在光阻材料中过滤器吸附机制的作用
Tetsu Kohyama
(ENTEGRIS,INC.,BillericaMA 01821,USA)
As semiconductormanufacturerswork to realize shrinking features,increasing importance is placed on strict control of particle and metal concentrations in process chemicals these contam inants ultimately a ffect yield and reliability.Historically,point-of-use filters have been used to m itigate the effects of particles and metal contam inants.However,some of the components in a solution are adsorbed by the filter membrane,which can either improve or reduce yield.In general,some volumemust be passed through the filter membrane to reach the equilibrium where the concentration of contaminants in the filtrate becomes the same as the one of the challenge.Most of the photoresists generally contain non-ionic fluorochemical surfactants as leveling agents. The concentrations of these surfactants are so low,only a few tens of parts-per-billion,that its change can cause the deterioration of the leveling properties.Moreover,polytetrafluoroethylene (PTFE)membranes absorb a lot of fluorochem ical surfactants because of their high affinities.Therefore,it is important to pay attention to the initial volume filtered through a membrane and make sure that steady state has been reached.On the other hand,adsorption can have a positive impact on yield because certain kinds of small particles,for example,ionic soft gel particles,can be captured only by adsorption mechanisms in some photoresist applications.In those cases,nylon filters can bemore effective than other filters for particle removal due to their unique adsorption property.But there is little understanding of how nylon filters work;what role does the adsorption play in nylon filtration?It is essential to understand the adsorption principle in nylon filtration before implementing the filter in appropriate applications.This report introduces our recentworks on a nylon adsorption study and tries to answer some of the existing questions regarding nylon filtration.
1 W hat is the Adsorption Equilibrium[1]?
Understanding of the concept of the equilibrium is required to figure out the adsorption phenomenon in the liquid filtration.Generally,the attachment of matters to a surface is called adsorption.Here the terms are defined.The capturing substance is called adsorbent and the adsorbed matter is called adsorbate(Figure 1).The reverse of adsorption is desorption.At the initial stage of the process,the adsorption of the matter to the surface is greater than the desorption of the matter from the surface.The concentration of the matter in liquid decreases.At one point,the adsorption rate becomes equal to the desorption rate.At this point,the adsorption and desorption have reached an equilibrium.The concentration of thematter in liquid w ill keep steady afterward.

Figure 1Image of the adsorption equilibrium in liquid.
2 Two Factors'Effect on Liquid Phase Adsorption[1]
In the equilibrium in the liquid phase,the quantity of the adsorption depends on the concentration of the matters and the temperature in the solution.The data[2]illustrates that the concentrations and the temperature have an effect on the adsorption amount for the active carbon in an aqueous solution.As the concentration of propionic acids increase,the adsorption amount increases.That is because there ismuch adhesion to activate carbon due to a lot of propionic acids.On the other hand,when temperature increases,the desorption rate increases so that it can cause the quantity of adsorption to reduce.Thus,as the temperature becomes higher,the adsorption amount usually decreases.
3 Classification of the Adsorption[3]
Table Ishows that the adsorption phenomenon is classified w ith enthalpy,the behavior of the heating and adsorption mechanism.Enthalpy is generally divided into two classifications:“Physical Adsorption”and“Chemical Adsorption(Chem isorption).”In addition,the behavior of the adsorbates can be defined as“Reversible,”“Associate Reversible(Quasireversible)”or“Irreversible.”But as for the accurate classification of these two phenomena,the criteria are so vague that they cannot be accurately expressed.Therefore,the interaction was generally sorted by adsorptionmechanism,and itwasadopted in this report.
There are four understoodmechanismsof adsorption.They include Intermolecular Force (Van Der Waals Force),Hydrogen Bonding (including Hydrophilic Interaction/Hydrophobic Interaction),Electrostatic Interaction (Electrostatic Force)and other chemical reactions.Table Ishows the classification of adsorption mechanisms.The mechanisms are ranked from the least effective to themost effective adsorption.Thus,“Intermolecular Force”is theweakestand“Others”the strongest.In addition,the table describes the correlationsw ith thegroup in each column.Forexample,“Intermolecular Force”is equivalent to“Reversible Adsorption”at the thermal behavior and“PhysicalAdsorption”at the division of the enthalpy.
