一种用于光催化氧化硫醚成亚砜的超薄卟啉的共价有机骨架纳米片
刘文博 党 辉 刘文苹 郁宗华 曹 韡 王 康*, 姜建壮*,
(1北京科技大学化学与生物工程学院化学与化学工程系,功能分子与晶态材料科学与应用北京市重点实验室,北京材料基因工程高精尖创新中心,北京 100083)
(2国民核生化灾害防护国家重点实验室,北京 102205)
0 Introduction
Selective oxidation of sulfides to sulfoxides is an important industrial reaction because the products are bioactive substances which are generally utilized in pharmaceutics[1-5]. To achieve the reaction, an oxidant such as dioxirane, oxone, singlet oxygen (1O2), and hydrogen peroxide is necessary[6-10]. Among which1O2,a reactive oxygen species, has recently attracted increasing attention owing to its excellent selectivity,high oxidation efficiency, ready availability, and environmental friendliness[11-15]. For the generation of1O2,efficient photosensitizers are critical because it is forbidden to directly excite3O2to1O2. So far, diverse homogeneous photosensitizers including organic conjugated compounds and organic metal complexes have been developed for the production of1O2[16-18].Porphyrin (Por)derivative is one of the most widely applied homogeneous photosensitizers owing to its superior1O2yield,high efficiency in light harvesting,and excellent chemical stability[13,19-21]. It is worth noting that the Por-based homogeneous photosensitizers usually suffer from the difficult product separation. To overcome this problem,immobilizing Por molecules on some substrates such as SiO2, Si, and polydimethylsiloxane has been utilized[22-25]. However, such immobilized heterogeneous photosensitizers usually display decreased activity in comparison to the corresponding homogeneous ones.As a consequence, it is still a great challenge to develop Por-based hetergeneous photosensitizers with high activity for photooxygenation.
Covalent organic frameworks (COFs) are a new class of porous materials with ordered two-dimensional(2D) or three-dimensional (3D) networks[26-28]. Owing to the intriguing architectures and unique properties including ordered porous structure, large surface area,highly adjustablility, good stability, and functionalities,COFs have attracted considerable interest in many fields such as gas storage and separation, sensing,catalysis, luminescence, and energy conversion[29-34]. In particular, 2D COF nanosheets (NSs), which possess high surface percentage and accessible active sites, are considered as an ideal platform for heterogeneous photo-/electro-catalysis[35-39]. We rationalize that 2D COF NSs fabricated by using Por molecules as building blocks could integrate the advantages of 2D COF NSs and Pors, resulting in superior performance in1O2generation as well as selective oxidation of sulfides to sulfoxides.
Very recently, we have developed a simple bottom-up synthetic method for fabricating ultrathin iminebased 2D COF NSs[37]. In the present case, Por-based COF-367 NSs were also prepared by this newly developed method. The potential application of ultrathin COF-367 NSs for the selective photooxidation of sulfides was investigated. We firstly demonstrate that COF-367 NSs exhibit high activities as photosensitizers for the1O2generation under the irradiation of visible light. Then COF-367 NSs were used as a photosensitizer for the oxidation of thioanisole into methyl phenyl sulfoxide upon visible-light irradiation with the turnover frequency (TOF) as high as 714 h-1. In addition, multiple cycles of the photooxidation reaction experiments prove the excellent stability of COF-367 NSs.
1 Experimental
1.1 General remarks
All the reagents and solvents were of reagent grade and used as received.1H NMR spectra were recorded on a Bruker DPX 400 spectrometer. IR spectra were recorded as KBr pellets using a Bruker Tensor 37 spectrometer with 2 cm-1resolution.UV-Vis absorption spectra were collected on an SHIMADZU UV-2600 spectrophotometer. Atomic force microscope (AFM)images were measured by a Bruker Multimode 8 system with a silicon cantilever by using tapping mode.All AFM images are shown in height mode without any image processing except flatting. Transmission electron microscopy (TEM) images were collected from HT7700 electron microscope at 100 kV. High resolution-TEM(HR-TEM) images were taken on a JEM-ARM200F electron microscope operated at 200 kV.The solar simulator was a SAN-EI ELECTRIC XES-40S1.
1.2 Photo-bleach experiments based on oxidation of 1,3-diphenylisobenzofuran
An oxygen-saturated dimethylformamide (DMF)solution (3.0 mL) of 1,3-diphenylisobenzofuran (DPBF,50 μmol·L-1) in the presence of 25 μg of COF-367 NSs was treated with a halogen lamp (20.0 mW·cm-2,λ>510 nm). The time-dependent absorption spectra of DPBF were recorded per three minutes for heterogeneous phase.
