侧脑室注射α-突触核蛋白对黑质和纹状体单胺氧化酶B表达的影响
宓晓晴 谢俊霞 宋宁
[摘要] 目的 探讨侧脑室注射α-突触核蛋白(α-syn)对小鼠黑质和纹状体区单胺氧化酶B(MAO-B)蛋白表达以及运动功能的影响。
方法 将8周龄雄性C57BL/6小鼠随机分为生理盐水组以及0.04、0.20和2.00 ng的α-syn组,给予侧脑室连续注射生理盐水或α-syn共7 d,采用转棒实验检测小鼠运动能力,采用免疫印迹法检测小鼠黑质和纹状体区酪氨酸羟化酶(TH)和MAO-B蛋白表达。
结果 与生理盐水组相比,0.20和2.00 ng的α-syn组小鼠在转棒上运动时间明显缩短(F=4.877,P<0.05),且纹状体区MAO-B蛋白表达水平显著升高(F=9.662,P<0.05),但0.04 ng的α-syn组小鼠运动水平和纹状体区MAO-B蛋白表达水平均没有明显变化(P>0.05)。各组小鼠黑质区TH和MAO-B蛋白表达水平比较差异均无显著性(P>0.05)。
结论 侧脑室注射α-syn可引起小鼠纹状体区MAO-B蛋白表达增加和运动功能障碍。
[关键词] α突触核蛋白;输注,脑室内;纹状体;黑质;单胺氧化酶;小鼠
[中图分类号] R338.2
[文獻标志码] A
[文章编号] 2096-5532(2021)05-0642-04
doi:10.11712/jms.2096-5532.2021.57.134
[开放科学(资源服务)标识码(OSID)]
[网络出版] https://kns.cnki.net/kcms/detail/37.1517.R.20210628.1725.016.html;2021-06-29 10:22:32
EFFECT OF INTRACEREBROVENTRICULAR INJECTION OF Α-SYNUCLEIN ON THE EXPRESSION OF MONOAMINE OXIDASE B IN THE SUBSTANTIA NIGRA AND THE STRIATUM
MI Xiaoqing, XIE Junxia, SONG Ning
(Department of Physiology and Pathophysiology, School of Basic Medicine, Qingdao University Medical College, Qingdao 266071, China)
[ABSTRACT] Objective To investigate the effect of intracerebroventricular injection of α-synuclein (α-syn) on the expression of monoamine oxidase B (MAO-B) in the substantia nigra and the striatum of mice.
Methods Male C57BL/6 mice, aged 8 weeks, were randomly divided into normal saline group and 0.04, 0.20, and 2.00 ng α-syn groups. After intracerebroventricular injection of normal saline or α-syn was given for 7 consecutive days, the rotarod test was used to evaluate motor ability, and Wes-
tern blotting was used to measure the protein expression levels of tyrosine hydroxylase (TH) and MAO-B in the substantia nigra and the striatum of mice.
Results Compared with the normal saline group, the 0.20 and 2.00 ng α-syn groups had a significant reduction in the time spent on the rotating rod (F=4.877,P<0.05) and a significant increase in the protein expression level of MAO-B in the striatum (F=9.662,P<0.05), while the 0.04 ng α-syn group had no significant changes in motor ability and the protein expression level of MAO-B in the striatum (P>0.05). There were no significant differences in the protein expression levels of TH and MAO-B in the substantia nigra between these groups (P>0.05).
Conclusion Intracerebroventricular injection of α-syn can increase the protein expression of MAO-B in the striatum of mice and induce impairment of motor ability.
