First-principles study of electronic structure and magnetic properties of Sr3Fe2O5 oxide*
Mavlanjan Rahman(买吾兰江热合曼) and Jiuyang He(何久洋)
School of Physics and Electronic Engineering,Xinjiang Normal University,Urumqi 830054,China
Keywords: Sr3Fe2O5,first-principles calculation,electronic structure,magnetism
1. Introduction
In recent years, people have used some unconventional methods such as high-pressure or low-temperature topology technology to prepare some novel oxides with special structures and properties.[1-4]A typical example is Dion-Jacobson type layered perovskite oxide, such as RbLaNb2O7.[5-7]In addition, theoretically predicted oxides with a square layered crystal structure have also been synthesized, such as CaCuO2[8,9]SrCuO2,[10]BaCuO2[11]LaNiO2,[12]and SrFeO2.[13]A more special one is the new oxide SrFeO2with infinite layered crystal structure prepared by Tsujimoto’s team under low temperature (~400 K) conditions using perovskite SrFeO3and reducing substance CaH2. The infinite layered structure is composed of two-dimensional FeO2layers with corner-sharing FeO4squares separated by Sr2+ions, and this quasi-two-dimensional structure has the same crystal structure with the high-temperature superconducting matrix SrCuO2.[14-16]SrFeO2shows G-type antiferromagnetic (AFM) order. Under high external pressure, it exhibits a spin transition from the high-spin stateS= 2 to the intermediate-spin stateS=1, almost simultaneously accompanied by an AFM-FM transition and a insulator-metal transition.[13]Subsequently,Liis Seinberg and Mar´ıa Retuertoet al.studied the crystal structure and magnetism of the compound SrFe1−xMxO2(M= Co, Mn) by synchrotron xray diffraction, neutron diffraction, and Mossbaur spectrometer. SrFe1−xMxO2(M= Co, Mn) can maintain the same layered crystal structure as SrFeO2when Fe site doping is less than 30%.[17,18]Fabio Denies Romeroet al. synthesized the compound SrFe0.5Ru0.5O2[19]which is isostructural with SrFeO2,and they found that the layered structure can be kept unchanged just for compounds with 1:1 mixture of iron and ruthenium on the B-cation site.
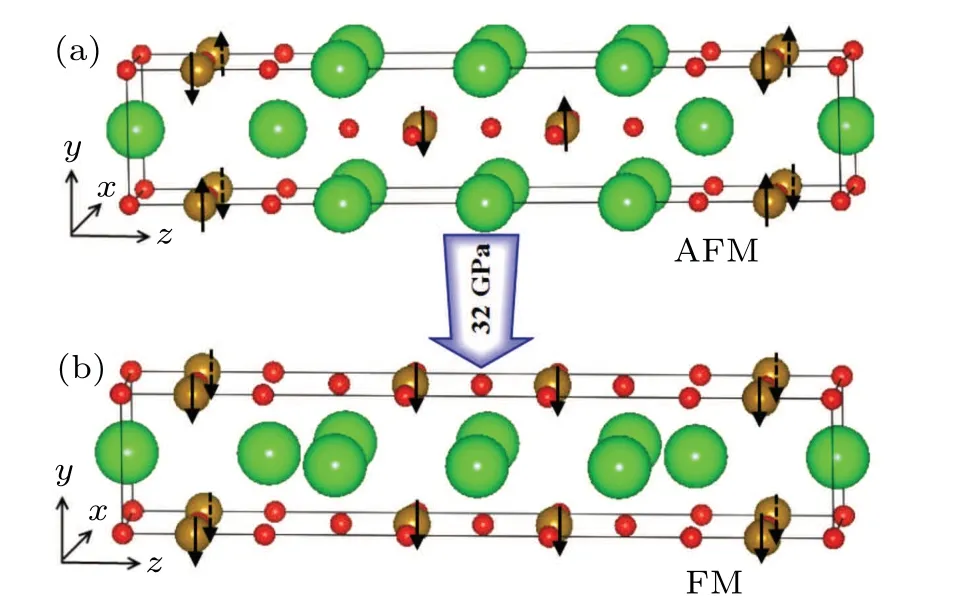
Fig.1. (a) The structure of Sr3Fe2O5 under ambient pressure, and(b)the structure of Sr3Fe2O5 under high pressure,the blue balls represent Sr atoms,the red balls represent O atoms,and the brown balls represent Fe atoms. Arrows indicate the spin orientations of Fe ions.