1.3 Photo-driven sulfide oxidation
The starting materials including sulfide substrate(1.0 mmol) and COF-367 NSs (the molar fraction was 0.05% based on Por unit) were suspended in 1.0 mL CD3OD in a 5.0 mL glass vessel. Before the reaction,oxygen was bubbled into the mixture for 5.0 min using an oxygen balloon. The reaction vessel in a water bath was then irradiated with a 25 W blue LED light (5.0 mW·cm-2, 420 <λem<490 nm) for the present photooxidation of sulfide derivatives, and the conversion was determined by1H NMR. The TOF was calculated using the following equation: TOF=ns/(nct), wherensis the amount of sulfide substrate conversion (mol),ncis the amount of the catalyst (based on Por unit,mol),andtis reaction time(h).
1.4 Stability test
In the heterogeneous recycle test,the photo-oxidation of sulfide (1.0 mmol) in the presence of COF-367 NSs (0.05% based on Por unit) was carried out. After each cycle of reaction, another sulfide (1.0 mmol) was added into the resulting mixture for the next cycle of reaction.
2 Results and discussion
As mentioned above,COF-367 NSs were prepared by means of the reported procedure (Fig.1a)[37]. As shown in the TEM image (Fig.1b), COF-367 NSs displayed a typical ultrathin 2D structure with the lateral sizes up to several micrometers. The thickness of COF-367 NSs,detected by AFM,wasca.1.0 nm,corresponding toca. 3 layers (Fig.1c). These results confirm the ultrathin nature of the as-prepared COF-367 NSs.Remarkably, the ultrathin structure improves the interactions between COF-367 NSs and the solvent molecules and in turn reduces the aggregation, resulting in the good dispersed nature of COF-367 NSs in various organic solvents(Fig.2).Actually,the excellent dispersibility of COF-367 NSs can be further confirmed by the fact that the absorption of COF-367 NSs in DMF was linear dependent on the concentration from 0.05 to 0.60 μg·mL-1(Fig.1d and 1e). In particular, it can be found that COF-367 NSs showed the very intense Por Soret band at 442 nm with several Q bands at 528,580, and 668 nm, very similar to the Por molecular monomer in DMF (Fig.1f), suggesting the outstanding absorption capability of COF-367 NSs. As a result,COF-367 NSs integrate the large-aspect-ratio 2D morphology and the high efficiency in light harvesting,which are supposed to be promising candidates for1O2generation. In addition, bulk COF-367 was also prepared for the purpose of comparison[40].
To evaluate the ability of COF-367 NSs in generating1O2, DPBF, a well-known1O2scavenger, was used to detect the1O2evolution upon visible-light irradiation(500 W halogen lamp,λ>510 nm). As can be found in Fig.3a, the absorption band of DPBF at 415 nm in an oxygen-saturated DMF solution gradually decreased under the irradiation in the existence of COF-367 NSs,suggesting a steady conversion of3O2into1O2.For comparision, photo-oxidation of DPBF was carried out in the absence of COF-367 NSs. As shown in Fig.3b, the absorption spectra of DPBF only showed a negligible decrease, revealing COF-367 NSs play the key role in the photocatalytic oxygenation of DPBF. Fig.3d exhibits the time-dependent plots of the absorbance of DPBF at 415 nm. It can be found that DPBF was completely expended in 24 min in the presence of COF-367 NSs,demonstrating the excellent performance of COF-367 NSs towards1O2generation. For the reference purpose,the ability of bulk COF-367 in generating1O2was also investigated under the same experiment condition(Fig.3c). In contrast, bulk COF-367 shows much lower photoactivity with onlyca.25% DPBF consumed within 24 min (Fig.3d), indicating the advantage of ultrathin 2D COF-367 NSs as a heterogeneous photocatalysis compared to the corresponding bulk material.
