Synthesis and Characterization of 3-Phenyl-5-(2-Phenylthio-Quinolin-3-Yl)-1-(4-Methoxyphenyl)-2-Thiazoyl)-Pyrazoline
ZENG Yong-ming,LIU Fang-ming
(College of Materials and Chemical Engineering,Hangzhou Normal University,Hangzhou 310036,China)
Synthesis and Characterization of 3-Phenyl-5-(2-Phenylthio-Quinolin-3-Yl)-1-(4-Methoxyphenyl)-2-Thiazoyl)-Pyrazoline
ZENG Yong-ming,LIU Fang-ming
(College of Materials and Chemical Engineering,Hangzhou Normal University,Hangzhou 310036,China)
The experiment synthetized the compound 3-phenyl-5-(2-phenylthio-quinolin-3-yl)-1-(4-methoxyphenyl)-2-thiazoyl)-pyrazoline,and determined its crystal structure by X-ray single-crystal diffraction.Moreover,the compound has been confirmed by IR spectra,1H NMR,MS and elemental analysis.
Quinolin;Thiazole;Pyrazoline;crystal structure;synthesis
0 Introduction
Heterocycles bearing nitrogen,sulphur constitute the core structure of a number of biologically interesting compounds.Quinoline ring systems represent a major class of heterocycles,which has aroused a growing interest in the organic and medicinal chemistry due to its important pharmacological properties[1],such as antimalarial[2-4],antimicrobial[5],antibacterial[6],antiproliferative[7]activities as well as many others[8-10].Meanwhile,Pyrazoline derivatives have also been reported in the literature to exhibit various pharmacological activities such as anti-inflammatory[11],antihypertensive[12],and antimicrobial[13-14].Pyrazole derivative celebrex is a potential anti-inflammatory drug[15].In addition,thiazole and its derivatives are known as substances with antiviral[16-17]antituberculosis[18]and anti-HIV activities[19].Penicillins containing a thiazole ring system(thiazolidine)[20]are also important naturally occurring products.Recently,in order to overcome the rapid development of drug resistance,considerable interest has been shown in thiazolyl-pyrazolin derivatives,which possess significant antibacterial activities.[21-22]
To enhanced potential activities,we report herein the synthesis and characterization of the title compound(Scheme 1),in which thiazole and pyrazoline ring are introduced to quinoline moiety.In order to confirm its structure,a single crystal of this compound was obtained from methanol,and themolecular structure was determined by X-ray diffraction.
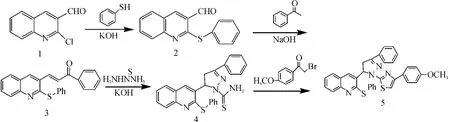
Scheme 1 The synthesis of title compounds
1 Experimental
1.1 Materials and synthesis
All the solvents and materials were of reagent grade and purified as required.Melting points were recorded on a mettler FP-5capillary melting point apparatus are uncorrected.Elemental analyses were performed on a Perkin-Elmer 2 400elemental analyzer.The IR spectra were measured on a Bruker Equinox 55FT-IR spectrophotometer with KBr disk in the range 400-4 000cm-1.The1H NMR spectra were determined on a Varian Inova-400spectrophotometer using with TMS as the internal reference and CDCl3as solvent.The results were found to be good agreement with the calculated values.The starting compound 1was prepared according to the previously reported procedures[23].
1.2 The synthesis of 2-phenylthio-3-formylquinoline(2)
Equimolar amounts of the benzenethiol(20mmol)and potassium hydroxide(20mmol)were dissolved in dimethyl sulfoxide.After dissolution of the reactants,a solution of 1(20mmol)in 10ml dimethyl sulfoxide was added dropwise.The mixture was refluxed(85~90℃)and monitored by TLC until the reaction was completed.After cooling,then poured into ice water and neutralized with dilute hydrochloric acid to pH=5~6.The resulting solid was collected by filtration and crystallized from DMF/ethanol to give 2in 88%yield;M.p.:123~124℃;1H NMR(400MHz,CDCl3)δ=9.58(s,1H,-CHO),8.24(s,1H,quinolin-H4),8.10-7.19(m,9H,Ar-H);IR(KBr):ν=1 705(C=O),1 626(C=N),762(C-S-C)cm-1;MS:m/z=265(M+),236,204,165,128,101.