Recently, Yamamotoet al. studied the special layered compound Sr3Fe2O5,[20,21]which has a ladder-type layered structure under ambient pressure. As shown in Fig. 1(a), it hasImmmspace group symmetry (No. 71) and the lattice constants area=3.51685(5) ˚A,b=3.95405(7) ˚A, andc=20.91727(36) ˚A. Sr3Fe2O5has an anti-ferromagnetic (AFM)structure and the Fe ion has a high spin stateS= 2 under the ambient pressure. The ladder-type layered structure transforms to an infinite layered structure under high pressure, as shown in Fig.1(b), and its symmetry group becomesAmmm.Further increasing pressure also induces a transition from antiferromagnetic (AFM) structure to ferromagnetic (FM) structure,accompanied by a spin transition from the high-spin stateS=2 to the intermediate-spin state ofS=1.[21,22]In order to better understand this compound, in this paper, we use firstprinciples calculations to study the electromagnetic structure and magnetism of Sr3Fe2O5under the ambient pressure and high pressure.
2. Calculation
We used the PWSCF program in the QUANTUM ESPRESSO calculation package to perform first-principles calculations.[23]Generalized gradient approximation plus Coulomb repulsive energy (GGA+U) method (super soft pseudo potential is used in the calculation)[24,25]is used in our calculation. When calculating the total energy and the density of state,we used a unit cell of 20 atoms. In the calculation,the G-type AFM(the nearest neighbor iron ions have the opposite spin direction)and the FM(the nearest neighbor iron ions have the same spin direction)structures are considered. The plane wave cut-off energy was set to be 400 eV,and a grid of 8 8 2 was taken for the Brillouin zone.These calculation parameters ensured that our calculation error is controlled within 1 meV for each atom. TheUwas set to be 4eV under the ambient pressure and 0 under high pressure.
3. Results and discussion
Firstly, we calculated the electronic structure and magnetic properties of Sr3Fe2O5under the ambient pressure. The results show that the ground state of the compound is a Gtype AFM structure with a magnetic moment of 3.6µBfor Fe ion,which is consistent with the experimental result.[20,21]For GGA+Ucalculations,we found that the compound remained the G-type AFM state whenUvaried from 2 eV to 5 eV under the ambient pressure and for matching the experimental spin moment we chose theU=4 eV.Figure 2 shows the total density of states for Sr3Fe2O5, the density of states of d orbitals and sp orbitals for Fe, and the density of states of sp orbitals for O.It can be seen that the compound is an insulator under the ambient pressure.The iron 3d orbital has a larger exchange splitting,which is larger than the crystal field splitting,therefore the Fe ion has a high spin stateS=2,which is the same as SrFeO2.The different lowest energy orbitals dx2−y2and dz2for ladder-type layered compound Sr3Fe2O5and infinite layered compound SrFeO2are due to different choice ofzaxis.The electronic structure of Sr3Fe2O5can also be qualitatively explained by the ionic model and the hybridization effects between the 3d orbital of Fe ion and the 2p orbital of O. The two layers in Sr3Fe2O5alternate in thezdirection, similar to a ladder-type structure. According to crystal field theory, the degeneracy of 3d orbitals is lifted due to the ligands. The orbital splitting diagram for square-planar coordination can be derived from the octahedral diagram.[26]The electronic difference between ladder-type layered compound Sr3Fe2O5and the infinite layered compound SrFeO2that the lowest energy orbital in the infinite layered structure is dz2,and the lowest energy orbital in the ladder-type layered structure is dx2−y2. This is because the six-coordinated Fe ion of the corresponding perovskite SrFeO3loses two oxygen atoms in thezdirection to form SrFeO2, which reduces the energy of the dz2orbital,while the Fe ion in Sr3Fe2O5has two fewer oxygen atoms in theydirection,resulting in reduction of dx2−y2orbital energy.It can be seen from Fig. 2(c) that there is a strong coupling between the 4s orbital and the dx2−y2orbital of Fe ion,which further reduces the energy of the dx2−y2orbital. In the laddertype structure,the order of increasing energy of 3d orbitals for Fe ion is dz2< dxz< dxy< dyz< dx2−y2. According to the molecular orbital theory,the bonding state has the property of a ligand,and its energy ordering is different or completely opposite to that of the crystal field.The anti-bonding state has the properties of transition metal ions and the ordering properties of the crystal field energy levels. The density of states(Fig.2)are obviously consistent with the results predicted by the crystal field theory and molecular orbital theory. We find that the anti-bonding state orbital appears in the range of−4 eV to 0 eV and the energy of the dx2−y2orbital is the lowest. The electronic structure is (dx2−y2)1(dyz)1(dxy)1(dxz)1(dz2)1for the up-spin electron, and (dx2−y2)1(dyz)0(dxy)0(dxz)0(dz2)0for the down-spin electron. It can be seen that the down-spin electrons on the d6orbital of the Fe ion occupy the dx2−y2orbital instead of occupying the dz2orbital like as SrFeO2.Therefore,the total electronic structure of the 3d orbital of Fe ion in Sr3Fe2O5is(dx2−y2)2(dyz)1(dxz)1(dxy)1(dz2)1.
