大分子杂质是双黄连注射剂导致类过敏反应的重要原因*
万绪莲,夏 恒,韩依伦,李律宇,陈 欢,黎勇坤,段为钢△
(1.云南中医药大学基础医学院,云南 昆明 650500;2.云南中医药大学中药学院,云南 昆明 650500;3.云南中医药大学第三附属医院,云南 昆明 650500)
Injection is a necessary dosage form used on emergent occasions or when drugs are with poor bioavailability.Drugs injected through subcutaneous tissue,musculartissue,orvein usually absorbed quickly and onset much faster than those via oral administration,though its delivery way is dangerous.Injections often cause more and/or heavier adverse reactions than other dosage forms,such as anaphylactoid reactions which mixed with anaphylactic reactions[1-2].Most anaphylactoid reactions usually happen less than 30 mins after administration,including itch,erythema,cough,hypotension,dyspnea,and shock;similar to those of anaphylactic reactions(allergy)[3].However,different from anaphylactic reaction,anaphylactoid reaction can be ignited by the first exposure to injections,while immunoglobin E(IgE)is not required to mediate such a reaction[3].Therefore,anaphylactoid reactions are called pseudo-allergic reactions[3-4].
Although the mechanisms of anaphylactic reactions are largely illustrated,those of anaphylactoid reactions are still elusive.As for anaphylactoid reactions,the substances igniting,the targets mediating,and the pathways participating in the reaction are not fully clear.It has been reported that substances with small molecular weight such as compound 48/80(C48/80)can ignite the reaction,while the reaction is likely mediated by a Mas related G-protein coupled receptor(MRGPRX2)[3-5].The receptor proved to be an orphan receptor,is highly expressed in mast cells.The activation of the receptor triggers mast cell degranulation.The released substances,including histamine,will activate their downstream effectors and trigger the anaphylactoid reactions.Therefore,most of the studies on anaphylactoid reaction focused on small molecules[6].
However,almost all injections can ignite anaphylactoid reactions as contrasted with their oral prepara tions.It seems that substances other than small molecules could also play a role in anaphylactoid reactions.For example,Shuanghuanglian injection(SHL),a traditional Chinese medicine injection made from the flower of Lonicera japonica,the root of Scutellaria baicalensis,and the fruit of Forsytnia suspensa,is widely used against virus infection and inflammation,without obvious anaphylactoid reactions[7-11].Three most frequent symptoms were in skin and mucosa,digestive system,and body temperature center[12].On the contrary,the similar reactions of its oral preparation with the same formula were seldom documented.The evidence suggests that the substances igniting anaphylactoid reactions can be kept out by walls of the gastrointestinal tract.Considering that macromolecular substances into blood flow can be prevented by the gastrointestinal tract[13],it can be suggested that macromo-lecular substances are at least partially responsible for the anaphylactoid reactions[14-15].
As mentioned above,C48/80 is a widely used tool reagent in studying anaphylactoid reaction[5,16].The agent is produced from N-methyl-p-methoxy phenylethylamine and formaldehyde.According to its synthesis route,C48/80 is a mixture of polymers with a theoretical molecular weight of 629.1 Da,and the agent is possible to contain macromolecular substances.Unfortunately,the macromolecules in the substance have not been taken seriously.
Though a study in 1976 reported that contaminated macromolecules may cause anaphylactoid reaction[17],the adverse reaction was yet ignored by pharmaceutical industries.Due to techniques of removing macromolecules are not widely introduced,all injections theoretically contain macromolecular impurities which may come from raw materials,contamination and excipients,as well as C48/80.Does macromolecular substances play an important part in anaphylactoid reactions?The main aim of the study is to illustrate that C48/80 and SHL really contain macromolecular impurities,and they play an important role in triggering anaphylactoid reactions.
1 Materials and Methods
1.1 Materials C48/80 was manufactured by Sigma-Aldrich.SHL was manufactured by Harbin Zhenbaodao Pharmaceutical Co.LTD(Harbin,China)and approved by China Food and Drug Administration(cFDA).Normal saline for injection(NS)was manufactured by Chenxin Pharmaceutical Co.LTD (Jining,China).Enzyme-linked immunosorbent assay(ELISA)kits for Guinea pig soluble complement terminal complex Sc5b-9 (Sc5b-9)(Lot.MM-8751501),complement factor B (CFb)(Lot.MM-8750901),mannose binding lectin(MBL)(Lot.MM-8751301),bradykinin(BK)(Lot.MM-8751801),β-hexosaminidase(β-HEX)(Lot.MM-196101),lysozyme (LZM)(Lot.MM-8752201),and histamine(HIS)(Lot.MM-033501)were manufactured by Jiangsu Enzyme industry Co.LTD(Yancheng,China).Cromolyn sodium (Lot.D16-J12G137501)and Evans Blue(Lot.L04D11 J133367)were manufactured by Shanghai Yuanye Biotechnology Co.LTD(Shanghai,China).Hematoxylin-eosin(HE)staining kits were manufactured by Boster Biological Technology Co.LTD(Wuhan,China).
