Standardization and Quality control parameters of Kayam Churna
Prajwal Gupta,Ashwini Kendle,Diksha Bhagat,Mahera Khan,Prabuddha Wakode,Shruti Shah,Sonali Patil*
1 Department of Bioanalytical Sciences,B.K.Birla College (Autonomous),Kalyan,Maharashtra,India.
Abstract Kayam Churna is proprietary ayurvedic formulation which is blend of seven ingredients which are Senna leaves (Cassia angustifolia),Black Salt,Mulethi(Glycyrrhiza glabra),Nishoth (Operculina turpethum),Ajwain(Trachyspermum ammi),Haritaki (Terminalia chebula) and Svarjikshara(Sodium bicarbonate) with Senna as a major ingredient.Standardization and stability studies play a vital role for each herbal formulation,so the research was carried out to prepare the formulation in the laboratory and to standardize the Churna,by means of its physicochemical properties,phytochemical analysis and microbiology study.The organoleptic and powdered characteristics were studied and compared with monographs.Various physicochemical analysis was carried out like bulk density,tapped density,percent compressibility,loss on drying,ash value and heavy metal determination.Phytochemical analysis was carried out to find the presence of tannins,alkaloids,glycosides,flavonoids and steroids.Microbiological analysis was evaluated using the Total Viable Count method.HPTLC Chromatographic technique was used for fingerprinting.Microscopic analysis of Formulation showed the presence of Vessels,Fibre,Calcium oxalate crystals,etc.Tannins,Alkaloids,flavonoids were found to be present in the formulation.Microbial count and all physicochemical parameters were found to be within the limits as per the WHO guidelines.Further studies can be carried out for finding out its efficacy and potency.
Keywords Herbal formulation;Standardization;Evaluation;Quality control tests;Fingerprinting
Background
In India,traditional medicine is science of Ayurveda,Siddha,Unani,also Yoga and Naturopathy,to treat for various conditions.Pharmacological studies and clinical trials lack somewhere in this healthcare system.So,Standardization of any formulation including herbal formulation becomes the essential factor to assess its purity,safety,stability and efficacy based on their concentration of active ingredients and formulation and dosage forms [1].The subject of herbal drug standardization is massively wide and deep.There is so much to know and so much seemingly contradictory theories on the subject of herbal medicines and its relationship with human physiology and mental function.For the purpose of research work on standardization of herbal formulations,a profound knowledge of the important herbs found in India and widely used in Ayurvedic formulation is of utmost importance.
In Ayurveda,Churna is fine powder of one ingredient or mixture of one or more ingredients in specific quantity,which is further dried,sieved,checked,mixed in proper way and stored in closed container.Kayam Churnais a well-known proprietary ayurvedic formulation that has been widely used for treating constipation,several gastrointestinal anomalies and other related disorders for decades (The Ayurvedic Pharmacopoeia of India).Owing to the strong laxative and digestive qualities of this herbal product,consuming it in prescribed quantities does really help in improving digestion and provide relief from constipation problems [2].Fabricated in accordance with the principles of Churna,Kayam Churnais a balanced composite of seven incredible ayurvedic ingredients which areCassia angustifolia,Black Salt,Operculina turpethum,Trachyspermum ammi,Terminalia chebula,Sodium BicarbonateandGlycyrrhiza glabra,withCassiaangustifolia(Senna) as a major ingredient each of which works in conjunction to treat a wide array of gastrointestinal disorders like constipation,gastritis,diarrhea,heartburn,peptic ulcer and gastro esophageal reflux disease,as shown in Figure 1 and Table 1.