1.3 The synthesis of(E)-3-(2-Phenylthio-quinolin-3-yl)-1-phenyl-2-propen-1-one(3)
Phenylethanone(20mmoles)was added to a solution of 2(20mmoles)in aqueous KOH(10ml,35% KOH)at 0℃.The reaction mixture was stirred for 2hat ice bath.After standing overnight,the resulting solid was collected by filtration,washed with water and recrystallized from ethanol to give pale yellow solid 3in 85%yield;M.p.:135-136℃;1H NMR(400MHz,CDCl3)δ=8.42(s,1H,quinolin-H4),8.15(d,1H,J=15.6Hz,Hβ),7.96(d,1H,J=15.6Hz,Hα),8.02-6.67(m,14H,Ar-H);IR(KBr):ν=1 659(C=O),1 609(C=N),760(C-S-C)cm-1;MS:m/z=366(M+),336,262,227,171,151,125.
1.4 The synthesis of 3-phenyl-5-(2-phenylthio-quinolin-3-yl)-1-thiocarbamoyl-pyrazoline(4)
A mixture of the appropriate 3(5mmol)and thiosemicarbazide(6mmol)in EtOH(50ml)was stirred and heated under reflux for 0.5h.After dissolution of the reactants,a solution of KOH(12.5mmol)in water(5ml)was added dropwise.The solution was refluxed for a further 4h,then poured into crushed ice,the formed solid product was filtered off,washed with ethanol,dried,and then crystallized from ethanol to give white solid 4(94%).M.p.:198.8-199.6℃;1H NMR(400MHz,CDCl3)δ=7.88(s,1H,quinolin-H4),7.22-7.59(16H,m,Ar-H and NH2),6.46(dd,1H,Hx,Jax=12.0Hz,Jbx=6.4Hz),3.99(dd,1H,Ha,Jax=12.0Hz,Jab=17.6Hz),3.38(dd,1H,Hb,Jbx=6.4Hz,Jab=17.6Hz);IR(KBr):ν=3 412,3 264(N-H),3 031(Ar-H),1 613,1 513,1 473(C=N,C=C),1 361(C=S)cm-1;MS:m/z=336(M+),262,227,200,171,108.
1.5 The synthesis of 3-phenyl-5-(2-phenylthio-quinolin-3-yl)-1-(4-(4-methoxyphenyl)-2-thiazoyl)-pyrazoline(5)
A mixture of 4(0.5mmol)and 2-bromo-1-phenyl-ethanone(0.5mmol)in ethanol(30ml)was stirred and refluxed for 1h.Upon standing the reaction mixture at room temperature for 3h.The precipitate was filtered off and recrystallized from anhydrous ethanol and benzene to give the title compound 5.M.p.:241.5-242.6;IR(KBr):ν=3 032(Ar-H),1 646,1 576,1 474(C=N,C=C),763(CS-C)cm-1;1H NMR(400MHz,CDCl3)δ=6.15-7.85(20H,m,Ar-H),6.16(dd,1H,Hx,Jax=11.04Hz,Jbx=4.98Hz),4.03(dd,1H,Ha,Jax=11.04Hz,Jab=16.79Hz),3.74(3H,s,CH3O),3.30(dd,1H,Hb,Jbx=4.98Hz,Jab=16.79Hz);MS:m/z=570(M+),465,304,262,236,163,127,77,57.