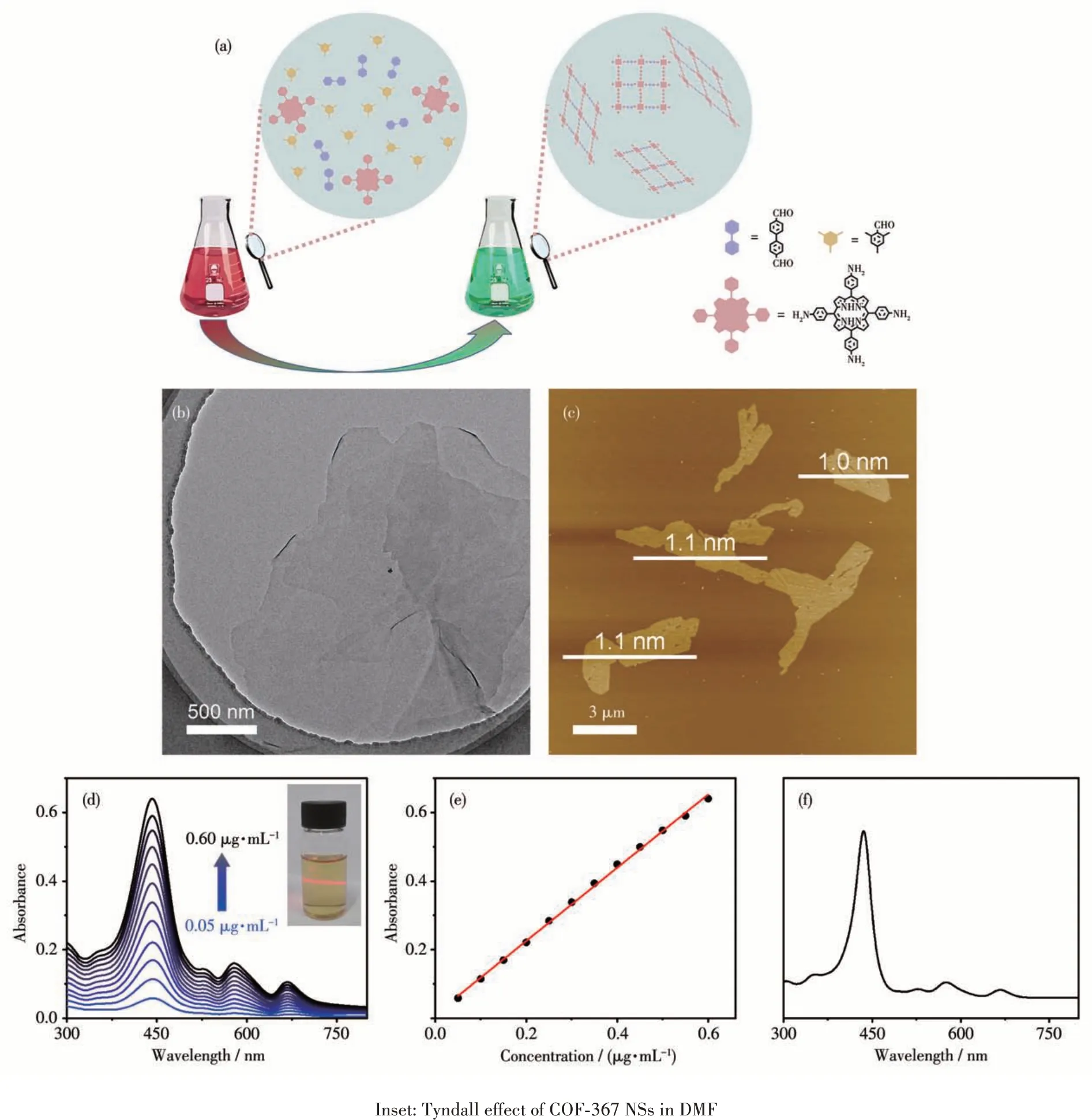
Fig.1 (a)Synthesis of COF-367 NSs;(b)TEM and(c)AFM images of COF-367 NSs;(d)UV-Vis absorption of COF-367 NSs n DMF with the concentration in a range of 0.05~0.60 μg·mL-1;(e)Plot of absorbance of COF-367 NSs dispersed in DMF at 442 nm vs the concentration;(f)UV-Vis absorption of tetrakis(4-aminophenyl)porphyrin in DMF
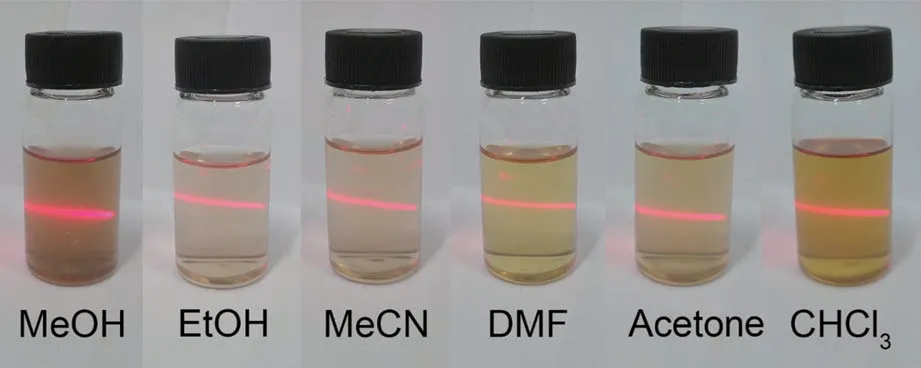
Fig.2 Photos of Tyndall effect of COF-367 NSs dispersed in various organic solvents
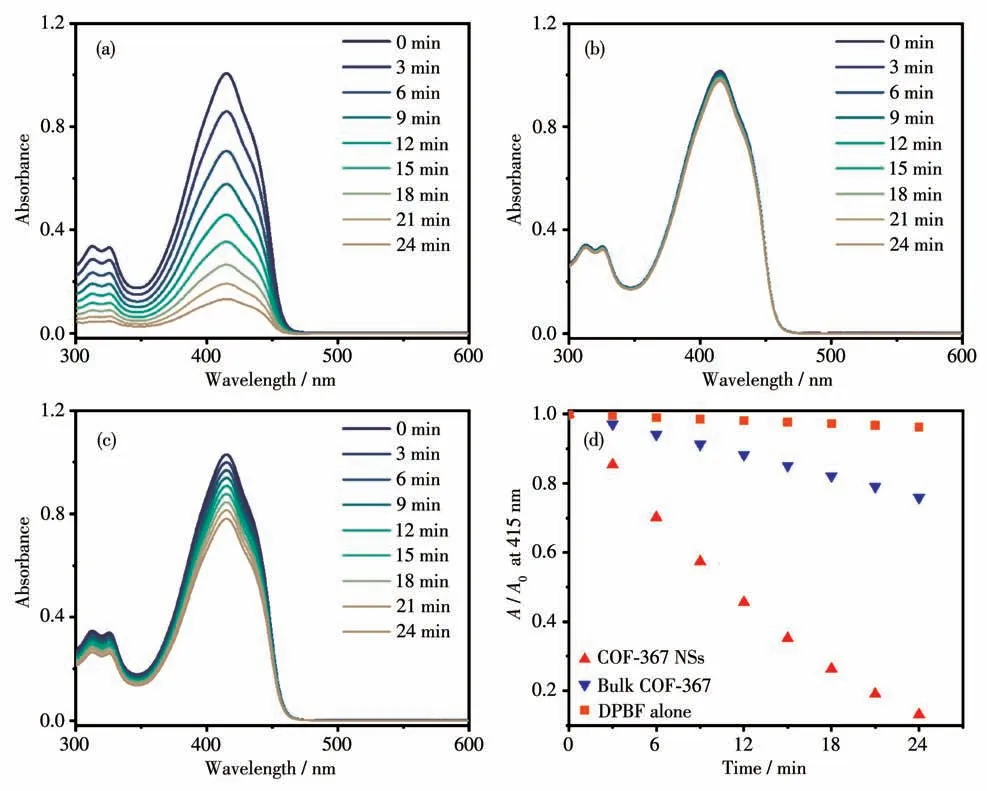
Fig.3 Time-dependent absorption spectra of DPBF in DMF under the irradiation at λ>510 nm(a)in the presence of COF-367 NSs,(b)without COF-367 NSs,(c)in the presence of bulk COF-367;(d)Time-dependent plots of the absorbance of DPBF at 415 nm in the presence of COF-367 NSs and bulk COF-367 as well as for DPBF alone
Considering the exceptional ability of COF-367 NSs in generating1O2, we further study COF-367 NSs as a heterogeneous catalyst in selective photocatalytic oxidation of sulfides to sulfoxides.The reaction was initially conducted with thioanisole in CD3OD in the presence of O2(101.3 kPa) and COF-367 NSs (Molar fraction 