Table 1 Composition of Kayam Churna

Figure 1 Ingredients of Kayam Churna (Left to right: Cassia angustifolia,Black salt,Operculina turpethum,Trachyspermum ammi,Terminalia chebula,Sodium Bicarbonateand Glycerrhiza glabra)
This paper deals with the study of standardization and to check quality control parameters ofKayam Churnaas herbal formulation.The monographs of ingredients are described below [3].
Cassia Angustifolia [4]
Chemical constituentsSenna leaf also contains other anthraquinone glycosides in small amounts.They are sennosides C and D,rhein,kaempferol,aloeemodin and isorhamnetin.It also contains phytosterol,mucilage,resin,myricyl alcohol,salicylic acid,chrysophanic acid and calcium oxalate.
Microscopic CharacteristicsTransverse section of leaflet through midrib shows an isobilateral structure,epidermal cells,straight walled containing mucilage,both surface bear scattered,unicellular hair,prismatic crystals of calcium oxalate present on large veins and clusters of calcium oxalate crystal distributed throughout the palisade and spongy tissues,mid rib biconvex,stomata,crystal sheath of parenchymatous cell containing prismatic crystal of calcium oxalate.
UsesSenna is used on a short-term basis to treat constipation.It also is used to empty the bowels before surgery and certain medical procedures.It increases activity of the intestines to cause a bowel movement.
Operculina Turpethum [5]
Chemical constituentsTurpeth contains 9%-13% resin,which is a mixture of α and β-turpethin,glycosides,and turpethin,besides coumarin,scopoletin,and sugars.
Microscopic CharacteristicsMature root shows thin cork,consisting of 3-5 rows of brown cells,thin-walled cells;some of the cortical cells become thick walled appearing as isolated,cellulosic fibres with pointed tips,oval to subrectangular,sclerenchymatous cells having wide lumen,xylem shows 3-5 radiating arms,xylem vessels in single or 2-3 groups,having simple pits on their walls,calcium oxalate crystals as prisms and rosettes found scattered in cortex,starch grains both simple having elliptical to spherical and compound having 2-4 components.
UsesIt is used internally to treat fevers,edema,anemia,constipation,hepatitis,ulcers,skin disorders,obesity,hemorrhoids,cough,asthma,paralysis,gout,and rheumatism.The root purgative and prescribed in scorpion sting and snake bite.Other Diseases healed by turpeth.Turpeth is also efficacious for other diseases like melancholia,leprosy,enlargement of spleen and paralysis.
Trachyspermum Ammi [6]
Chemical constituentsThe major component was thymol(39.1%) followed by oleic acid (10.4%),linoleic acid(9.6%),gamma-terpinene (2.6%),p-cymene (1.6%),palmitic acid (1.6%),and xylene (0.1%).
Microscopic characteristicsTransverse section of fruit shows two hexagonal structures attached with each other by carpophores,epicap consist of a single layer of tangentially elongated tabular cells,having thick walled,unicellular trichomes,Oily,Greyish brown,endosperm consists of thick-walled cells filed with of oil globules,embryo small and circular composed of polygonal thin walled cells,characterized.
Uses: Ajwain is used in traditional medicine for controlling high blood pressure.Ajwain is known to keep your lungs and pharynx cleans so that blockage doesn't trouble you.Ajwain helps in the reduction of weight gain.
Terminalia Chebula [7]
Chemical constituents: A number of glycosideshave been isolated from haritaki,including the triterpenesarjun glucosideI,arjungenin,and the chebulosides I and II.Other constituents include an coumarin conjugated with gallic acids called chebulin,as well as other phenolic compounds including ellagic acid,2,4-chebulyl-β-D-glucopyranose,chebulinic acid,gallicacid,ethylgallate,punicalagin,terflavin A,terchebin,luteolin,and tannic acid.
Microscopic CharacteristicsTransverse section of pericarp shows epicarp consisting of one layer of epidermal cells inner tangential and upper portions of radial wall thick,mesocarp,2-3 layers of scleroids in group and vascular bundles scattered brownish in colour,under microscope shows a few fibres,vessels with simple pits sclereids of various shapes and sizes but mostly elongated,endocarp consist of thick walled sclereids of various shapes and sizes.
Uses: Haritaki is a magical remedy that holds high significance in treating countless health problems including indigestion,gastritis,lung disease,obesity,impotence,cough,cold,asthma,vision defects,urinary tract infections and hair,and skin problems.
Glycyrrhiza Glabra [8]