1.6 Crystal data and structure determination
A single crystal suitable for X-ray diffraction study was cultivated in a test tube from CH2Cl2-CH3CH2OH mixture by a slow evaporaion method at room temperature.A single crystal of the title compound having approximate dimensions of 0.399mm×0.368mm×0.256mm was mounted on a Bruker CCD diffractometer equipped with a graphite-monochromatic MoKα(λ=0.71073Å)radiation using anωscan mode at 296(2)K.In the range of 1.77<2θ<55.04,a total of 23 251reflections were collected and 6 397were unique with Rint=0.034,of which 4 331were observed with I>2σ(I)and used in the structure determination and refinements.The structure was solved by direct methods with SHELX97and refined by SHELXL97[24-25].All non-hydrogen atoms were refined with anisotropic thermal parameters.Other H atoms were placed in the calculated positions.A full-matrix least-squares refinement gave the final R=0.0463and wR=0.1758,S=1.05,(Δρ)max=0.26and(Δρ)min=-0.24e/Å3.
CCDC-805818contains the supplementary crystallographic data for this paper.These data can be obtained free of charge at http://www.ccdc.cam.ac.uk/conts/retrieving.html.
2 Results and disussion
The synthetic pathway for the target compound is outlined in Scheme 1.
Compound 2was prepared by the reaction of the benzenethiol with 1in dimethyl sulfoxide under refluxing.Furthermore,at the room tempature,compound 2was subsequently treated with phenylethanone in the presence of potassium hydroxide in ethanol afforded smoothly the(E)-3-(2-Phenylthio-quinolin-3-yl)-1-phenyl-2-propen-1-one 3.Then,3-phenyl-5-(2-phenylthio-quinolin-3-yl)-1-thiocarbamoyl-pyrazoline 4was synthesized by the reaction ofα,β-unsaturated ketone 3and thiosemicarbazide in refluxing ethanol.Finally,the title compound 5was prepared by condensation of pyrazoline 4with1-(4-methoxylphenyl)-2-bromoethanone.The1H NMR,MS and elemental analysis for the product are in good agreement with the title compound.The IR spectra of 3exhibit C=O stretching 1 659cm-1.In the1H NMR spectra,the quinoline-H4were observed as doublets of doublets at 8.42ppm,the protons belonging to the aromatic ring were seen atδ8.02-6.67as multiplet signals.The protons ofα,β-unsaturated carbonyl compounds appears as two doublets at 8.16ppm for Hαand 7.96ppm for Hβwith 15.6Hz as coupling constant,which supports E-form geometrically stereochemical isomer.The IR spectra of compound 4showed the stretching band of N-H and C=S at 3 412,3 264cm-1and 1 361cm-1.The IR spectra of 5showed strong stretching vibration at 1 576cm-1due to the presence of C=N and sharp absorption,and strong absorption at 763cm-1for C-S-C single bond.In addition,the1HNMR spectrum of compound 5,a characteristic singlet of the quinoline-H4is recorded atδ7.86cm-1.Three distinct of doublets of doublets atδ3.30ppm(Ha),δ4.03ppm(Hb)andδ6.16ppm(Hx)are attributed to the CH2(Ha,Hb)and CH(Hx)protons of the ABX system in the pyrazoline ring.The mass spectrum of the titled compound revealed the existence of the molecular ion peaks and significant fragmentat ion peaks,which is strong evidence for the structure.