0.05%) upon 25 W blue LED irradiation. As can be seen in Fig.4a, 4c and Table 1, the thioanisole was converted to methyl phenyl sulfoxide with high efficiency(>99%) and high selectivity (98.8%), revealing the efficient photocatalytic activity of COF-367 NSs towards oxidizing sulfides to sulfoxides with the TOF calculated as high as 714 h-1. In sharp contrast, bulk COF-367 exhibited an inferior efficiency with a conversion of 48% in 3 h reaction period (Fig.4a,4d and Table 1),in line with the trend of1O2generation efficiency, further indicating the superiority of Por-based COF-367 NSs as a heterogeneous photocatalyst. Towards further understanding the role of COF-367 NSs,a series of control experiments were performed in the absence of visible-light irradiation,COF-367 NSs,and O2,respectively. As can be seen in Table 2, all these experiments generated negligible sulfoxide, revealing the photocatalyst nature of COF-367 NSs in photocatalytic oxidation of thioanisole to methyl phenyl sulfoxide. Besides the photocatalytic activity,the recycling stability is another considerable indicator to indicate the performance of a heterogeneous photocatalyst. As shown in Fig.4b, the thioanisole conversion had no obvious decrease even after six runs of thioanisole oxidation, proving the considerable stability of COF - 367 NSs. In addition,COF-367 NSs maintain the ultrathin COFs structure after multiple cycles as confirmed by the TEM and AFM images as well as FT-IR spectra, further indicating the excellent stability of COF-367 NSs (Fig.5). Inspired by the high photocatalytic performance of COF-367 NSs in the oxidation of thioanisole to methyl phenyl sulfoxide,the general applicability of COF-367 NSs in selective photooxidation of sulfides was investigated. A wide scope of substrates were used to test the photocatalytic activity and selectivity of COF-367 NSs under the same reaction conditions with the data listed in Table 1. As can be seen, when thioanisole bearing either an electron-donating (e.g.methoxy and methyl)or an electron-withdrawing (e.g.Br) group was used as a substrate, all reactions exhibited high conversion (>99%)and high selectivity (>97%) under 0.05% loading of COF-367 NSs.In addition,excellent yield and selectivity for aliphatic sulfoxide were also obtained under