酒精性肝损伤模型大鼠炎性因子及肝功能的影响[J].中国生化药物杂志,2015,10(35):15-21.
[20] 王宫,魏文增,吴华蒿.牛樟芝滴丸对大鼠非酒精性脂肪肝的干预作用[J].福建中医,2018,49(4):41-43.
[21] López-Sánchez GN,Dóminguez-Pérez M,Uribe M,et al. Non-alcoholic fatty liver disease and microRNAs expression,how it affects the development and progression of the disease [J]. Ann Hepatol,2020:S1665-2681(20)30053-3.
[22] 张方.与肝脏脂质代谢异常相关的转录因子的研究进展[J].现代临床医学,2014,40(3):163-166.
[23] Kersten S. Peroxisom proliferator activated receptors and obesity [J]. Eur J Pharmacol,2002,440(2/3):223-234.
[24] 魏文增,王宫,吴华嵩.牛樟芝滴丸对大鼠非酒精性脂肪肝相关基因表达的影响[J].中国中医药科技,2020, 27(2):186-189.
[25] Torres JL,Novo-Veleiro I,Manzanedo L,et al. Role of microRNAs in alcohol-induced liver disorders and non-alcoholic fatty liver disease [J]. World J Gastroenterol,2018,24(36):4104-4118.
(收稿日期:2020-06-01)