Guinea pigs weighing 250~350 g and female Sprague-Dawley(SD)rats weighing 200 g were provided by Kunming Medical University.Animals were raised at 22℃temperature,at 45%~55% humiditycontrolled conditions,and under natural light.
A multiple microplate reader of K6600A was manufactured by Beijing Kai’ao Technology Development Co.Ltd (Beijing,China).A lyophilizer of FDU-1100 was manufactured by EYELA(Tokyo,Japan).Ultra-filters of 10 kDa (1808013VS)were manufactured by Sartorius AG(Hamburg,Germany).Ultrapure water was obtained from a Milli-Q water purification system(EMD Millipore Group,Darmstadt,Germany).Other instruments or reagents used in the present study,if not mentioned,were made in China.
1.2 Ethical approval and consent to participate Animal experiments were approved by the Animal Care and Use Committee of Jiangsu Provincial Institute of Materia Medica,Nanjing Tech University,Nanjing China(IACUC-20170830-02),and all the animals were treated under Guidelines for Ethical Review of Laboratory Animal Welfare of China(GB/T 35892-2018).Animal experiments were in accordance with ARRIVE guidelines(https://arriveguidelines.org).
1.3 Macromolecular Substance Preparation The original C48/80 was dissolved in water for injection to obtain a 5%solution.The colorless solution was gradually added to the upper tube of the ultra-filter of 10 kDa.The ultra-filter was spun at 3 500 g and 4℃until more than a half volume was filtered through.Then,the solution passed through was collected as Component B containing substances with molecular weight below 10 kDa,and the solution in the upper tube was recovered by mixing with the water for injection and spun again.When the solution in the upper tube was diluted six times and ultrafiltered,the retained solution in the upper tube was collected as Component A containing substances with molecular weight above 10 kDa.
The two components mentioned above were dried by the lyophilizer.Briefly,the solution was put in a plastic bottle of 250 mL,and frozen at-40 ℃overnight.Then,the frozen solution was put into the chamber of the lyophilizer and lyophilized for more than 48 h.Then,the bottle was weighed every 3 h,and when the total weight of the bottle did not decrease the lyophilization was ended.The net weight of the powder was calculated by subtracting the bottle weight from the total weight.The powder diluted with NS to an arranged concentration was used for subsequent studies.
Similarly,components of the original SHL injection were prepared.Briefly,SHL was added to the upper tube of the ultra-filter of 10 kDa,and spun at 3 500 g and 4 ℃ until nine tenths of the volume was filtered through.The part retained in the upper tube was collected as component A of SHL and the part passed through was com ponent B.The obtained solutions,components A and B,were used directly without dilution or concentration[16].
1.4 Systemic anaphylactoid reactions in guinea pigs evoked by macromolecules The original C48/80 and its components were dissolved in NS(0.75 mg/mL).Every 6 guinea pigs,including 3 male guinea pigs and 3 female guinea pigs (without pregnancy),were randomly arranged in a group to detect anaphylactoid reactions.Guinea pigs were intravenously administered with original C48/80 or its components (0.25 mg/kg).Animals administered with NS of the same volume were served as a negative control.According to Chi nese Pharmacopoeia (CP)of the 2015 edition(Book 4)[19],the symptoms appeared in 30 mins after administration were observed and their blood was immediately drawn without anticoagulation to obtain serum samples.The symptom scores were recorded according to Table 1.If an animal appeared two or more symptoms,only the higher or the highest score was used[13,16].