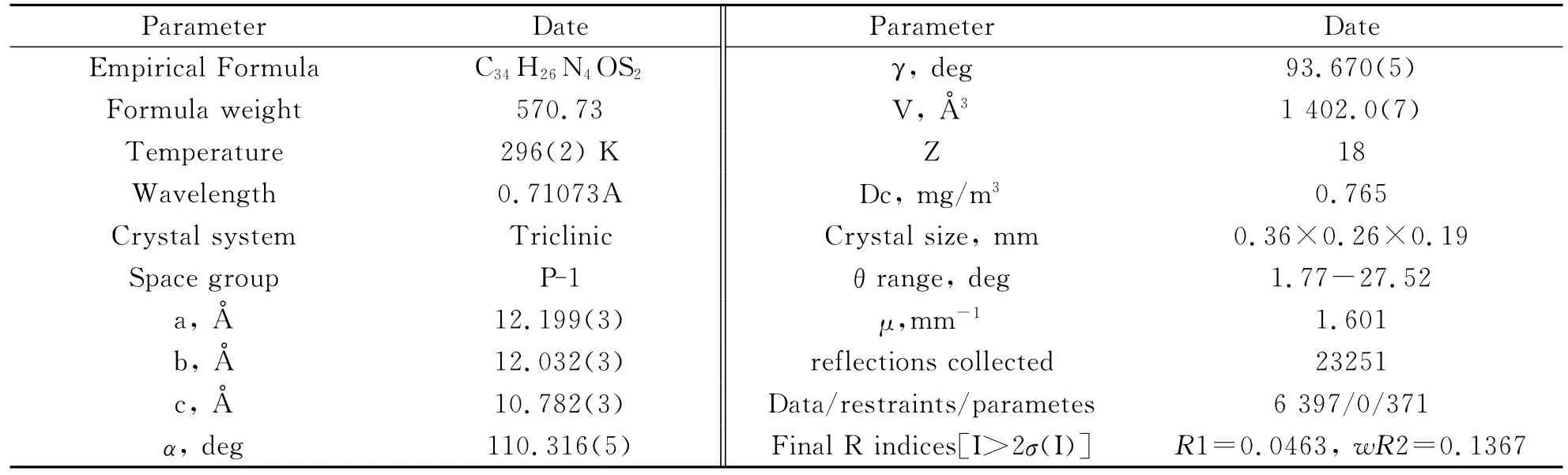
Tab.1 Crystal data and structure refinement for compound 5
In order to confirm the configuration of the product,a single crystal of the title compound was cultured for X-ray diffraction analysis.Crystal data and refinement details for the structure determination are presented in Tab.1.The selected bond lengths and bond angles are listed in Tab.2 and the hydrogen bond geometry in Tab.3.The C-H…πstacking interactions in the crystal are given in Tab.4.The molecular structure of the title compound with atomic numbering scheme is shown in Fig.1 and Fig.2depicts the molecular packing in the unit cell.
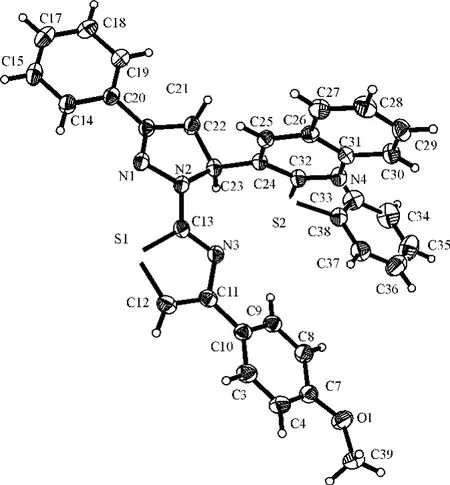
Fig.1 The molecular structure of the title compound
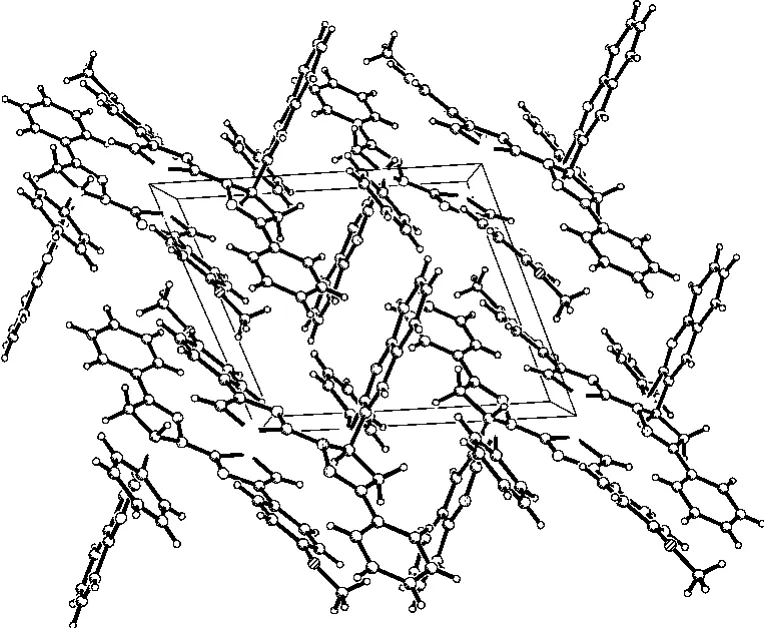
Fig.2 The molecular packing in the unit cell
The five-membered ring of pyrazoline shows an envelope conformation,which is almost planar,however,the attached C(9)atom slightly deviates(0.065Å)from the ring plane.The dihedral angle between the pyrazoline ring and the C(14)~C(20)phenyl ring is 12.36°,confirming that these two planes are almost planar.However,The pyrazoline ring is twisted to the quinoline ring,making a dihedral angle of 80.86°.The thiazole ring is almost planar to the pyrazoline ring and the plane of C(3),C(4),C(7),C(8),C(9)and C(10),and they form a dihedral angle of 16.01°and 21.67°,respectively.
