The Synthesis of the Thiazolyl-Pyrazoline Derivatives with 1,2,3-Triazole Moiety
CHEN Fei, LIU Fang-ming, CHEN Sen-lin, DONG Zhi-qiang
(College of Materials and Chemical Engineering, Hangzhou Normal University, Hangzhou 310036, China)
The Synthesis of the Thiazolyl-Pyrazoline Derivatives with 1,2,3-Triazole Moiety
CHEN Fei, LIU Fang-ming, CHEN Sen-lin, DONG Zhi-qiang
(College of Materials and Chemical Engineering, Hangzhou Normal University, Hangzhou 310036, China)
Novel pyrazoline derivatives with 1,2,3-triazole and thiazole moiety were synthesized by the cyclization of 3,5-diphenyl-1-thiocarbamoylpytazolines(1), and 2-bromo-1-(2-phenyl-1,2,3-triazol-4-yl) ethanone (2) in ethanol. The structure of these compounds was confirmed with IR,1H NMR, mass spectral and elemental analysis.
1,2,3-triazoles; pyrazoline; thiazole
0 Introduction
In recent years, interest in the nitrogen- and sulfur-containing heterocyclic compounds significatively increased mainly due to their versatile agricultural and biological activities. Pyrazoline derivatives have been proven to show a broad spectrum of biological activities including fungicidal, insecticidal, antiproliferative and Cannabinoid receptor antagonists[1-4]. In addition, the 1,2,3-triazole motif makes up the core structure of some compounds with pharmaceutical activities such as anti-influenza, antibacterial, treating cryptosporidiosis and Gaucher disease[5-8]. Furthermore, the thiazole moiety is present in a number of drugs as a pharmacophore, and there are numerous examples of biological activities in the literature including antimicrobial, insecticidal, antibacterial and antiviral activity[9-12].
In our previous papers[13-14], we reported a series of novel pyrazoline derivatives with both thiazole and 1,2,3-triazole in the molecule with a view to obtain higher bioactivity leading compounds. In this publication, we tried to replace the 1-aryl-2-bromoethanones with 2-bromo-1-(2-phenyl-1,2,3-triazol-4-yl) ethanone and synthesized some new thiazolyl-pyrazoline derivatives bearing 1,2,3-triazole moiety, 2-(3,5-diaryl-4,5-dihydro-1H-pyrazol-1-yl)-4-[2H-(2-phenyl-1,2,3-triazol)-4-yl] thiazoles . The synthetic route is shown in Scheme 1.
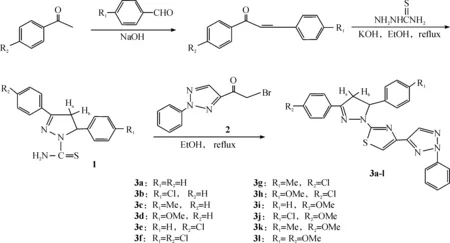
Scheme 1 The synthesis route of title compounds
1 Experimental
1.1 Materials and synthesis
All the solvents and materials were of reagent grade and purified as required. Reactions were monitored by TLC. Melting points were determined by use of a Mettler FP-5 melting point apparatus and are uncorrected. IR spectra were recorded as KBr pellets on a Bruker Equinox 55 FT-IR spectrophotometer.1H NMR spectra were recorded on a Bruker 400 MHz spectrometer using TMS as internal reference and CDCl3as solvent. Mass spectra were acquired with an Agilent 5975 Instrument (EI, 70 eV).
1.2 The synthesis of 3,5-diphenyl-1-thiocarbamoylpyrazoline (1)
Corresponding chalcone[15](7 mmol) and thiosemicarbazide (0.764 g, 8.4 mmol) was refluxed in ethanol (40 mL). After dissolution of the reactants, a solution of KOH (12.5 mmol) in water (5 mL) was added dropwise. The solution was refluxed for a further 4 h. The reaction mixture was allowed to cool, poured into crushed ice, and the solid mass separated out was filtered, washed with cold ethanol, dried, and crystallized from ethanol/water.