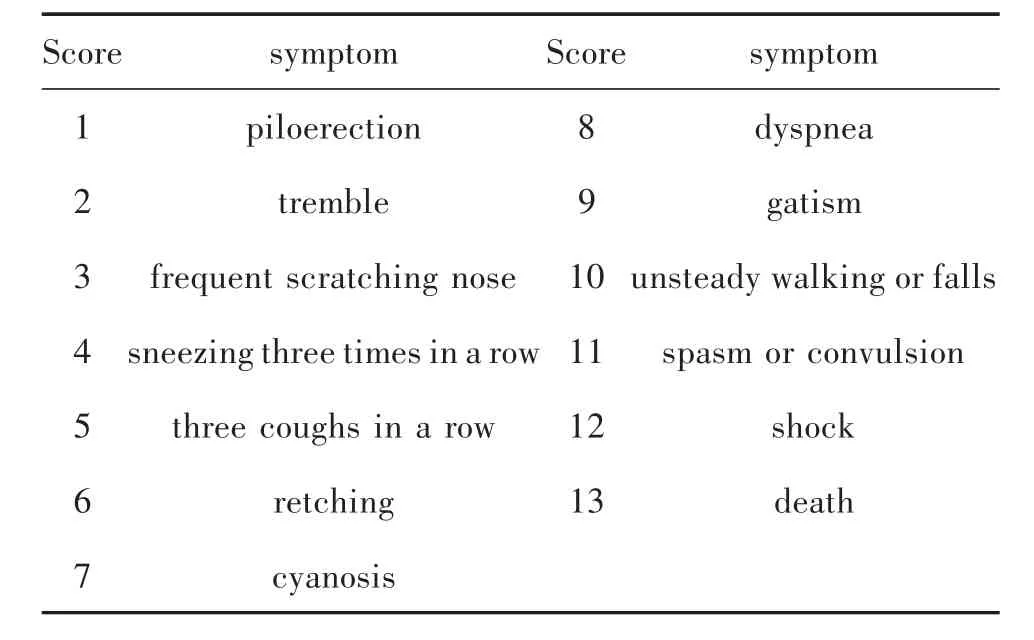
Tab.1 Scores of symptoms
Similarly,guinea pigs were intravenously administered with the original SHL injection and its components(3.4 mL/kg).Their symptoms appeared in 30 min after administration were also recorded according to Table 1 and their blood was collected 30 min after administration.
The coagulated blood was spun at 3 000 g and 4℃for 5 min to obtain serum.SC5b-9,CFb,MBL,BK,β-HEX,LZM,and HIS in the serum were determined using ELISA kits according to protocols.
1.5 Substances released from guinea pig leukocytes evoked by macromolecules Guinea pigs were anesthetized with urethane(1.0 g/kg)by intraperitoneal injection.Their abdomens were opened and their anticoagulated blood was drawn via the abdominal aortae.The blood was spun at 1 000 g and 4℃for 5 min to remove red blood cells,and the volume was recovered by adding NS.The suspension of 5 mL containing the whole white leukocytes was transferred to a six-well plate.When the original C48/80 or its components of 20 μg/mL were added in the suspension and mixed,the plate was kept at 37 ℃ for 30 min.The suspension of 1 mL was sampled and spun at 1 000 g and 4℃for 5 min to obtain its supernatant,and β-HEX,LZM and HIS in it were determined using ELISA kits[16].
Similarly,the suspension of 5 mL containing the whole leukocytes was transferred to a new six-well plate,and SHL or its components of 0.5 mL were added and mixed.The plate was also kept at 37℃.Thirty minutes later,β-HEX,LZM and HIS in the supernatant of the reaction suspension were determined using ELISA kits.
1.6 Passive cutaneous anaphylactoid(PCA)reaction evoked by macromolecules in guinea pigs Every 4 guinea pigs,including 2 male guinea pigs and 2 female guinea pigs(without pregnancy)weighing about 300 g were randomly arranged in a group.They were pretreated with cromolyn sodium(50 mg/kg)or NS of the same volume by intraperitoneal injection for 5 days.The day before the macromolecular injection,their back fur was removed with a pair of scissors.On the next day,they were intravenously injected with 1 mL Evans Blue solution(0.5%,dissolved in NS).Fifteen minutes later,the original C48/80 or its components(0.75 mg/mL)of 0.1 mL were subcutaneously injected at one point.The administration of NS of the same volume was served as a negative control.Thirty minutes later,the animals were anesthetized with ure thane(1.0 g/kg),their back skin was cut,and the bluestaining areas were photographed.The Evans Blue in the skin(4.00 cm2)was extracted using 4 mL acetonenormal saline solution (acetone∶normal saline=7 ∶3)for 24 h.The extract was spun at 3 000 g for 5 min,and the Evans Blue in the supernatant was determined by the multiplate reader at 610 nm.Similarly,PCA reaction evoked by the original SHL and its components of 0.1 mL were tested[18].
After Evans Blue was extracted,a piece of skin around the injection point was selected.The skin fixed by the acetone solution was routinely dehydrated with ethanol and embedded in paraffin.The skin was cut at 5 μm and stained with HE staining kits.The section was scanned using a fluorescence microscope in a light mode.