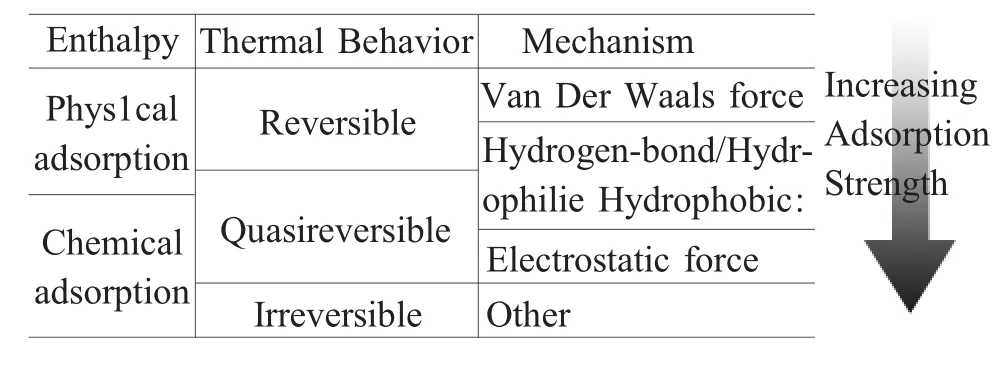
Table 1Classification adsorption
4 Experiment
4.1 Test Procedure
ACYLATION OF TERM INAL AM INE
GROUP ON NYLON MEMBRANES
Nylon membrane discs w ith 47mm diameter were soaked in Tetrahydrofuran(THF)of 40m L in a Perfluoroalkoxy (PFA)vessel.An acylating agent of 100uL of trifluoroacetyl imidazole was added to the vesseland left in a static condition atambient temperature for 24hours.THFwas removed from the vessel and the membranes were rinsed w ith isopropyl alcohol(IPA)three times.The membranes were air-dried and acylated nylonmembraneswere prepared.
SINGLE-PASS FILTRATION TESTING
The test setup for a single-pass filtration test is shown in Figure 2.A 47mm disk coupon was placed in themembrane holder and propylene glycol mono-methyl ether acetate (PGMEA)w ith triethanolam ine of 60×10-6was passed through the membrane.The concentration before and after filtration was measured by gas chromatography-mass spectroscopy (GC-MS).The testing was implemented at 20kPa under room temperature.
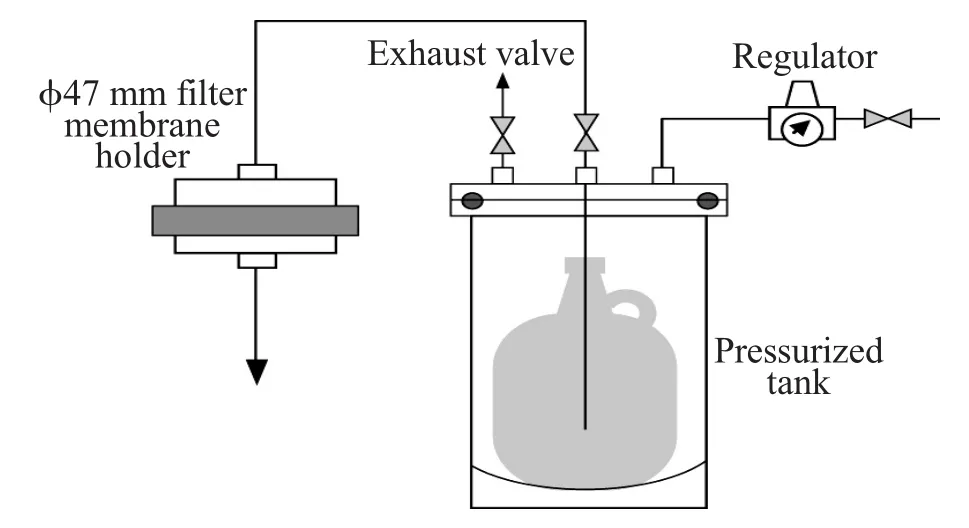
Figure 2Test setup.