the same reaction conditions. In particular, mustard gas,the most notorious vesicant agent, is a typical sulfide[15,41]. The selective oxidation of mustard gas into corresponding nontoxic sulfoxide is regarded as a promising detoxification route[15]. Herein, 2-chloroethyl ethyl sulfide (CEES), a simulant of sulfur mustard gas[2,12,41-42],was also used as a substrate. As shown in Table 1 and Fig.6,the oxidation degradation of CEES into 2-chloroethyl ethyl sulfoxide (CEESO) could be finished in 1.8 h with a high conversion (>99%) by the photocatalytic system of COF-367 NSs.
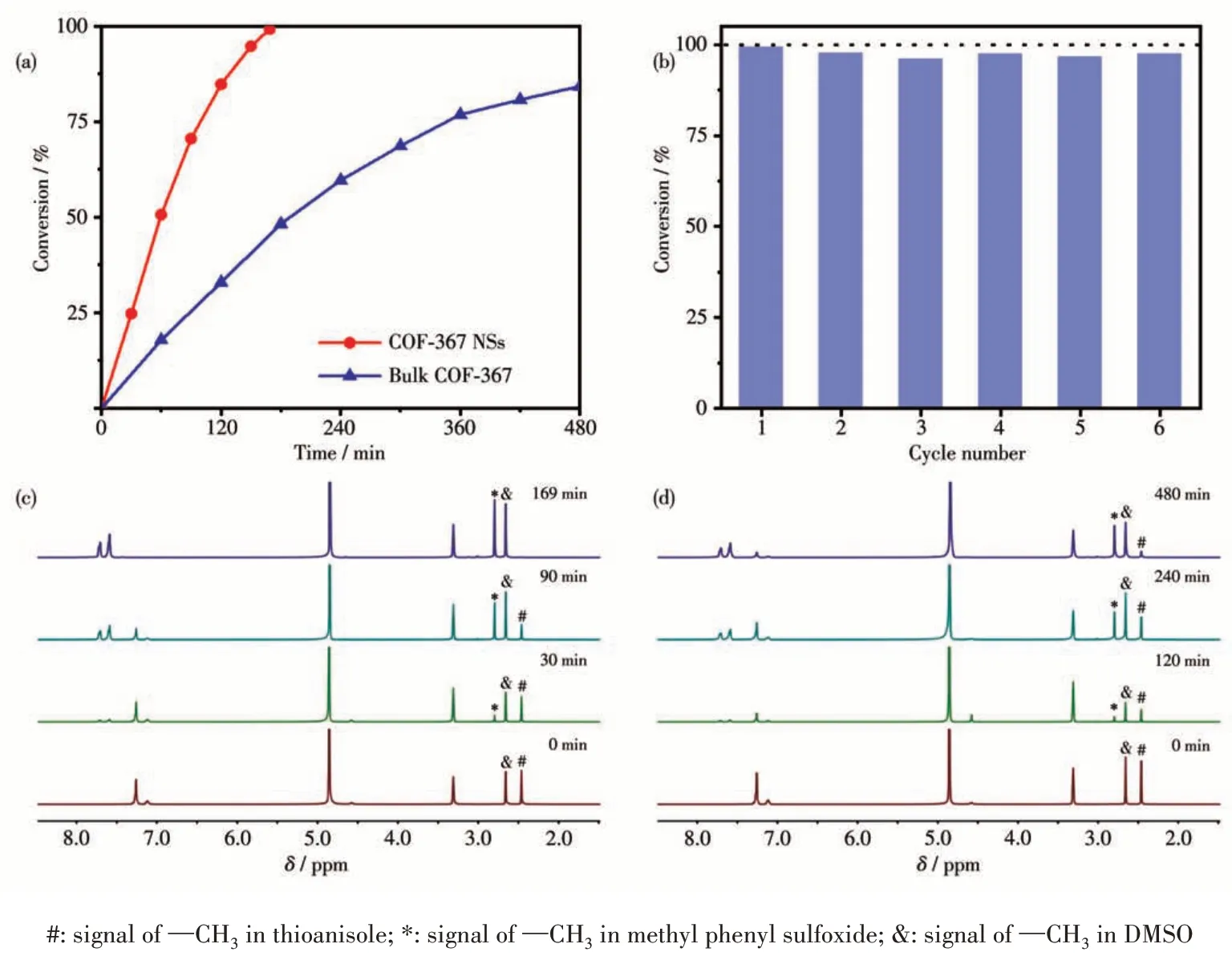
Fig.4 (a)Time-dependent plots of thioanisole conversion for COF-367 NSs and bulk COF-367;(b)Recyclability of COF-367 NSs in photo-oxidation reaction of thioanisole to methyl phenyl sulfoxide;Time-dependent 1H NMR spectra of thioanisole conversion for(c)COF-367 NSs and(d)bulk COF-367
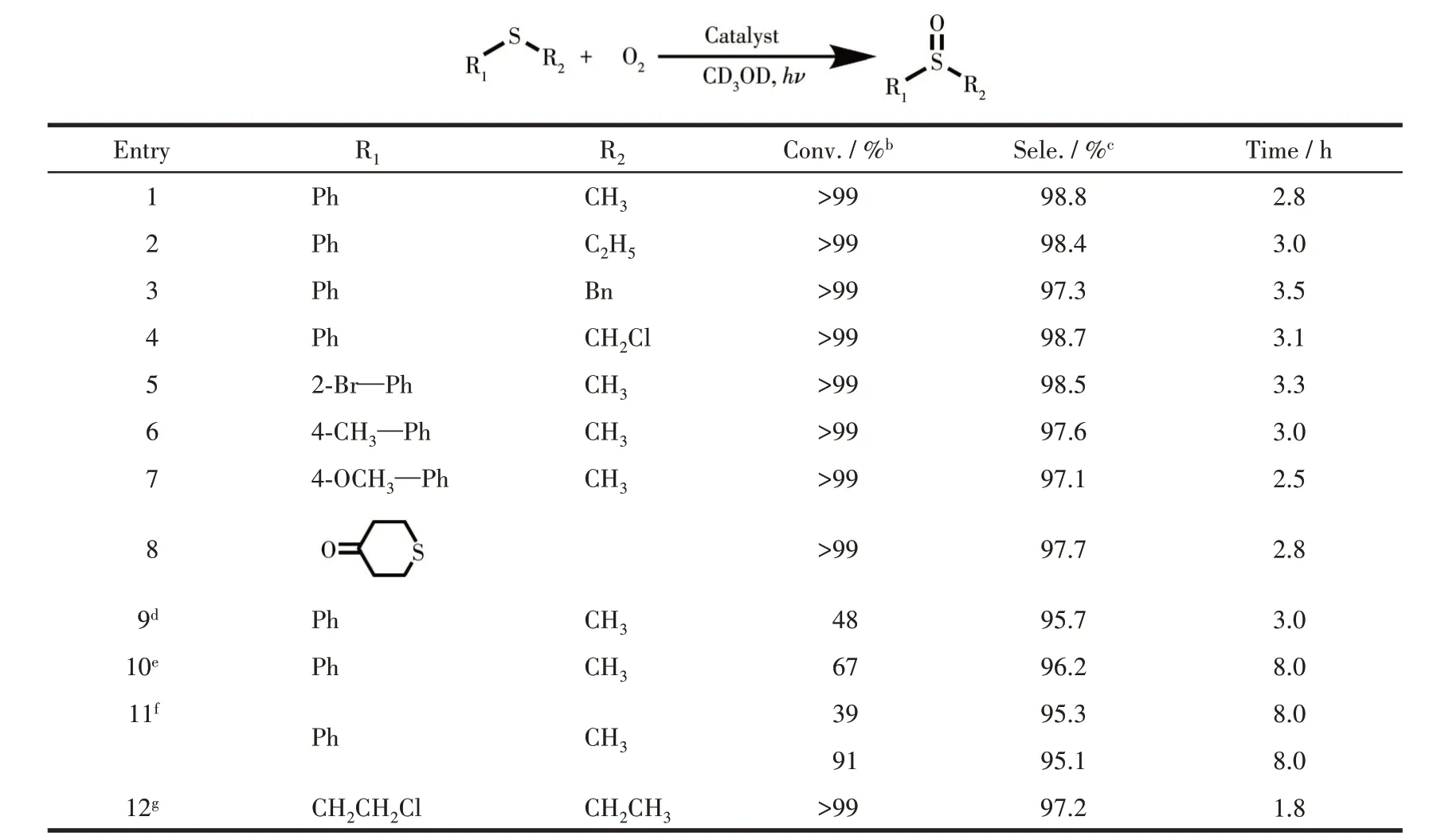
Table 1 Results for various photocatalytic oxidation of sulfides to sulfoxidesa
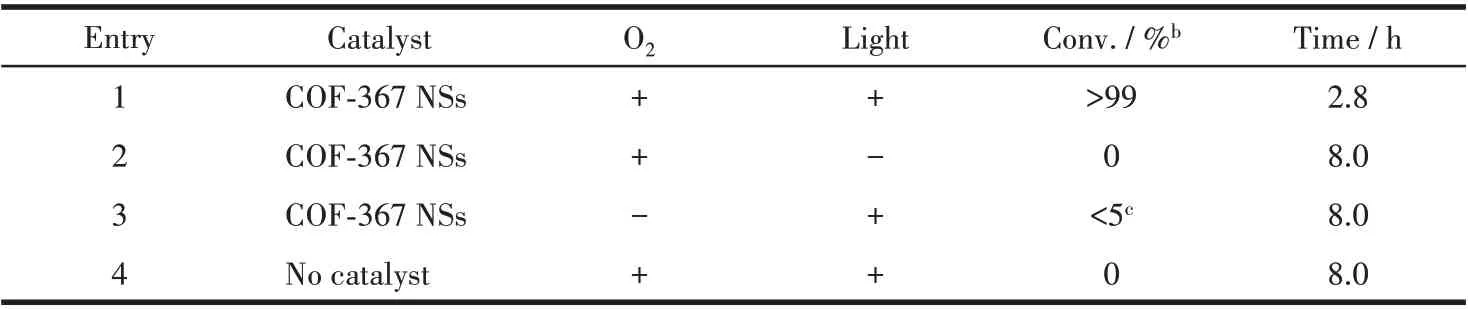
Table 2 Photo-oxidation reaction of thioanisole under various conditionsa