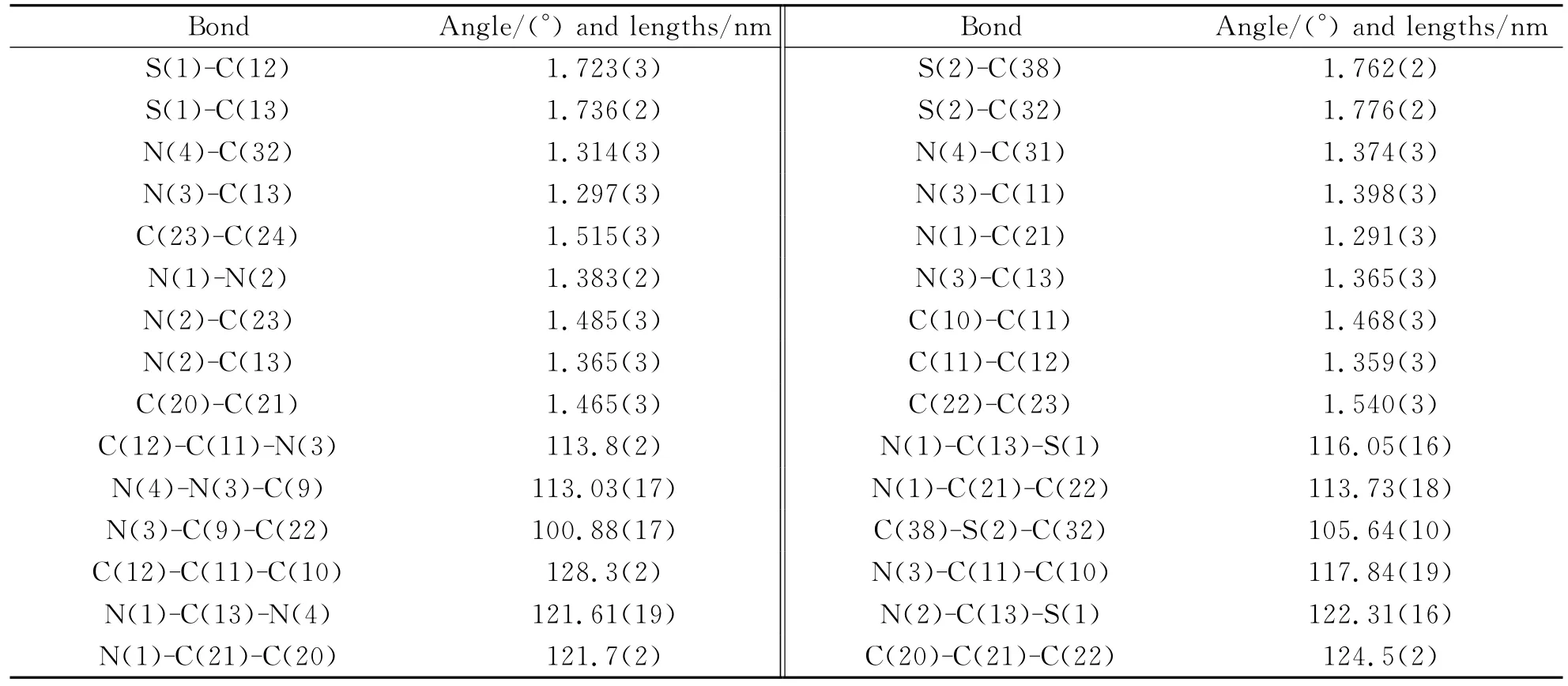
Tab 2. Selected bond lengths(Å)and angels(°)of compounds
Within the molecule,the C(13)-N(3)(1.365(3)Å)bonds are remarkably shorter than the normal N-C bond(1.47Å),but close to the typical C=N bond(1.33Å),whichreveals the existence of a typical conjugated system.At the same time,the sum of C(9)-C(11)-N(3),C(12)-C(11)-N(3)and C(9)-C(11)-C(12)bond angles is 359.98°,showing that atoms C(11)is of sp2hybridization.