[KEY WORDS] alpha-synuclein; infusions, intraventricular; corpus striatum; substantia nigra; monoamine oxidase; mice
帕金森病(PD)是第二大常見的神经退行性疾病,其确切病因至今尚未完全明了[1-3],其主要病理特征为黑质致密部多巴胺(DA)能神经元缺失和路易小体(LB)形成,临床主要表现有肌强直、静止性震颤、运动迟缓以及姿势不稳等[4-7]。α-突触核蛋白(α-syn)是SNCA编码的由140个氨基酸组成的小分子蛋白质,是LB的主要成分[8-10]。大量研究结果表明,PD等突触核蛋白病病人脑脊液中的α-syn水平较正常人显著降低[11-12]。然而,长期追踪调查发现,PD病人脑脊液中的α-syn水平会随着疾病进展逐渐回升,而且α-syn水平的升高与PD病人后期运动功能的下降存在紧密联系[13-14]。单胺氧化酶B(MAO-B)是一种线粒体膜蛋白,是单胺类神经递质的主要氧化脱氨酶,可分解DA产生3,4-二羟基苯基乙醛(DOPAL)等代谢产物[15-16]。有文献报道,α-syn以及α-syn 1~103片段均可直接结合MAO-B而增强其酶活性,最终导致DA能神经元变性[17]。
本文通过给予小鼠侧脑室注射不同浓度α-syn,以评价其运动功能以及纹状体和黑质区MAO-B蛋白表达的变化。现将结果报告如下。
1 材料和方法
1.1 实验动物及主要试剂
实验动物:SPF级雄性C57BL/6小鼠,8周龄,体质量(20±2)g,购自北京维通利华实验动物技术有限公司,饲养于可自由饮水取食、室温(19±2)℃、湿度(50±5)%、昼夜循环光照(12 h/12 h)的清洁环境中。主要试剂:α-syn购自美国rPeptide公司;酪氨酸羟化酶(TH)一抗购自德国Sigma公司;MAO-B一抗购自美国GeneTex公司;Rabbit Anti-β-actin购自中国博奥森公司;Goat Anti-Rabbit IgG二抗购自中国爱必信公司。
1.2 动物分组与处理
将40只实验小鼠随机分为生理盐水组(A组)以及0.04、0.20和2.00 ng α-syn组(B、C、D组),每组10只。小鼠用异戊烷麻醉后固定在脑立体定位仪上。剪开小鼠颅脑背侧皮肤,用体积分数0.03的过氧化氢溶液擦拭颅骨表面至颅缝和前后囟清晰可见。确定坐标(右侧侧脑室立体定位坐标为前囟后0.3 mm、右旁开1.0 mm、深度2.2 mm)后,将长5.2 mm的套管垂直埋入侧脑室2.2 mm。以1 μL/min的流量注射生理盐水或α-syn 2 μL,每天1次,连续7 d。
1.3 转棒实验
小鼠在旋转棒上适应2 min后,将旋转棒转速设置为4~40 r/min,使小鼠随旋转棒自主运动,记录小鼠在旋转棒上运动的时间。测量2次(间隔30 min)取平均值。
1.4 免疫印迹法检测TH和MAO-B蛋白表达
小鼠断头,根据小鼠大脑图谱取纹状体和黑质样本并称质量。按照25 μL/mg的比例向样本中加入蛋白裂解液,充分研磨后以12 000 r/min离心20 min,取上清,用BCA试剂盒检测蛋白浓度,加入1/4体积的Loading Buffer后95 ℃金属浴5 min。处理好的蛋白样本进行聚丙烯酰胺凝胶电泳后转膜(0.45 μm 的PVDF膜)。以50 g/L的脱脂奶粉室温封闭2 h后加入一抗TH(1∶3 000)、MAO-B(1∶1 000)、β-actin(1∶10 000)4 ℃孵育过夜,次日用山羊抗兔二抗(1∶10 000)室温孵育1 h,ECL方法显影。用Image J软件分析条带灰度值,TH和MAO-B蛋白表达水平以目的蛋白与内参β-actin条带灰度值的比值来表示。
1.5 统计学处理
应用Prism 5软件进行统计学分析。计量资料以±s形式表示,采用单因素方差分析(one-way ANOVA检验)进行多组均数的比较,继以Tukey方法进行两两均数间的比较。P<0.05表示差异有统计学意义。
2 结 果