1.3 The synthesis of 2-(3,5-diaryl-4,5-dihydro-1H-pyrazol-1-yl)-4-[2H-(2-phenyl-1,2,3-triazol)-4-yl] thiazoles (3a-3l)
A mixture of 2-bromo-1-(2-phenyl-1,2,3-triazol-4-yl) ethanones 2 (1 mmol) and equivalent amounts of 3,5-diphenyl-1-thiocarbamoylpyrazoline1was refluxed in anhydrous ethanol (20 mL). After completion of the reaction (monitored by TLC), the mixture was allowed to cool. Then the solid product was collected. The product was recrystallized from DMF/ethanol.
3a: Yield 82%, m.p. : 191~192 °C. Yellow solid, IR, ν/cm-l: 2808, 2715 (CH2, CH), 1632, 1596, 1495 (C=N, C=C), 760 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.88 (s, 1H, 1,2,3-triazole-H), 8.08~6.98 (m, 15H, Ar-H), 7.15(s, 1H, thiazole-H), 5.71 (dd, 1H, Hx, Jax= 6.4Hz, Jbx= 11.9Hz), 3.93 (dd, 1H, Hb, Jbx= 11.9Hz, Jab= 17.2Hz), 3.33 (dd, 1H, Ha, Jax= 6.4Hz, Jab= 17.2Hz); MS (EI) m/z (%): 448 (M+), 344, 242, 103, 91, 77; Analyses (%), C26H20N6S: C, 69.62; H, 4.49; N, 18.74; S, 7.15; Found: C, 69.64; H, 4.48; N, 18.75; S, 7.15.
3b: Yield 86%, m.p. : 165~166 °C. Yellow solid, IR, ν/cm-l: 2856, 2808, 2713 (CH3, CH2, CH), 1631, 1596, 1496 (C=N, C=C), 756 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.88 (s, 1H, 1,2,3-triazole-H), 8.08~6.88 (m, 14H, Ar-H), 7.16 (s, 1H, thiazole-H), 5.71 (dd, 1H, Hx, Jax= 6.4Hz, Jbx= 11.7Hz), 3.95 (dd, 1H, Hb, Jbx= 11.7Hz, Jab= 17.4Hz), 3.34 (dd, 1H, Ha, Jax= 6.4Hz, Jab= 17.4Hz); MS (EI) m/z (%): 482 (M+), 374, 242, 138, 103, 91, 77; Analyses (%), C26H19ClN6S: C, 64.66; H, 3.97; Cl, 7.34; N, 17.40; S, 6.63; Found: C, 64.65; H, 3.97; Cl, 7.35; N, 17.42; S, 6.62.
3c: Yield 81%, m.p. : 158~159 °C. Yellow solid, IR, ν/cm-l: 2808, 2712 (CH2, CH), 1632, 1596, 1498 (C=N, C=C), 756 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.93 (s, 1H, 1,2,3-triazole-H), 8.09~6.94 (m, 14H, Ar-H), 7.13 (s, 1H, thiazole-H), 5.66 (dd, 1H, Hx, Jax= 6.2Hz, Jbx= 11.9Hz), 3.88 (dd, 1H, Ha, Jax= 6.2Hz, Jab= 17.2Hz), 3.32 (dd, 1H, Hb, Jbx= 11.9Hz, Jab= 17.2Hz), 2.34 (s, 1H, CH3); MS (EI) m/z (%): 462 (M+), 358, 242, 117, 103, 91, 77; Analyses (%), C27H22N6S: C, 70.11; H, 4.79; N, 18.17; S, 6.93; Found: C, 70.10; H, 4.78; N, 18.19; S, 6.93.
3d: Yield 82%, m.p. : 116~117 °C. Yellow solid, IR, ν/cm-l: 2808, 2717 (CH2, CH), 1632, 1596, 1494 (C=N, C=C), 758 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.93 (s, 1H, 1,2,3-triazole-H), 8.09~6.90 (m, 14H, Ar-H), 7.12 (s, 1H, thiazole-H), 5.67 (dd, 1H, Hx, Jax= 6.4Hz, Jbx= 11.8Hz), 3.88 (dd, 1H, Ha, Jax= 6.4Hz, Jab= 17.3Hz), 3.33 (dd, 1H, Hb, Jbx= 11.8Hz, Jab= 17.3Hz), 3.85 (s, 1H, OCH3); MS (EI) m/z (%): 478 (M+), 374, 242, 134, 119, 103, 91, 77; Analyses (%), C27H22N6OS: C, 67.76; H, 4.63; N, 17.56; O, 3.35; S, 6.70; Found: C, 67.78; H, 4.64; N, 17.54; O, 3.33; S, 6.71.