Chemical constituents:Glycyrrhizine (6-8%) [Sweet instant 50 times more than sucrose].Liquiritin and isoliquiritin are responsible for yellow colour.Glucose,sucrose,asparagin,gum,protein,fats,resins,traces of tanin.Glycyrrhizinic acids are produces glycyrrhitinic acid and glycyrrhitic on hydrolysis.
Microscopic CharacteristicsTransverse section of stolon shows cork of 10-20 or more layers of tabular cells,outer layers with reddish brown amorphous contents,inner 3 or 4 rows having thicker,colourless walls,secondary cortex usually of 1-3 layers of radially arranged parenchymatous cells containing isolated prisms of calcium oxalate,secondary phloem a broad band,radially arranged groups of 10-15 fibres,vessels about 80-200μ in diameter with thick yellowpitted thickened walls.
Uses: Mulethi suggested uses include adrenocortical insufficiency,arthritis,bronchitis,dry cough,peptic ulcers,gastritis,infections (bacterial/viral),prostate cancer,sore throat,systemic lupus erythematosus,and upper respiratory inflammation.
Materials and Methods
Collection of Ayurvedic Formulation Ingredients
Proprietary ayurvedic formulationKayam Churnaconsists of seven ingredients in the powdered form,which areCassia angustifolia,Black Salt,Operculina turpethum,Trachyspermum ammi,Terminalia chebula,Sodium Bicarbonate andGlycyrrhiza glabrawere collected in powdered form from Ayurvedic Shop located in Kalyan,Maharashtra,India.Powdered ingredients were analyzed for their powder characteristics using Monograph of respective ingredient.
Composition &Preparation of Kayam Churna in Laboratory
Kayam Churnawas prepared as per standard procedure as per ayurvedic formulary of India,all the ingredients were weighed proportionally and were dried in shade and cleaned by hand sorting then crushed using grinder and passed through mesh.Individual ingredient was weighed as per standard and was mixed using the blender,further mixed formulation was unloaded,weighed and packed in container and labeled as ‘Kayam Churna.Further,it was stored at Room Temperature,for further analysis.
Proximate analysis of raw material and formulation
Organoleptic Analysis:Various characteristics like color,odor,taste and appearance were studied.
Phytochemical Analysis:Qualitative analysis was done using aqueous and methanol extracts after 24 hours of each ingredient and formulation in 1:9 (formulation/ingredients:methanol/water) ratio,presence of tannins,alkaloids,glycosides,flavonoids,steroids,phlobatotannins and Saponins were accessed [9].
(a) Tannins: 1ml aqueous/ methanol extract + 0.1% FeCl3dropwise
(b) Alkaloids: 1ml aqueous/ methanol extract+ 1 ml conc.HCl + Hager reagent
(c) Glycosides: 1ml aqueous/ methanol extract + 0.5 ml GAA + dropwise dil.FeCl3
(d) Flavonoids: 1 ml aqueous/ methanol extract + 1 ml NH3+ conc.H2SO4Solution
(e) Steroids:1ml aqueous/ methanol extract + 1ml CHCl3conc.H2SO4drop
(f) Phlobatotanins: 1ml aqueous/ methanol extract + boil with 1ml 1% HCl
(g) Saponins: 1ml aqueous/ methanol extract + few drops of olive oil + shake
Physiochemical Analysis:It was carried out to test Bulk density,tapped density,compressibility,loss on drying,and ash value [10].
(a) Microscopic Evaluation: Powdered Formulation was added on grease free slide with minute quantity and 1-3 drops of Safranin was added and kept for few seconds,post that placing cover slip on slide,it was observed on 10X and 45X.Monograph of herbal formulation was referred.
(b) Bulk density: It is the ratio of the mass of an untapped powder sample and its volume including the contribution of the interparticle void volume.The bulk density is expressed in grams per millilitre (g/ml)although the international unit is kilogram per cubic metre (1 g/ml=1000 kg/m3) because the measurements are made using cylinders.It may also be expressed in grams per cubic centimetre (g/cm3).
(c) Tapped Density: An increased bulk density attained after mechanically tapping a container containing the powder sample.The tapped density is obtained by mechanically tapping a graduated measuring cylinder or vessel containing the powder sample.After observing the initial powder volume or mass,the measuring cylinder or vessel is mechanically tapped,and volume or mass readings are taken until little further volume or mass change is observed
(d) Powder Compressibility: The inter particulate interactions influencing the bulking properties of a powder are also the interactions that interfere with powder flow,a comparison of the bulk and tapped densities can give a measure of the relative importance of these interactions in a given powder.Such a comparison is often used as an index of the ability of the powder to flow,for example the Compressibility index or the Hausner ratio