1.7 Pulmonary vascular permeability affected by macromolecules Female rats were intraperitoneally treated with 10 mg cromolyn sodium or NS of the same volume.Thirty minutes later,they were anesthetized with urethane(1.0 g/kg).Their abdomens and chests were opened,and the superior and inferior venae cava were ligated with artery clamps.NS was perfused into the right ventricle under the pressure of 1 500 mmH2O.Their abdominal aortae were cut for discharging.When the pulmonary circulation was perfused for 5 min,the color of the lungs became light and the almost colorless perfusate was discharged from their aortae.Then,the original C48/80 or its components(62.5 μg/mL)of 1 mL were perfused into the system and kept for 10 min.After that,Evans Blue solution (50 mg in 1000 mL NS)of 1 mL was perfused into the system and kept for 10 min.Finally,the system was perfused with NS for 5 min,and the lungswereremoved from theirchestsand photographed.The lung stained in blue suggests the vascular permeability[18].
Similarly,the effect of the original SHL and its components of the same volume(without dilution)on pulmonary vascular permeability were tested.
1.8 Statistics Values were expressed as mean±standard deviation(SD)or median±inter-quartile range(IQR).If data exhibited the normal distribution,one-way analysis of variance(ANOVA)was performed to compare means between groups.If there was a significance,a post-hoc test(equal variance)or Tamhane’s test(unequal variances)was used to compare means between every two groups.If data did not exhibit the normal distribution,a non-parametric test of Mann-Whitney test was used to compare their median values between every two groups.Statistical significance was accepted at P<0.05.
2 Results
2.1 Preparation of components of C48/80 and SHL injection By ultrafiltration and lyophilization,the yield of component A(above 10 kD)of C48/80 was 12.5%,and that of component B(below 10 kD)was 87.5%;suggesting that there are adequate macromolecular substances in the original C48/80.The original SHL was a transparently yellow-brown solution.Interestingly,component A of SHL was much darker than the original,while component B was much lighter,suggesting that there are colored macromolecular substances in the original SHL.
2.2 Systemic anaphylactoid reactionsevoked in guinea pigs by macromolecules The intravenously injected original C48/80(0.25 mg/kg)caused a moderate systemic anaphylactoid reaction.Its component A caused an even more serious reaction,while its component B caused a much weaker reaction(Fig.1A).Similar phenomena can be seen in SHL experiment because its component A also caused heaver anaphylactoid reactions(Fig.1B).The results suggested that macromolecular substances are one of the main factors responsible for the anaphylactoid reactions caused either by C48/80 or SHL.