3e: Yield 83%, m.p. : 192~193 °C. Yellow solid, IR, ν/cm-l: 2802, 2713 (CH2, CH), 1631, 1597, 1495 (C=N, C=C), 757 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.97 (s, 1H, 1,2,3-triazole-H), 8.08~7.28 (m, 14H, Ar-H), 7.15(s, 1H, thiazole-H), 5.86 (dd, 1H, Hx, Jax= 5.8Hz, Jbx= 11.6Hz), 3.92 (dd, 1H, Ha, Jax= 5.8Hz, Jab= 17.6Hz), 3.34 (dd, 1H, Hb, Jbx= 11.6Hz, Jab= 17.5Hz); MS (EI) m/z (%): 482 (M+), 344, 242, 137, 103, 91, 77; Analyses (%), C26H19ClN6S: C, 64.66; H, 3.97; Cl, 7.33; N, 17.40; S, 6.64; Found: C, 64.67; H, 3.97; Cl, 7.36; N, 17.39; S, 6.61.
3f: Yield 81%, m.p. : 187~188 °C. Yellow solid, IR, ν/cm-l: 1632, 1596, 1493 (C=N, C=C), 752 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.90 (s, 1H, 1,2,3-triazole-H), 8.06~7.32 (m, 13H, Ar-H), 7.16 (s, 1H, thiazole-H), 5.73 (dd, 1H, Hx, Jax= 6.2Hz, Jbx= 12.0Hz), 3.90 (dd, 1H, Ha, Jax= 6.2Hz, Jab= 17.4Hz), 3.29 (dd, 1H, Hb, Jbx= 12.0Hz, Jab= 17.4Hz); MS (EI) m/z (%): 516 (M+), 378, 242, 138, 103, 91, 77; Analyses (%), C26H18Cl2N6S: C, 60.35; H, 3.51; Cl, 13.70; N, 16.24; S, 6.20; Found: C, 60.36; H, 3.52; Cl, 13.70; N, 16.22; S, 6.20.
3g: Yield 80%, m.p. : 274~275 °C. Yellow solid, IR, ν/cm-l: 1631, 1595, 1493 (C=N, C=C), 751 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.90 (s, 1H, 1,2,3-triazole-H), 8.06~6.92 (m, 13H, Ar-H), 7.15 (s, 1H, thiazole-H), 5.72 (dd, 1H, Hx, Jax= 6.2Hz, Jbx= 12.0Hz), 3.91 (dd, 1H, Ha, Jax= 6.2Hz, Jab= 17.2Hz), 3.32 (dd, 1H, Hb, Jbx= 12.0Hz, Jab= 17.2Hz), 2.33 (s, 1H, CH3); MS (EI) m/z (%): 496 (M+), 358, 242, 117, 91, 77; Analyses (%), C27H21ClN6S: C, 65.25; H, 4.26; Cl, 7.13; N, 16.91; S, 6.45; Found: C, 65.25; H, 4.24; Cl, 7.12; N, 16.93; S, 6.46.
3h: Yield 82%, m.p. : 163~164 °C. Yellow solid, IR, ν/cm-l: 2805, 2716 (CH2, CH), 1632, 1596, 1493 (C=N, C=C), 757 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.90 (s, 1H, 1,2,3-triazole-H), 8.06~6.90 (m, 13H, Ar-H), 7.16 (s, 1H, thiazole-H), 5.71 (dd, 1H, Hx, Jax= 6.2Hz, Jbx= 11.8Hz), 3.91 (dd, 1H, Ha, Jax= 6.2Hz, Jab= 17.3Hz), 3.32 (dd, 1H, Hb, Jbx= 11.8Hz, Jab= 17.3Hz), 3.85 (s, 1H, OCH3); MS (EI) m/z (%): 512 (M+), ; Analyses (%), C27H21ClN6OS: C, 63.21; H, 4.13; Cl, 6.91; N, 16.38; O, 3.12; S, 6.25; Found: C, 63.19; H, 4.14; Cl, 6.92; N, 16.36; O, 3.12; S, 6.27.