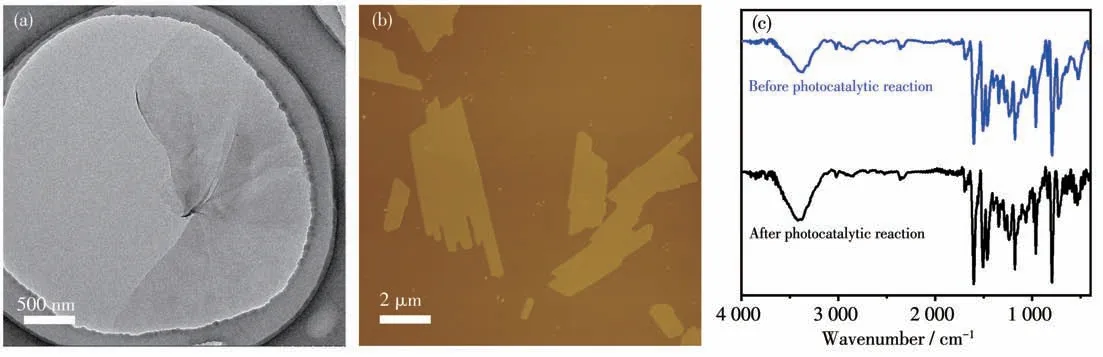
Fig.5 (a)TEM image,(b)AFM image,and(c)FT-IR spectra of COF-367 NSs after six cycles of photo-oxidation reaction of thioanisole
Toward further assessing the application of COF-367 NSs in selective oxidation of sulfides to sulfoxides, the photocatalytic reaction was carried out in air.As can be found in Table 1,COF-367 NSs can still convert 67% of thioanisole to methyl phenyl sulfoxide with a selectivity of 96.2% in 8 h under 0.05% loading and achieve a conversion of 95%with a high selectivity of 95.7% in 14 h. Moreover, we further carried out the photocatalytic oxidation of sulfides to sulfoxides in air under the sun light by using a solar simulator. The yield of methyl phenyl sulfoxide dropped to 39% in 8 h owing to the decrease in light intensity. Nevertheless,when the amount (molar fraction) of COF-367 NSs was increased to 0.2%, the corresponding yield sharply increased to 91% in 8 h with an excellent selectivity of 95.1% under the same reaction conditions. As a conclusion,all the results indicate that COF-367 NSs are a promising hetergeneous photosensitizer for selective oxidation of sulfides to sulfoxides.