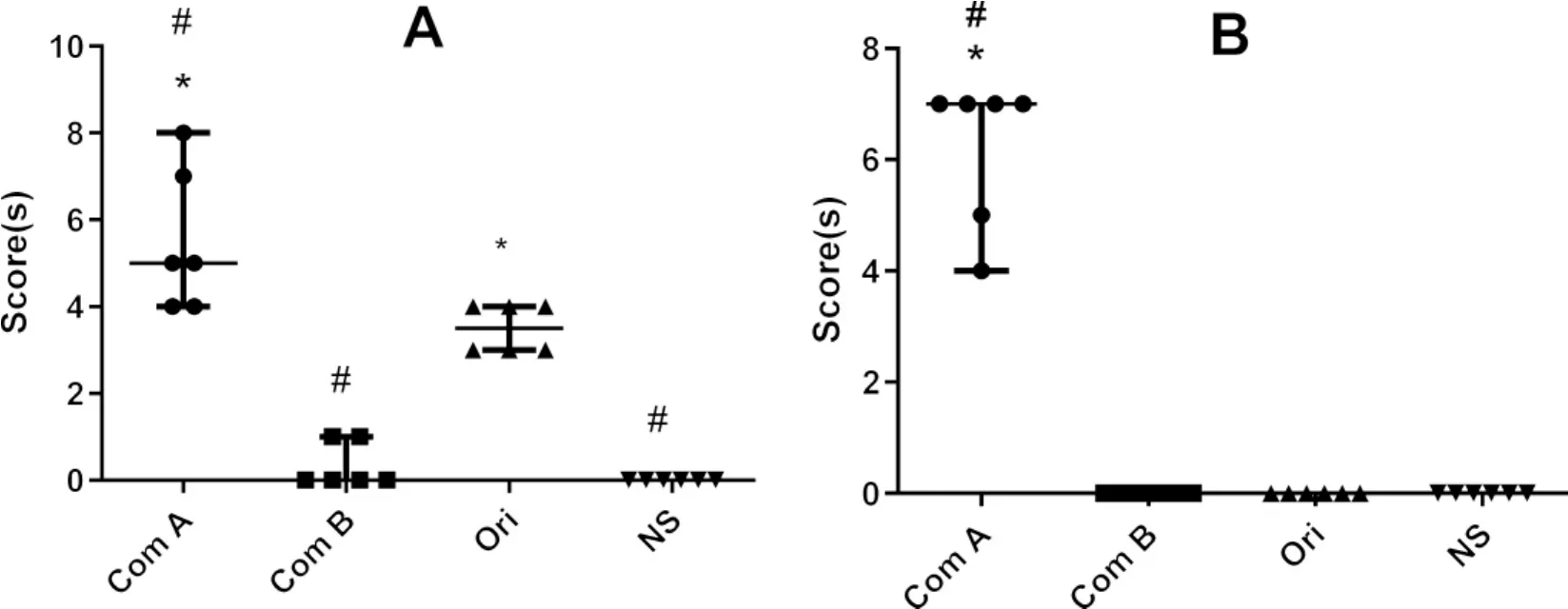
Fig.1 Systemic anaphylactoid reactions evoked by intravenously injected macromolecules of C48/80(A)and Shuanghuanglian injection(SHL)(B)in guinea pigs(n=6)
To understand the mechanism of the systemic anaphylactoid reactions,some factors in their serum were detected.By comparing with the negative control(NS),the original C48/80 and its component A tended to increase all the factors involved in Fig.2,though they did not significantly affect the levels of SC5b-9(Fig.2A),MBL(Fig.2C),and LZM(Fig.2F).By comparing with the original C48/80,component A significantly increased the levels of CFb (Fig.2B),BK(Fig.2D),β-HEX(Fig.2E).However,SHL is different from C48/80.By comparing the negative control,the original SHL and its component A tended to decrease the factors mentioned above.By comparing with the original SHL,component A of SHL did not increase the levels of the above factors in serum (Fig.2),either.Sometimes,the component A significantly decreased SC5b-9(Fig.2A),CFb(Fig.2B),LZM(Fig.2F),and HIS(Fig.2G).
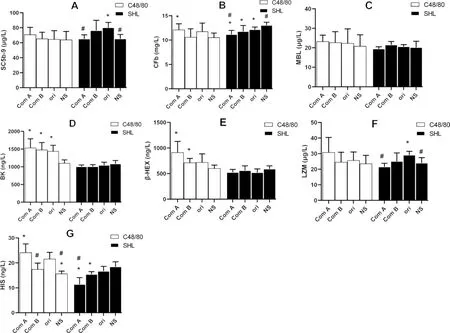
Fig.2 Serum factors affected by C48/80 and Shuanghuanglian injection(SHL)in guinea pigs(mean±SD,n=6)
To investigate whether the elevation of some factors mentioned above are derived from blood leukocytes,an in vitro experiment was performed.By comparing the original C48/80,its component A did not increase the release of β-HEX (Fig.3A)and LZM(Fig.3B),but significantly increased the release of HIS(Fig.3C);partly agreeing with the results in Fig.2 and suggesting that the anaphylactoid reactions caused by macromolecular substances in C48/80 is associated with the release of histamine from leukocytes.As for SHL,its component A did not increase the release of β-HEX(Fig.3A),LZM(Fig.3B)and HIS(Fig.3C);suggesting that the anaphylactoid reactions caused by macromolecular substances of SHL is less relevant to the blood leukocytes.
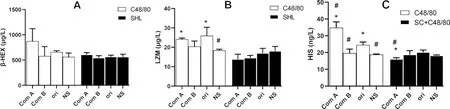
Fig.3 Substances released from blood leukocytes(mean±SD,n=3)
2.3 PCA reaction evoked by macromolecules in guinea pigs PCA reaction is believed to be a sensitive method to detect anaphylactoid reactions.Since anaphylactic or anaphylactoid reactions increase vascular permeability,the leached Evans Blue from vessels can be used to assess the intensity of the reaction.According to the results in Fig.4,normal saline for injection resulted almost no PCA reactions.However,the original C48/80 and its component A caused strong PCA reactions,and the reaction caused by its component B was much weaker(Fig.4A and 4C).It should be noted thatcomponentA caused the strongest PCA reaction among C48/80 groups.The reactions were partly prevented by cromolyn sodium(Fig.4B and 4C).