(e) Loss on drying: Initial weight of crucible was taken;the weight of crucible was again taken again after adding 2 gm of sample.The sample was kept for 3 hours in hot air oven at specific temperature It measures the amount of water and volatile matters in a sample when the sample is dried under specified conditions.Once completed take the final weight of crucible and determine the loss of drying value in terms of percentage.
(f) Total Ash value: 2gram of formulation in crucible was taken,then keep the ayurvedic formulation in Muffle Furnace at 500°C for 15 mins,once completed,compared the initial and final weight for determining the ash value
(g) Heavy metal determination: Presence of lead,Cadmium and Bismuth were checked as qualitative analysis
Microbiological analysis:Total Viable count (TVC) was used for microbiological analysis,1 gram of formulation was weighed and was transferred to the sterile test tube,10-fold serial dilution was made up to 10-5,nutrient agar was used for Total viable count,with spread plate technique and 0.1 ml of diluted sample from 10-3to 10-5was used for plating and incubated for 24 hours at 36°C,the count on next day actually represents the number of colony forming unit (CFU) per ml of the sample.A TVC is achieved by plating dilution of the culture until 30-300 colonies exist in a single plate
Preparation of Sample for Chromatographic analysis:All the plant powder and formulation were prepared by adding 1 gram of formulation/ ingredients in 10 ml methanol and stored overnight for 24 hours and post that extract was purified/extracted using Whatman filter paper no 1,further stored in refrigerator till further use
High Performance Thin Layer Chromatography(HPTLC):HPTLC was performed on precoated silica Gel 60 F254 with plate size of 10 × 10 cm,mobile phase used was 2-Propanol: Ethyl acetate: Water: Formic acid(8:8:5.8:0.2),scanning speed of 20mm/s,Sample applicator CAMAG Linomat 5,development twin trough chamber of 10 × 20 cm,detection was done using CAMAG Visualizer and data was analyzed using winCATS Planar Chromatography Manager [11].
Result and Discussion
The proprietary ayurvedic formulation ofKayam Churnawas made in lab and various parameters like organoleptic,phytochemical,physiochemical analysis and chromatographic parameters were observed and compared with the standard limits,analyzed both qualitatively and quantitatively as per Ayurvedic parameters.The similar method was used by Vats S.,et al.,2022 for preparation and standardization ofVrihatsamsharkar Churna[12].Formulation made and packed in container showed in Figure 2.Microscopic Evaluation was done to check the parameters as per monograph under microscope at 10 X,the result showed presence of Fibres,Vessels and Starch grains which is showed in Figure 3,the microscopic characteristics of raw materials were studied.Contamination was not observed,so the raw materials are pure.Therefore,it can be used for preparation of formulation.Organoleptic analysis also can be considered as macroscopic analysis,it is also done to check its purity and appearance whose parameters are mentioned in Table 2;The phytochemical analysis was done as per the procedure and analyzed qualitatively for both aqueous and methanolic extract and showed presence of Tannins,Alkaloids,Flavonoids and Saponins in theKayam ChurnaPowder which is elaborated in Table 3 and Table 4.Physicochemical analysis was done and calculated using standard formulas and all the values obtained is mentioned in Table 5;Heavy metal analysis was also carried out and it’s showed absence of lead,bismuth and cadmium phosphate in the formulation as shown in Table 6;10-fold serial dilutions were made of the formulation for microbiological analysis and spread plate technique was performed for the analysis of microbial contamination.The number of colonies in 103&104concentrations were seen to be present in the plates and were found to be in the limits as shown in Figure 5 and Table 7.HPTLC fingerprinting of formulation and ingredients showed the presence of various phytoconstituents as shown in Figure 6.India can emerge as the major country and play the lead role in the production of standardized,therapeutically effective Ayurvedic formulation.India needs to explore the medicinally important plants.This can be achieved only if the herbal products are evaluated and analyzed using sophisticated modern techniques of standardization such as UV-visible,TLC,HPLC,HPTLC,GC-MS,spectrofluorimetric and other methods [13].The above studies throw the light on the use of these techniques for standardization ofKayam Churna.