Tab.3 Hydrogen bonded geometry,distances and angles are given in(Å)and(°)
X-ray crystal structure determination indicates that there are two independent molecules in the unit.There exist kinds of hydrogen bonding interactions in the crystal structure of complex.The C(9)--H(9)..S(2)and C(18)--H(18)..N(1)are intramolecular hydrogen-bonding interactions.Furthermore,the p…πstacking interactions between the neighboring molecules are also observed(Fig.2and Table 4).The C(9)atoms are involved in p…πinteractions with the phenyl ring(centroid Cg2,C3,C4,C7,C8,C9and C10,symmetry code:-X,1-Y,-Z)and the C(12)atoms with the pyrazoline ring(centroid Cg2,symmetry code:X,Y Z)which increase ulteriorly the stability of the 3Dsupramolecular architecture of the polymer.

Tab.4 C-H...πHydrogen Bonds for the Title Compound(Å,°)
[1]Baruah B,Bhuyan P.Synthesis of some complex pyrano[2,3-b]-and pyrido[2,3-b]quinolines from simple acetanilides via intramolecular domino hetero Diels-Alder reactions of-oxa-1,3-butadienes in aqueous medium[J].Tetrahedron,2009,65:7099-7104.
[2]Allahyari R,Strother A,Fraser I M,et al.Synthesis of certain hydroxy analogues of the antimalarial drug primaquine and their in vitro methemoglobin-producing and glutathione-depleting activity in human erythrocytes[J].Med Chem,1984,27:407-410.
[3]Miller D S,Laszlo J.Mechanism of action of streptonigrin in leukemic cells[J].Cancer Res,1967,27:632-638.
[4]Wu Du.Towards new anticancer drugs:a decade of advances in synthesis of campto thecins and related alkaloids[J].Tetrahedron, 2003,59:8649-8687.
[5]Azad M,Munawar M A,Siddiqui H L.Antimicrobial activity and synthesis of quinoline-based chalcones[J].J Appl Sci,2007(7):2485-2489.
[6]Chen Yehlong,Fang Kuochang,Sheu Jiayuh,et al.Synthesis and antibacterial evaluation of certain quinolone derivatives[J].Med Chem,2001,44:2374-2377.
[7]Tseng C H,Tzeng C C.Synthesis and antiproliferative evaluation of certain indeno[1,2-c]quinoline derivatives[J].Bio Med Chem,2008,16(6):3153-3162.
[8]Sona S S,Sadaphal S A.Synthesis and antibacterial screening of new 4-((5-(difluoromethoxy)-1H-benzo[d]imidazol-2-ylthio)methyl)tetrazolo[1,5-a]quinoline derivatives[J].Het Chem,2010,47:441-445.
[9]Klingenstein R,Melnyk P.Similar structure activity relationships of quinoline derivatives for antiprion and antimalarial effects[J].Med Chem,2006,49:5300-5308.