Figure 3shows another test setup.Two 47mm diskswere placed in the holder.An aqueous solution or PGMEA involving sodium benzoate of 1.0×10-6was filtered through the membrane.The concentration before and after filtration was measured by Ion-Chromatography.The testing was done at 10m L/m in.at room temperature.
4.2 Soaking testing
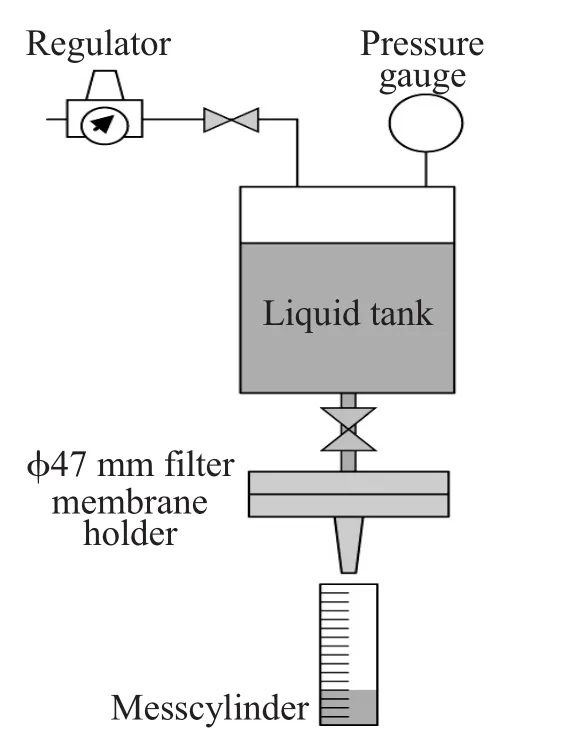
Figure 3Test setup.
The procedure was to soak 47mm coupon disks in a solventat room temperature and themeasurement was done after the adsorption equilibrium.The testing about the PAG was to put the two coupons in a 20m L solventm ixture (propylene glycolmono methyl ether[PGME]:PGMEA=7:3)including 50×10-6of triphenysulfonium perfluoro-1-butanesulfonate.The concentration wasmeasured by high performance liquid chromatography(HPLC)-monitor wavelength at 268nm.In Figure 8,a coupon was immersed in the ArF photoresist solution (25.5%polymers in PGMEA).After that,the sample was washed w ith PGMEA many times to thoroughly remove adherent polymers.Itwas then analyzed by Fourier Transform Infrared Spectroscopy (FT-IR)to investigate what was adsorbed.The anionic polymer Eudragit1100shown in Figure 4was dissolved in PGMEA w ith trace amounts of methanol and water.Five discs of nylon membrane were soaked in the solution overnight.The membrane samples were w ithdrawn and thoroughly cleaned by methanol.The disc samples were then soaked in 0.1N HCI/IPA overnight.Five nylon membrane discs were extracted in 0.1N HCI/IPA only as a control.The extract solutionswere analyzed by FT-IR after pre-concentration.