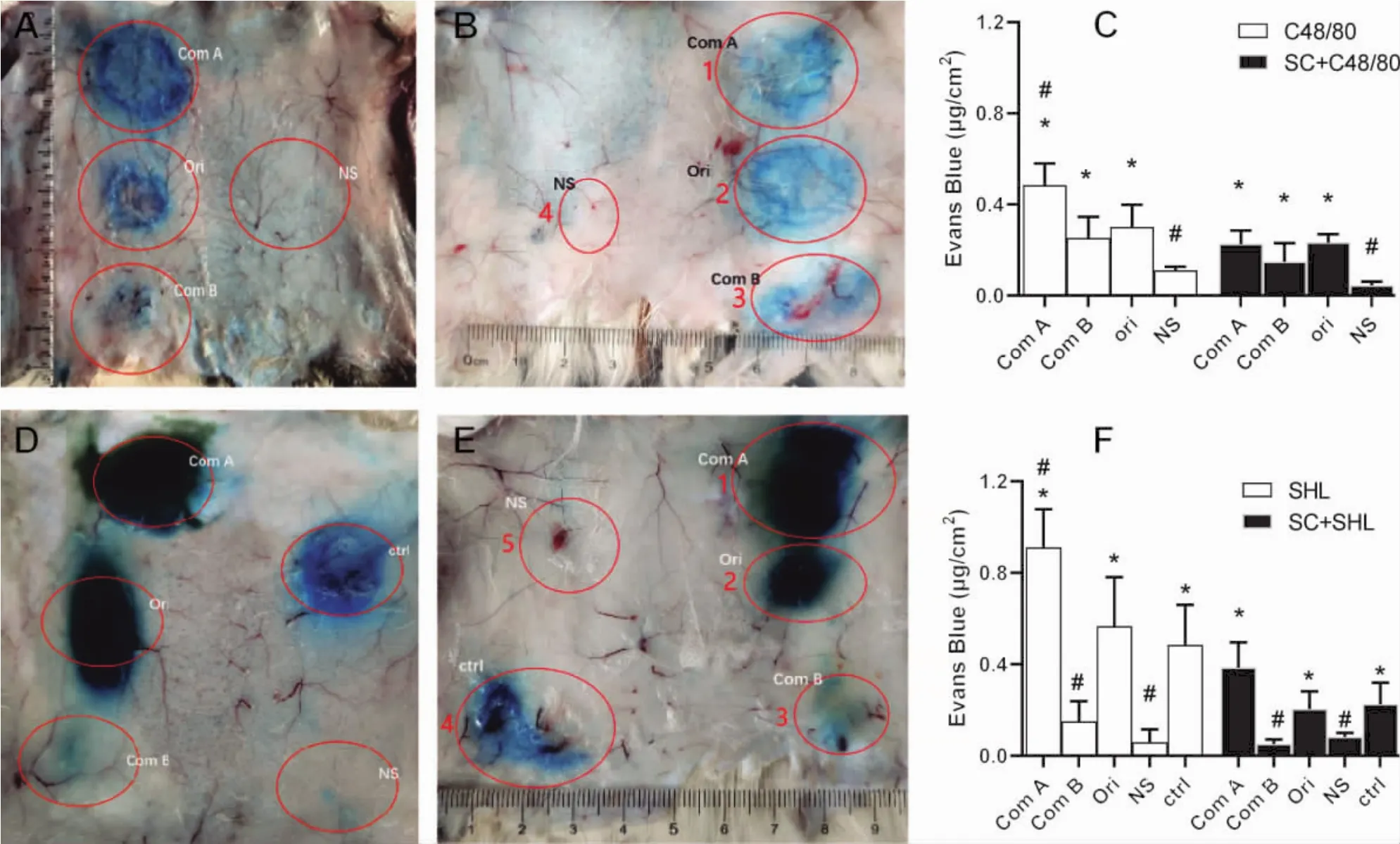
Fig.4 Guinea pig’s back skin was stained with Evans Blue after passive cutaneous anaphylactoid(PCA)reaction (mean±SD,n=4)
As for SHL,similar results were shown in Fig.4D,4E and 4D.The original SHL and its components caused even stronger PCA reactions than C48/80,respectively.Among the three SHL groups,component A caused the strongest PCA reaction,and component B caused the weakest.The reactions caused by SHL can also be partly antagonized by cromolyn sodium.
Considering PCA reactions are acute injury on skin,regeneration rather than necrosis can be observed in the area near to the injection point.Local hydropic degeneration can be observed in the areas of skin with severe PCA reactions(Fig.5).The degree of hydropic degeneration was generally consistent with the results of PCA.