2.1 侧脑室注射不同浓度α-syn对小鼠行为学的影响
生理盐水组以及0.04、0.20和2.00 ng α-syn组小鼠在旋转棒上运动的时间分别为(221.867±38.855)、(213.905±63.634)、(145.556±54.844)和(138.111±40.932)s,4组比较差异有统计学意义(F=4.877,P<0.05)。两两比较结果显示,与生理盐水组相比较,0.04 ng α-syn组小鼠运动时间无明显变化(P>0.05),而0.20和2.00 ng α-syn组小鼠的运动时间明显缩短,差异具有统计学意义(q=3.907、4.288,P<0.05)。
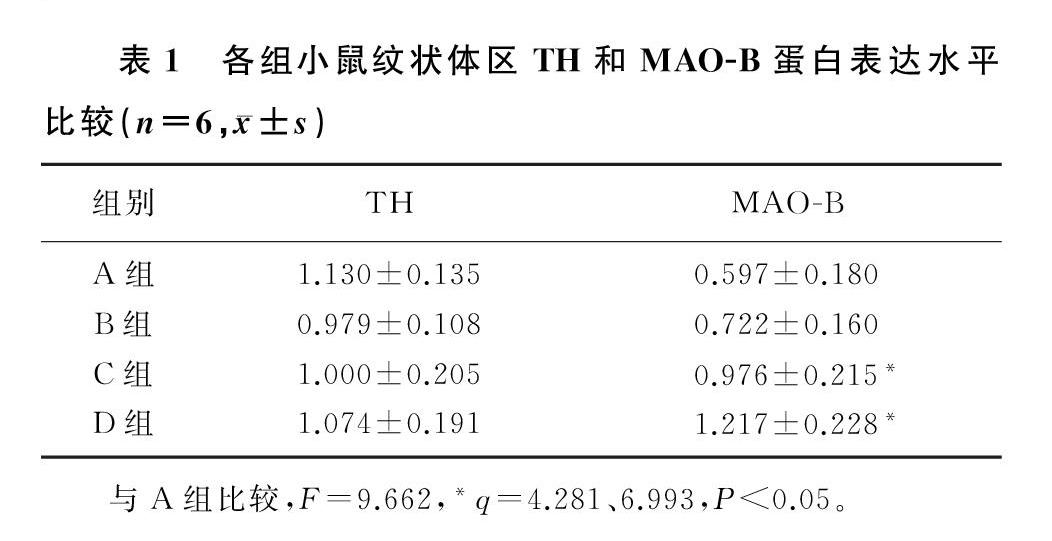
2.2 侧脑室注射不同浓度α-syn对纹状体区TH和MAO-B蛋白表达的影响
本研究4组小鼠纹状体区TH蛋白表达比较差异均无统计学意义(P>0.05)。4组小鼠纹状体区MAO-B蛋白表达比较差异有统计学意义(F=9.662,P<0.05)。两两比较结果显示,与生理盐水组相比较,0.04 ng α-syn组小鼠纹状体区MAO-B蛋白表达水平没有明显的变化(P>0.05),0.20和2.00 ng的α-syn组小鼠纹状体区MAO-B蛋白表达水平显著升高(q=4.281、6.993,P<0.05)。见表1。
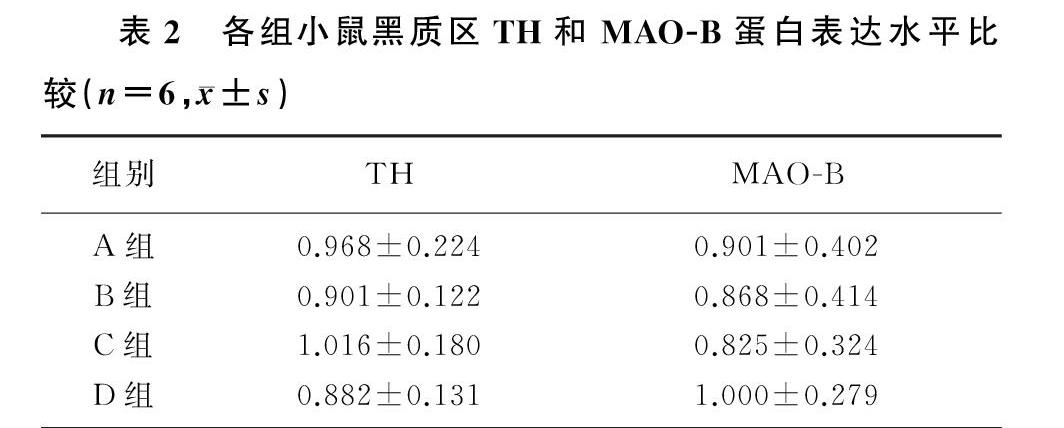
2.3 侧脑室注射不同浓度α-syn对黑质区TH和MAO-B蛋白表达的影响
本研究4组小鼠黑质区TH和MAO-B蛋白表达比较差异均无统计学意义(P>0.05)。两两比较结果显示,与生理盐水组相比,各浓度α-syn组小鼠黑质区TH和MAO-B蛋白表达水平均没有明显改变(P>0.05)。见表2。
3 讨 论
编码α-syn的SNCA是首個被人们发现的与PD相关的常染色体显性遗传基因[18]。无论是在遗传性PD还是在散发性PD中,α-syn均可以在DA能神经元中积聚形成LB[19]。生理状态下的α-syn与A组比较,F=9.662,*q=4.281、6.993,P<0.05。
通常被认为是舒展的可溶性结构,但当其浓度升高时α-syn易聚合折叠形成寡聚体,对神经元膜有毒性作用,可改变膜渗透性,导致钙大量内流,引起膜除极;也可引起细胞氧化损伤,导致细胞死亡[20]。细胞内的α-syn还可以被释放到细胞外。人的脑脊液和血浆中存在一定浓度的α-syn[21]。大量研究发现,PD病人脑脊液中的α-syn水平降低[11-12],但随着疾病进展,α-syn水平回升且与病人的认知、运动功能紧密相关[13-14]。
单胺氧化酶是在中枢神经系统和外周神经系统中催化DA、5-羟色胺和去甲肾上腺素等单胺类神经递质的主要氧化脱氨酶。MAO-B通过分解DA产生DOPAL并产生活性氧(ROS)物质和内源性神经毒素[16]。年龄相关的MAO-B表达增加与自由基损伤和ROS增加存在密切关联,可导致神经元线粒体功能降低,导致黑质致密部神经元活力降低以至神经变性[22]。MAO-B抑制剂常被作为有效的PD治疗药物[23-24]。本研究结果表明,侧脑室注射α-syn 7 d可引起C57BL/6小鼠纹状体区MAO-B蛋白表达升高和运动功能障碍,但黑质区MAO-B蛋白表达不变。MAO-B主要位于星形胶质细胞,在星形胶质细胞激活时MAO-B蛋白表达明显上调,因此MAO-B可能作为星形胶质细胞激活的生化标记物[25]。α-syn可以通过Toll样受体4(TLR4)激活星形胶质细胞[26]。因此我们推测,纹状体MAO-B表达升高可能与侧脑室注射的α-syn到达纹状体后激活星形胶质细胞有关,这尚需在后续实验中进一步证实。此外,MAO-B是DA酶解途径之一,当MAO-B表达增加时,一方面MAO-B可促进DA