5 Results and Discussion
Estimation of the Components'Effect on Yields
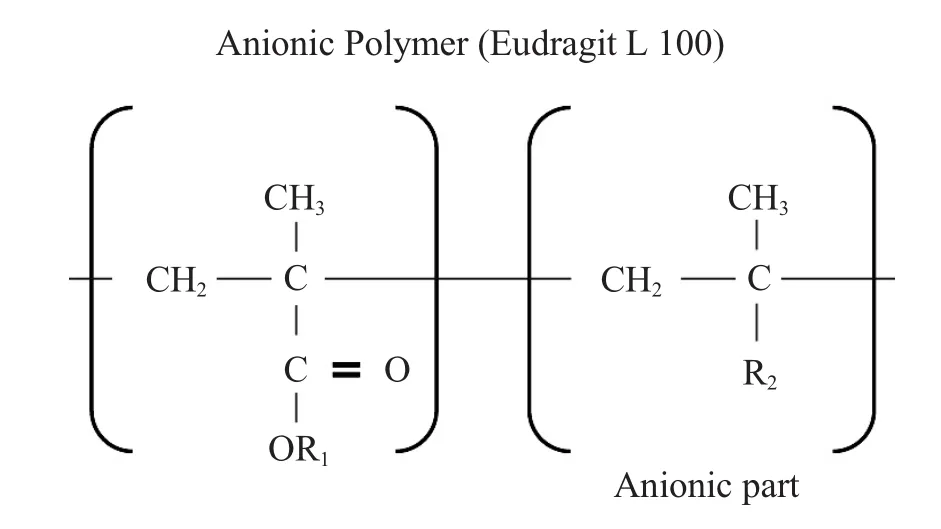
Figure 4Chemicalstructure ofanionic polymer Eudragit L 100.
Figure 5represents the typical components of a photoresist solution.Nylon filters are w idely used for the filtration or purification for photoresist solutions,and adsorption of resist components can occur.That's because nylonmolecules can work as an ion-exchangerdue to both cation and anion groups on the ends of molecules.In addition,the repetition of the amide structure also gives itshydrophilic property.Therefore,ithappens that the hydrophilic or the ionicmaterials in PGMEA and cyclohexanone can adhere to nylon.

Figure 5Representative components of a photoresist solution.
In this experiment,each nylon and ultra-highmolecular-weight polyethylene(UPE)membrane was soaked in the solventm ixture(PGME:PGMEA=7:3)w ith 50×10-6PAG.The PAG's concentration was measured after adsorption reached the equilibrium.The result shows that the concentrations of both solutions don't decrease significantly (Figure 6).Since there is PAG of about (1000~2000)×10-6in the products,it is thought that the adsorption doesn't have a significant influence.
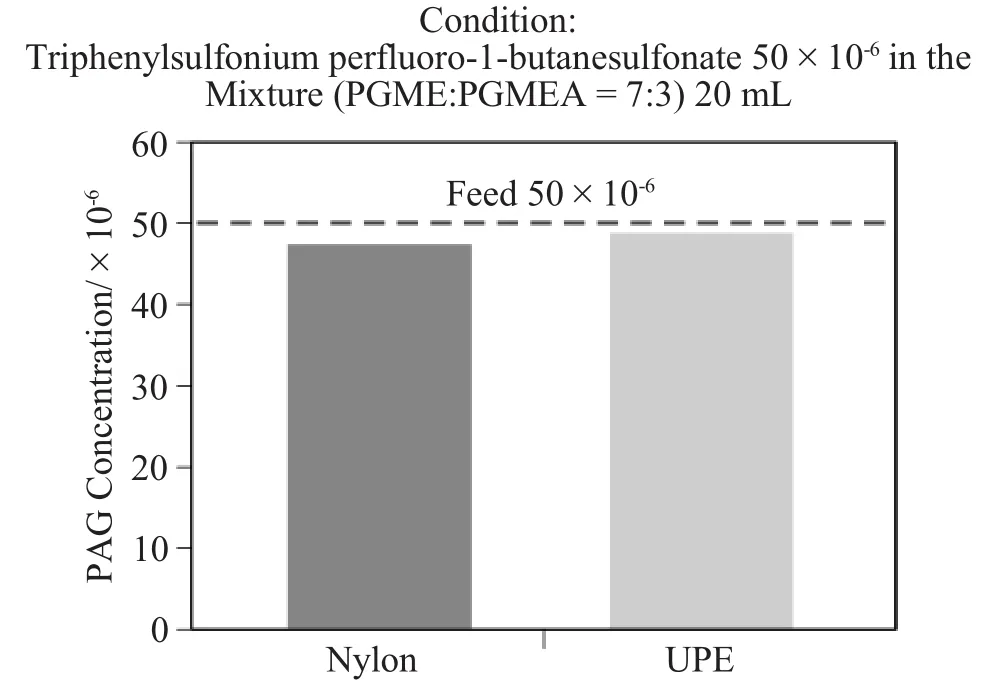
Figure 6Adsorption testing of the PAG on nylon and UPE membranes.