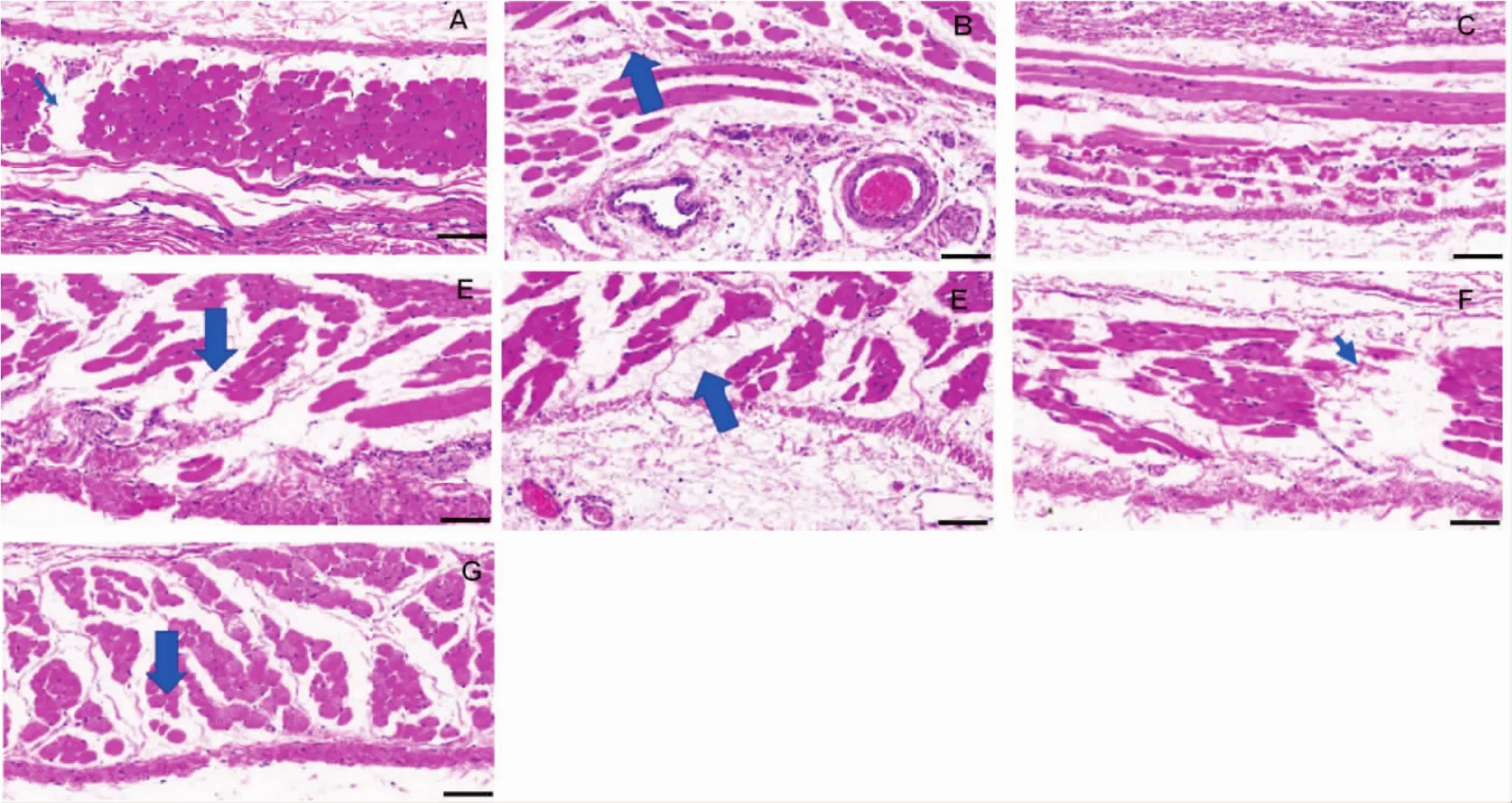
Fig.5 Hematoxylin-eosin(HE)staining on guinea pig’s skin section.The fields were near to the injection sites
2.4 Pulmonary vascular permeability affected by macromolecules To exclude the role of blood cells that may mediate vascular permeability,pulmonary perfusion experiment was carried out in female rats,and the results were showed in Fig.6.The lungs treated by NS was only slightly stained by Evans Blue(Fig.6A).The lungs treated by the original C48/80 and its component A was even heavily stained by the dye(Fig.6B and 6C).Unsurprisingly,the lungs treated by component B of C48/80 was almost negatively stained by the dye.The increased vascular permeability caused by macromolecular substances in C48/80 can almost be fully prevented by cromolyn sodium(Fig.6 E).