分解;另一方面,DA分解产生的DOPAL还可以激活δ分泌酶,在N103位置裂解α-syn。α-syn以及α-syn 1~103片段均可直接结合MAO-B而增强其酶活性[17,27]。我们推测,MAO-B表达和活性的增加,可能在未造成DA能神经元损伤之前(本实验观察到黑质和纹状体区TH表达均不变)显著促进了纹状体区DA的分解,造成了小鼠运动功能障碍。与其他脑区相比较,黑质中星形胶质细胞分布相对较少[28],这可能是侧脑室注射α-syn未引起黑质区MAO-B蛋白表达变化的原因。
综上所述,侧脑室注射α-syn可引起C57BL/6小鼠纹状体区MAO-B蛋白表达增加和运动功能障碍。本文研究结果为进一步探讨脑脊液中α-syn的变化影响PD疾病进程提供了新的实验思路和理论依据。
[参考文献]
[1]SPILLANTINI M G, SCHMIDT M L, LEE VIRGINIAM Y, et al. Alpha-synuclein in Lewy bodies[J]. Nature, 1997,388(6645):839-840.
[2]BI M X, DU X X, JIAO Q, et al. Expanding the role of proteasome homeostasis in Parkinsons disease: beyond protein breakdown[J]. Cell Death & Disease, 2021,12(2):154.
[3]BI M X, JIAO Q, DU X X, et al. Glut9-mediated urate uptake is responsible for its protective effects on dopaminergic neurons in Parkinsons disease models[J]. Frontiers in Molecular Neuroscience, 2018,11:21.
[4]STEFANOVIC A N D, STCKL M T, CLAESSENS M M A E, et al. α-Synuclein oligomers distinctively permeabilize complex model membranes[J]. The FEBS Journal, 2014,281(12):2838-2850.
[5]VEYS L, VANDENABEELE M, ORTUO-LIZARN I, et al. Retinal α-synuclein deposits in Parkinsons disease patients and animal models[J]. Acta Neuropathologica, 2019,137(3):379-395.
[6]BUCCIANTINI M, GIANNONI E, CHITI F, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases[J]. Nature, 2002,416(6880):507-511.
[7]YAN M H, WANG X L, ZHU X W. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease[J]. Free Radical Biology and Medicine, 2013,62:90-101.
[8]LASHUEL H A, OVERK C R, OUESLATI A, et al. The many faces of α-synuclein: from structure and toxicity to the-rapeutic target[J]. Nature Reviews Neuroscience, 2013,14(1):38-48.
[9]FELINSKI E A, QUINN P G. The coactivator dTAF(Ⅱ)110/hTAF(Ⅱ)135 is sufficient to recruit a polymerase com- plex and activate basal transcription mediated by CREB[J].