[10]Suzuki T,Fukazawa N.Structure-activity relationship of newly synthesized quinoline derivatives for reversal of multidrug resistance in cancer[J].Med Chem,1997,40:2047-2052.
[11]Nasr M N A,Said S A.Novel 3,3a,4,5,6,7-hexahydroindazole and arylthiazolylpyrazoline deriva-tives as anti-inflammatory agents[J].Arch der Pharm,2003,336:551-559.
[12]Turan Z G,Chevallet P.Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity[J].Eur Med Chem,2000,35:635-641.
[13]Turan Z G,Ozdemir A.Synthesis and antimicrobial activities of some 1-[(N,N-thiocarbamo ylthio)acetyl]-3,5-diaryl-2-pyrazolines[J].Phosphorous Sulfur Silicon Relat Elem,2005,180:2717-2724.
[14]Kaplancikli Z A,Turan Z G,Ozdemir A.Synthesis and antimicrobial activity of some thiazolyl-pyrazoline derivatives[J].Phosphorous Sulfur Silicon Relat Elem,2007,182:749-764.
[15]Gans K R,Galbraith W.Anti-inflammatory and safety profile of DuP 697,a novel orally effective prostaglandin synthesis inhibitor[J].Pharm Exp Ther,1990,254:180-187.
[16]El-Sabbagh O I,Baraka M M.Synthesis and antiviral activity of new pyrazole and thiazole derivatives[J].Eur Med Chem,2009,44:3746-2753.
[17]Crute J J,Grygon C A.Herpes simplex virus helicase-primase inhibitors are active in animal models of human disease[J].Nat Med,2002(8):386-391.
[18]Ashtekar D R,Fernandes F.A rapid method for the evaluation of new antituberculous agents[J].Chemotherapy,1987,33:22-27.
[19]Sakamoto T,Cullen M D.Synthesis and anti-HIV activity of new metabolically stable alkenyldiarylmethane non-nucleoside reverse transcriptase inhibitors incorporating N-methoxy imidoyl halide and 1,2,4-oxadiazole systems[J].Med Chem,2007,50:3314-3321.
[20]Gupta R R,Kumar M,Gupta V.Heterocyclic chemistry of five membered heterocycles[M].Berlin,Heidelber,New York:Springer-Verlag,1999:416.
[21]Abdel-Wahab B F,Abdel-Aziz H A,Ahmed E M.Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles[J].Eur Med Chem,2009,44:2632-2635.
[22]Ozdemir A,Turan Z G.Synthesis and antimicrobial activity of 1-(4-aryl-2-thiazolyl)-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives[J].Eur Med Chem,2007,42:403-409.
[23]Devi I,Baruah B.α-cyclisation of tertiary amines:synthesis of some novel annelated quinolines via a three-component reaction under solvent-free conditions[J].Syn lett,2006(16):2593-2596.
[24]Sheldrick G M,SHELXTL.Structure Determination Software Suite.Version 5.0[CP].Siemens Industrial Automation,Analytical Instrumentation,USA,1995.
[25]Siemens,XSCANS and SHELXTL(Version 5.0)[CP].Siemens Analytical X-ray Instruments Inc.,Madison,Wisconsin,USA 1995.
1-(4-甲氧基苯-2-噻唑基)-3-苯基-5-(2-苯硫基-3-喹啉基)-2-吡唑啉的合成与表征
曾永明,刘方明
(杭州师范大学材化学院,浙江杭州310036)
合成了目标化合物1-(4-甲氧基苯-2-噻唑基)-3-苯基-5-(2-苯硫基-3-喹啉基)-2-吡唑啉,并利用单晶X射线衍射法测定了它的晶体结构.另外,其结构经IR,1H NMR和MS及元素分析确证.
喹啉;吡唑啉;噻唑;晶体结构;合成
10.3969/j.issn.1674-232X.2012.02.007
O621.3Article characterA
1674-232X(2012)02-0126-06
Received date:2011-07-06
LIU Fang-ming(1966—),male,professor,engaged in synthesis of heterocyclic compound.E-mail:fmliuf859@sohu.com