On theotherhand,the am ine quencheradsorption isn't confirmed on the UPEmembranes,but itsadsorption occurred w ith nylonmembranes(Figure 7).Ultimately,thismeans that additional purging through a nylon membrane is required before equilibrium is reached.This increases the costofwasted chemistry.
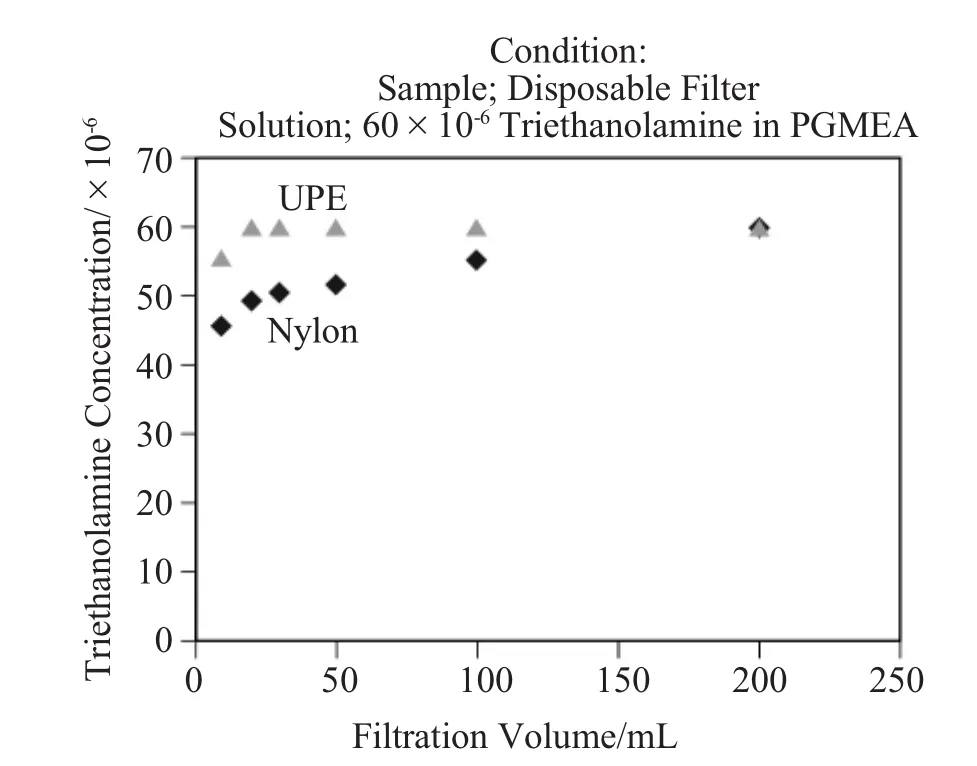
Figure 7Adsorption testing of the quencher on nylon and UPEmembranes.
In addition,it is possible for adsorption of some of the resist polymers on nylon filters to occur because of the partial formation of anionic polymers by hydrolysis of branch units.Figures 8and 9show that the resist polymers remained on the membrane surface,although the samples were flushed several times w ith PGMEA.Thus,the nylon membrane captured some of the anionic polymers,although it is difficult to tell how much of the polymer was trapped.