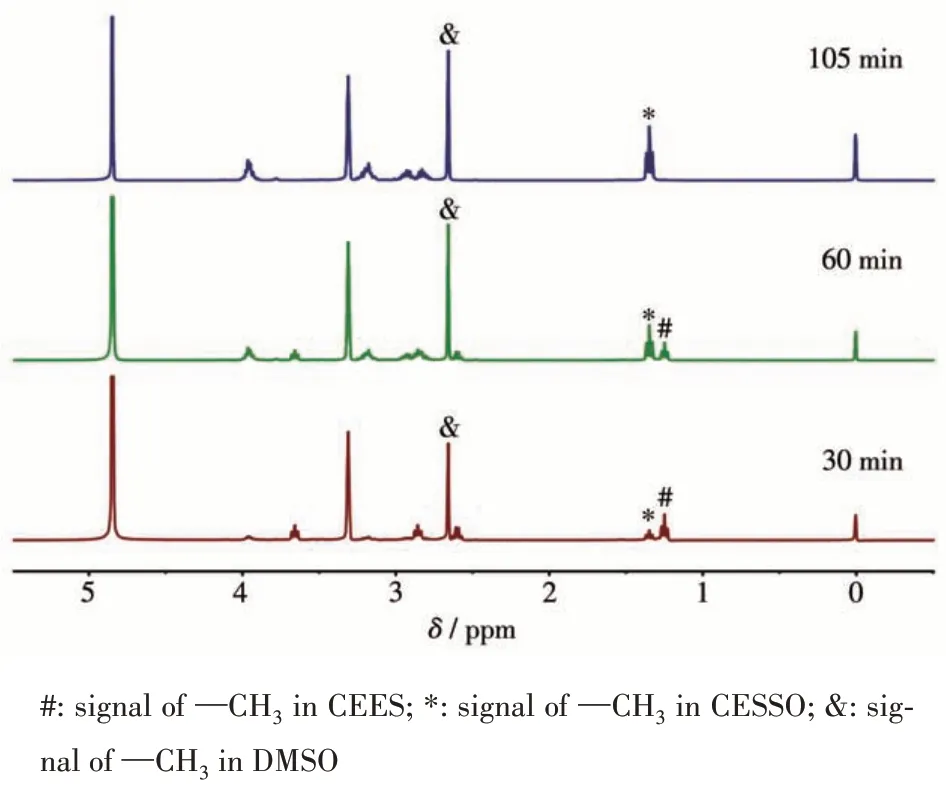
Fig.6 Time-dependent 1H NMR spectra of CEES conversion for COF-367 NSs
3 Conclusions
In summary, we investigate the potential application of ultrathin COF-367 NSs as a hetergeneous photosensitizer for selective photocatalytic oxidation of sulfides to sulfoxides. The ultrathin structure of COF-367 NSs results in good dispersibility in various organic solvent. This, in combination with the high efficiency in light harvesting of the Por units, endows the good performance of COF-367 NSs towards1O2generation upon visible-light irradiation.In particular,it is further demonstrated COF-367 NSs could be an excellent heterogeneous catalyst in photocatalytic oxidation of sulfides to sulfoxides with high yield and selectivity as well as good recycling stability. This work might provide a new thinking in the design and synthesis towards effective catalytic materials for heterogeneous photooxidation reaction and even detoxification of chemical warfare agents.
Acknowledgements:We thank the financial support from Beijing Natural Science Foundation (Grant No.2194091), Natural Science Foundation of China (Grants No. 21631003,21871024), and Fundamental Research Funds for the Central Universities(Grant No.FRF-BD-19-016A).