Figure 2 Prepared formulation of Kayam Churna.

Figure 3 Microscopic analysis of Kayam Churna Powder.(a) Fibres (b) Vessels (c) starch grains

Figure 4 Prepared Extract for phytochemical and chromatographic analysis.(a) Methanol extract of Kayam Churna and its ingredients,(b) Aquous extract of Kayam Churna and its ingredients

Table 2 Organoleptic Analysis of Kayam Churna Formulation.

Table 3 Phytochemical analysis of Kayam Churna in aqueous extract.

Table 4 Phytochemical analysis of Kayam Churna in methanolic extract

Table 5 Physicochemical Properties

Table 6 Heavy Metal Determination Qualitatively

Table 7 Microbiological analysis with the media of nutrient agar

Figure 5.Microbiological Evaluation of Kayam Churna on Nutrient Agar
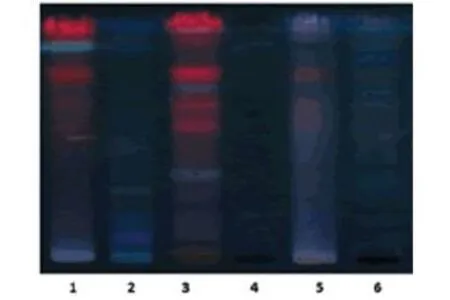
Figure 6.HPTLC fingerprinting of Kayam Churna and its ingredients.From left to Right: Cassia angustifolia,Glycyrrhiza glabra, Operculina turpethum,Trachyspermum ammi,Terminalia chebula and Kayam Churna Formulation
Conclusion and Future prospective
In the present study,the quality of raw materials,intermediates obtained during the preparation ofKayam Churnaand the final product was assessed by modern scientific procedures.Values of proximate analysis of the raw materials used were found to be within the permissible pharmacopoeial limits.
The results of present study can be used as a quality control method for characterization ofKayam Churnain industry to check their uniformity.The obtained values of physical and chemical parameters for the finished product can be adopted to lay down new pharmacopoeial standards to be followed in the traditional preparation ofKayam Churnawith batch-to-batch consistency.
Data availability or Code availability
Correspondence and requests for materials should be addressed to sonali1386@gmail.com (Dr.Sonali Patil).
Abbreviations
TVC,Total Viable count;CFU,colony forming unit;HPTLC,High Performance Thin Layer Chromatography;TNTC,Too Numerous To Count;GAA,Glacial Acetic Acid.
Acknowledgments
The authors would like to acknowledge B.K.Birla College(Autonomous) for providing the facility for carry out research.
Author contributions
The methodology was carried out at center by Prajwal Gupta,Ashwini Kendle,Diksha Bhagat,Mahera Khan and Prabuddha Wakode.Shruti Shah guided for physicochemical analysis.Sonali Patil is the guide for the research and corresponding author.
Competing interests
Authors acknowledge B.K.Birla College (Autonomous),Kalyan for facility provided to carry out this research work.
 TMR Modern Herbal Medicine2022年3期
TMR Modern Herbal Medicine2022年3期
- TMR Modern Herbal Medicine的其它文章
- Traditional Chinese medicine as monotherapy or combined with oseltamivir in the treatment of H1N1 influenza: a systematic review and meta-analysis
- The genus Strobilanthes: phytochemistry and pharmacology
- Niaoduqing granules inhibits TGF-β1-induced epithelial-mesenchymal transition in human renal tubular epithelial HK-2 cells
- Effects of Euphorbiasteroid on gene expression in lung cancer cells based on gene chip
- A review of the ethnobotanical value,phytochemistry,and pharmacology of Physalis pubescens L.