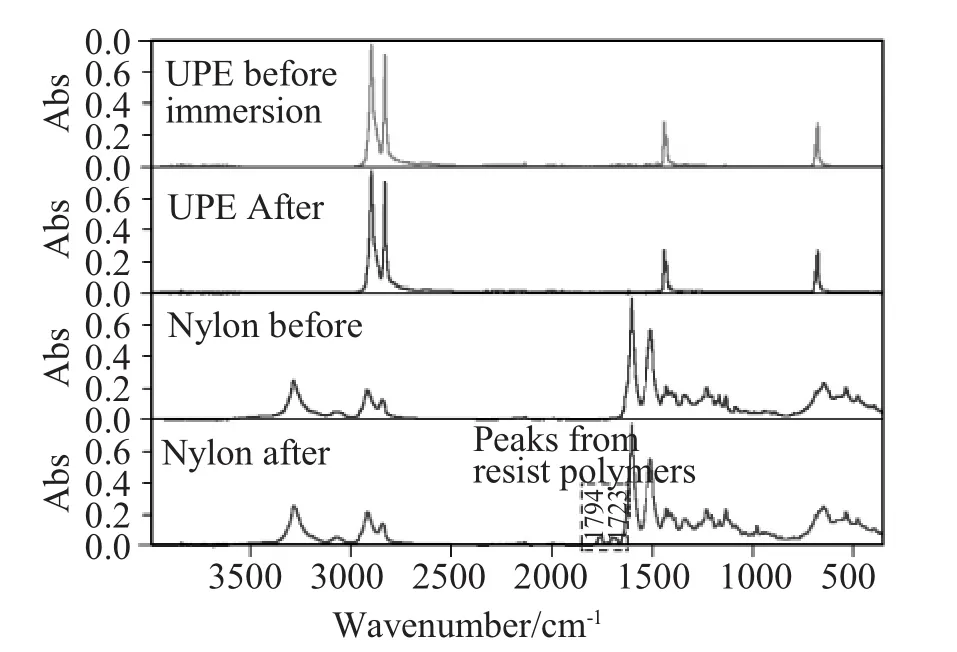
Figure 8Adsorption testing of ArF resist polymers on nylon and UPEmembranes.
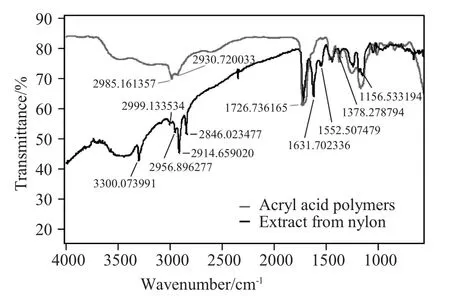
Figure 9The spectrum of the extract from nylon membranes after the soaking in the anionic polymer Eudragit L100.
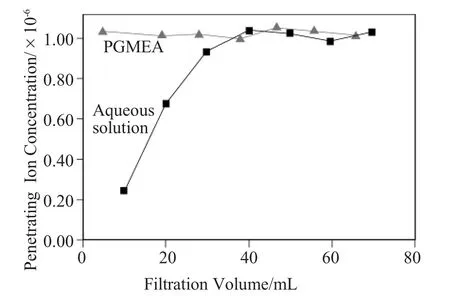
Figure 10Adsorption testing of sodium benzoate on nylon membranes in an aqueous solution and PGMEA.
This adsorption mechanism is related to ion exchange interactions,which the follow ing two experi-ments proved.Figure 10shows totally different results between an aqueous solution and PGMEA.Sodium benzoate dissolved in an aqueous solution exists as an anion form.However,it is insoluble in PGMEA due to its low dielectric constant[4].In addition,acylation of the term inal am ine group reduces the capability to capture the anion in an aqueous solution,which means that the am ine group is effective to remove the anion groups (Figure 11).Thus,the electrostatic forces between the amine group and Benzoate anion can work more effectively in an aqueous solution than in PGMEA.