Proceedings of the National Academy of Sciences of the United States of America, 2001,98(23):13078-13083.
[10]WEN A Y, SAKAMOTO K M, MILLER L S. The role of the transcription factor CREB in immune function[J]. Journal of Immunology (Baltimore, Md:1950), 2010,185(11):6413-6419.
[11]HALL S, HRFELT A, CONSTANTINESCU R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders[J]. Archives of Neurology, 2012,69(11):1445-1452.
[12]MOLLENHAUER B, LOCASCIO J J, SCHULZ-SCHAEFFER W, et al. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with Parkinsonism: a cohort study[J]. The Lancet Neurology, 2011,10(3):230-240.
[13]HALL S, SUROVA Y, HRFELT A, et al. Longitudinal measurements of cerebrospinal fluid biomarkers in Parkinsons disease[J]. Movement Disorders: Official Journal of the Movement Disorder Society, 2016,31(6):898-905.
[14]MAJBOUR N K, VAIKATH N N, EUSEBI P, et al. Longitudinal changes in CSF alpha-synuclein species reflect Parkinsons disease progression[J]. Movement Disorders: Official Journal of the Movement Disorder Society, 2016,31(10):1535-1542.
[15]MASATO A, PLOTEGHER N, BOASSA D, et al. Impaired dopamine metabolism in Parkinsons disease pathogenesis[J]. Molecular Neurodegeneration, 2019,14(1):35.
[16]MEISER J, WEINDL D, HILLER K. Complexity of dopamine metabolism[J]. Cell Communication and Signaling: CCS, 2013,11(1):34.
[17]KANG S S, AHN E H, ZHANG Z T, et al. A-synuclein stimulation of monoamine oxidase-B and legumain protease mediates the pathology of Parkinsons disease[J]. The EMBO Journal, 2018,37(12):e98878.
[18]DENG H, WANG P, JANKOVIC J. The genetics of Parkinson disease[J]. Ageing Research Reviews, 2018,42:72-85.
[19]RECASENS A, DEHAY B, BOV J, et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein patho-
logy and neurodegeneration in mice and monkeys[J]. Annals of Neurology, 2014,75(3):351-362.
[20]DANZER K M, KREBS S K, WOLFF M, et al. Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology[J]. Journal of Neurochemistry, 2009,111(1):192-203.
[21]EL-AGNAF O M, SALEM S A, PALEOLOGOU K E, et al. Alpha-synuclein implicated in Parkinsons disease is present in extracellular biological fluids, including human plasma[J]. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 2003,17(13):1945-1947.
[22]KOPPULA S, KUMAR H, KIM I S, et al. Reactive oxygen species and inhibitors of inflammatory enzymes, NADPH oxidase, and iNOS in experimental models of Parkinsons disease[J]. Mediators of Inflammation, 2012, 2012:823902.
[23]NAOI M, MARUYAMA W, SHAMOTO-NAGAI M. Rasagiline and selegiline modulate mitochondrial homeostasis, intervene apoptosis system and mitigate α-synuclein cytotoxicity in disease-modifying therapy for Parkinsons disease[J]. Journal of Neural Transmission (Vienna, Austria:1996), 2020,127(2):131-147.
[24]YOUDIM M B H. Monoamine oxidase inhibitors, and iron chelators in depressive illness and neurodegenerative diseases[J]. Journal of Neural Transmission (Vienna, Austria:1996), 2018,125(11):1719-1733.
[25]TONG J C, RATHITHARAN G, MEYER J H, et al. Brain monoamine oxidase B and A in human Parkinsonian dopamine deficiency disorders[J]. Brain: a Journal of Neurology, 2017,140(9):2460-2474.
[26]SORRENTINO Z A, GIASSON B I, CHAKRABARTY P. α-Synuclein and astrocytes: tracing the pathways from homeostasis to neurodegeneration in Lewy body disease[J]. Acta Neuropathologica, 2019,138(1):1-21.
[27]WU Z R, XIA Y Y, WANG Z H, et al. C/EBPβ/δ-secretase signaling mediates Parkinsons disease pathogenesis via regulating transcription and proteolytic cleavage of α-synuclein and MAOB[J]. Molecular Psychiatry, 2021,26(2):568-585.
[28]SONG N, WANG J, JIANG H, et al. Astroglial and microg-lial contributions to iron metabolism disturbance in Parkinsons disease[J]. Biochimica et Biophysica Acta Molecular Basis of Disease, 2018,1864(3):967-973.
(本文編辑 马伟平)