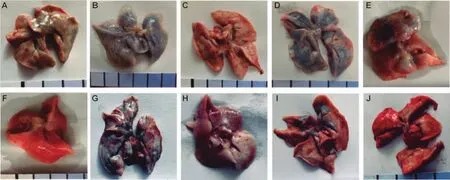
Fig.6 Rat lungs stained with Evans Blue after perfusing with normal saline for injection(A),component A of C48/80(B),component B of C48/80(C),the original C48/80(D),cromolyn and component A of C48/80(E),cromolyn and component B of C48/80(F),component A of Shuanghuanglian injection(SHL)(G),component B of SHL(H),the original SHL(I),and cromolyn and component A of SHL(J).
The lungs treated with the component A of SHL was also heavily stained by Evans Blue(Fig.6G),while those treated with the component B was only slightly stained(Fig.6H).The elevation in vascular permeability caused by the component A was almost fully prevented by cromolyn sodium(Fig.6J).
3 Discussion
The present study proved that C48/80 and SHL contain macromolecular impurities.Though C48/80 is a mixture of polymers,it was used to be believed that the agent only contains small molecules.However,more than ten percent of macromolecular substances were obtained by ultrafiltration.The role of the macromolecular substances in triggering anaphylactoid reaction was not noticed before.Similarly,like other herbal injections[13],macromolecular impurities were proved in SHL.The macromolecular substances in component A of SHL were not isolated in the present study,and the component would contain both the enriched macromolecular substances and other small molecules.Theoretically,component A of SHL should have a higher solids content than the original.In fact,our previous study[20]proved that the solids content of component A was only slightly and insignificantly higher than that of the original,while that of component B was slightly and insignificantly lower.Therefore,as for SHL,the solids dose of the original SHL and its components can be considered the same,and the difference in anaphylactoid reaction should be caused by the substances with different molecular weights.In addition,similar results were reported in an excipient(polysorbate 80)for injection[18].
Components A of C48/80 caused more serous anaphylactoid reactions than the original(Fig.1),suggesting that macromolecular substances may play an important role in triggering the reactions.The reactions might asso ciate with the activation of complement system because component A of C48/80 tended to elevate the level of SC5b-9,CFB,and MBL,though some of which did not reach a significant high level(Fig.2).Considering there was an elevation of BK in serum,the macromolecular substances of C48/80 activate bradykinin system.The anaphylactoid reaction might associate with the activation of mast cells,too,because component A of C48/80 elevated the level of β-HEX,LZM,and HIS both in vivo(Fig.2)and in vitro(Fig.3),which are most likely to be released from mast cells[21].
Different from C48/80,the macromolecular substances in SHL caused even more serous anaphylactoid reactions than that in C48/80,but rarely elevated the levels of the serum factors(Fig.2).Further,SHL groups tended to inhibit β-HEX,LZM,and HIS in vitro(Fig.3).The possible explanation is that SHL has pharmacological effects against virus and inflammation[22],and the serum factors that can be elevated by C48/80 were inhibited by SHL.The results suggested that the mechanism of SHL could be different from that of C48/80 in triggering anaphylactoid reactions.
Compared with anaphylactoid reaction ignited by intravenous administration,PCA reaction is a more sensitive test with visual results.PCA reaction seldomly causes animal death,which were supported by the present study.The advantage of PCA reaction is that the degree of anaphylactoid reaction can be quantitatively evaluated according to the exudation of Evans Blue.This means that the principle of the test is to evaluate vascular permeability.Due to the limited understandingofanaphylactoid reaction,intravenous investigation is still necessary for intravenous injections,since PCA reaction cannot find a lethal reaction of an intravenous injection.
The macromolecular substances from C48/80 and SHL enhanced the vascular permeability in the pulmonary perfusion tests(Fig.6).Considering blood cells including leukocytes,were removed by perfusate,and large molecules are unlikely to cross the vascular wall into the interstitial space to activate mast cells where they reside,it can be deduced that the increase of vascular permeability is likely to be resulted from a direct effect on epithelia.
Cromolyn sodium is a mast cell stabilizer with anti-inflammatory activity.The mechanism may be linked tointerferences with the antigen-stimulated calcium transport across the mast cell membrane,thereby inhibiting mast cell release of histamine,leukotrienes,and other substances that cause anaphylactoid reactions[23].Considering cromolyn sodium also reduces vessel permeability[24],it is understandable that the PCA reactions and the increase of vascular permeability caused by macromolecular substances from C48/80 and SHL were prevented by cromolyn sodium.
4 Conclusion
All the results suggested that macromolecular substances are one of the main factors responsible for anaphylactoid reactions caused both by C48/80 and SHL.The direct increase of vascular permeability participates the anaphylactoid reactions ignited by C48/80 and SHL,and mast cell degranulation is also involved in the reaction of C48/80.