The G-type AFM state of Sr3Fe2O5under ambient pressure comes from AFM exchange interactions. According to the Fe 3d electronic configuration(dx2−y2)2(dyz)1(dxy)1(dxz)1(dz2)1, we see that the exchange interaction is due to the dxy, dxz, dyz, dz2orbitals of two adjacent Fe ions, while the full occupied dx2−y2orbitals do not form the exchange interaction. The unpaired electrons on the two dxyorbitals along thexdirection form anti-ferromagnetic coupling through the Pπbond, and the unpaired electrons on the two adjacent dxz, dyz, dz2orbitals along thezdirection form anti-ferromagnetic coupling with the weakerπbond.If the unpaired electrons on adjacent orbitals form an antiferromagnetic coupling,the electrons can be delocalized over theM-O-M(orM-M) unit, thus lowering the kinetic energy. On the contrary, if a ferromagnetic coupling is formed,this delocalization is forbidden by Pauli exclusion principles,therefore,the energy is more costed. In the same way,the exchange coupling of dyzand dxyorbitals along theydirection is also an anti-ferromagnetic. Therefore, the system forms a G-type anti-ferromagnetic structure, which is consistent with the G-K principle.[27]

Fig.2. (a)Total density of states(DOS),(b)density of states of d orbital for Fe2+,(c)density of states of sp orbital for Fe2+,and(d)density of states of sp orbital for O2−under ambient pressure.

Fig. 3. (a) Total density of states (DOS) and (b) density of states of d orbital for Fe2+ under high pressure.
Experimental results show that the ladder-type layered structure of Sr3Fe2O5becomes an infinite layered structure under the external pressure around 32 GPa. A step block of the compound changes from the position (0.5 0.5 0.5) to the position (0 0.5 0.5), but still maintains the four-coordinated layered structure of Fe-O,and the corresponding space group changes fromImmmtoAmmm,[22]as shown in Fig.1. We calculated the electronic structure and magnetic structure of the infinite layered compound Sr3Fe2O5,and the results show that the ground state of the compound is a FM state,the magnetic moment of Fe ions is 2.2µB,and the density of states(Fig.3)show that the compound is a conductor. These are consistent with the experimental results.[20,21]
It can be seen from Fig.3(b)that the electronic structure(dx2−y2)2(dyz)1(dxy)1(dxz)1(dz2)1under ambient pressure no longer exists.Under high pressure,the electronic structure can be approximately written as (dx2−y2)2(dyzdxz)3(dxy)1(dz2)0(ignoring the fractional part). According to the electron occupation,the intralayer exchange effect between adjacent unoccupied dz2orbitals disappears. The interaction between adjacent dxyorbitals still maintains the original anti-ferromagnetic coupling along thexandydirections. However, under high pressure,the interactions between adjacent dxzdyzorbitals are FM in thex,y,andzdirections. Along thexandydirections,the AFM interaction of the dxyorbital competes with the FM interaction of the dxzand dyzorbitals,and the FM interaction is exceeded.Therefore,the compound exhibits a FM structure.
4. Conclusions
We performed first-principles calculations based on the generalized gradient approximation of the density functional theory plus Coulomb repulsive energyU(DFT+U),and studied the magnetic and electronic structure of the ladder-type layered compound Sr3Fe2O5under ambient pressure and high pressure. The electronic structure of the 3d orbital for Fe ion under ambient pressure is:(dx2−y2)2(dyz)1(dxz)1(dxy)1(dz2)1. The ladder-type layered structure changes to an infinite layered structure when pressurized, that is, one of the stepped blocks changes from (0.5 0.5 0.5) position to (0 0.5 0.5) position, but still maintains the Fe-O four-coordinated layered structure. Further pressure changes the G-type AFM state to the FM state. We explained the magnetic state according to the G-K rule. We found the spin transition from theS=2 toS=1 under high pressure.These results well accounted for the experimental finding.
- Chinese Physics B的其它文章
- Numerical investigation on threading dislocation bending with InAs/GaAs quantum dots*
- Connes distance of 2D harmonic oscillators in quantum phase space*
- Effect of external electric field on the terahertz transmission characteristics of electrolyte solutions*
- Classical-field description of Bose-Einstein condensation of parallel light in a nonlinear optical cavity*
- Dense coding capacity in correlated noisy channels with weak measurement*
- Probability density and oscillating period of magnetopolaron in parabolic quantum dot in the presence of Rashba effect and temperature*