Figure 11Adsorption testing of sodium benzoate on nylon,acylated nylon and UPEmembranes in an aqueous solution.
The above experimental results show that nylon can capture ionic matters based on adsorption.It is generally believed that some impurities and particles(especially,gel-like particles)in some photoresists are ionic matters.Therefore,nylon filters would remove such impurities in the photoresist,which could lead to improved yields.Figure 12illustrates the adsorption events when the photoresist w ith various components passes through nylon filters.
6 Conclusion
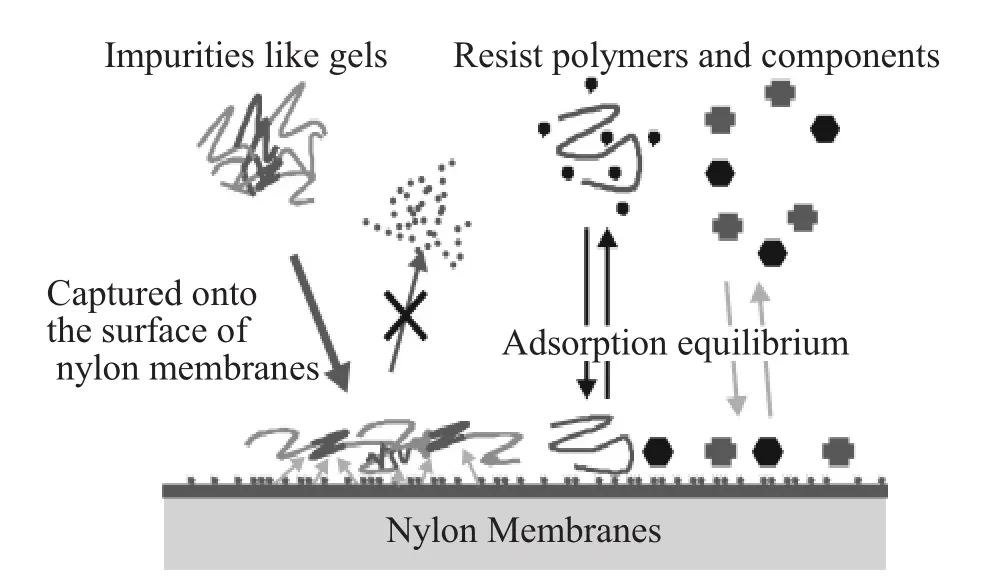
Figure 12Schematic image of the component adsorption in photoresists on nylon membranes.
In conclusion,adsorption behavior depends on the affinity of thematerials.For example,in the case of the photoresists'adsorption,there is a significant difference between nylon and UPE membranes.Therefore,it isnecessary to choose filters in consideration of the adsorption when the solutions are chosen.In addition,initial prim ing conditionsmay require increased attention in order to reach equilibrium and avoid wasted expensive chem istry.In the future,it is assumed that the understanding of the adsorption w ill becomemore important,as there is an increasing demand for control of particles,metals and impurities.There is stilla significantamountof uncertainty about how trace amounts of impurities affect yield,so additional studies aboutadsorption properties are required.
7 Acknow ledgements
Iwould like to thank several resist vendors who provided samples for testing utilized in this study.I also would like to thank Y.Shimada,M.Amari,H.Zhang and J.Braggin of Entegris,Inc.for useful discussions and support.
:
[1]Seiichi,K.,Tatsuo,I.and Ikuo A.Chem istry of the Adsorption[M].Maruzen Publishing Co.,Ltd,Nihonbashi,1-30(1991).
[2]Yuzo,S.,Motoyuki,S.and Kaoru,F.Activate Carbon[Z].Kodansha Scientific Ltd,Shinjyuku,77(2000).
[3]Israelachvili,J.N.An Intermolecular Force and Surface Power[Z].MacGraw-HillCompanies,Ginza,Part I(1991).
[4]Israelachvili,J.N.An Intermolecular Force and Surface Power[Z].MacGraw-Hill Companies,Ginza,30(1991).