3i: Yield 78%, m.p. : 192~193 °C. Yellow solid, IR, ν/cm-l: 2808, 2718 (CH2, CH), 1632, 1592, 1516 (C=N, C=C), 758 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.92 (s, 1H, 1,2,3-triazole-H), 8.02~6.94 (m, 13H, Ar-H), 7.12 (s, 1H, thiazole-H), 5.67 (dd, 1H, Hx, Jax= 6.5Hz, Jbx= 11.8Hz), 3.88 (dd, 1H, Ha, Jax= 6.5Hz, Jab= 17.6Hz), 3.32 (dd, 1H, Hb, Jbx= 11.8Hz, Jab= 17.6Hz), 3.87 (s, 1H, OCH3); MS (EI) m/z (%): 478 (M+), 344, 242, 133, 103, 91, 77; Analyses (%), C27H22N6OS: C, 67.76; H, 4.64; N, 17.56; O, 3.34; S, 6.70; Found: C, 67.75; H, 4.63; N, 17.57; O, 3.33; S, 6.70.
3j: Yield 82%, m.p. : 165~166 °C. Yellow solid, IR, ν/cm-l: 2808, 2713 (CH2, CH), 1631, 1592, 1514 (C=N, C=C), 758 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.92 (s, 1H, 1,2,3-triazole-H), 8.02~6.94 (m, 13H, Ar-H), 7.12 (s, 1H, thiazole-H), 5.65 (dd, 1H, Hx, Jax= 6.2Hz, Jbx= 11.8Hz), 3.91 (dd, 1H, Ha, Jax= 6.2Hz, Jab= 17.2Hz), 3.32 (dd, 1H, Hb, Jbx= 11.8Hz, Jab= 17.2Hz), 3.87 (s, 1H, OCH3); MS (EI) m/z (%): 512 (M+), 374, 242, 134, 119, 103, 91, 77; Analyses (%), C27H21ClN6OS: C, 63.21; H, 4.13; Cl, 6.91; N, 16.38; O, 3.12; S, 6.25; Found: C, 63.22; H, 4.13; Cl, 6.91; N, 16.37; O, 3.10; S, 6.27.
3k: Yield 82%, m.p. : 153~154 °C. Yellow solid, IR, ν/cm-l: 2864, 2806, 2719 (CH3,CH2, CH), 1631, 1592, 1513 (C=N, C=C), 752 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.93 (s, 1H, 1,2,3-triazole-H), 8.09~6.94 (m, 13H, Ar-H), 7.12 (s, 1H, thiazole-H), 5.67 (dd, 1H, Hx, Jax= 6.4Hz, Jbx= 11.8Hz), 3.88 (dd, 1H, Ha, Jax= 6.4Hz, Jab= 17.3Hz), 3.32 (dd, 1H, Hb, Jbx= 11.8Hz, Jab= 17.3Hz), 3.86 (s, 1H, OCH3), 2.32 (s, 1H, CH3); MS (EI) m/z (%): 492 (M+), 358, 242, 133, 117, 91, 77; Analyses (%), C28H24N6OS: C, 68.27; H, 4.91; N, 17.06; O, 3.25; S, 6.51; Found: C, 68.29; H, 4.94; N, 17.03; O, 3.25; S, 6.49.
3l: Yield 82%, m.p. : 181~182 °C. Yellow solid, IR, ν/cm-l: 2807, 2715 (CH2, CH), 1632, 1592, 1514 (C=N, C=C), 757 (C—S—C);1H NMR (CDCl3, 400 MHz)δ: 7.93 (s, 1H, 1,2,3-triazole-H), 8.09~6.87 (m, 13H, Ar-H), 7.13 (s, 1H, thiazole-H), 5.67 (dd, 1H, Hx, Jax= 6.2Hz, Jbx= 12.0Hz), 3.89 (dd, 1H, Ha, Jax= 6.2Hz, Jab= 17.4Hz), 3.33 (dd, 1H, Hb, Jbx= 12.0Hz, Jab= 17.4Hz), 3.86 (s, 1H, OCH3), 3.87 (s, 1H, OCH3); MS (EI) m/z (%): 508 (M+), 374, 242, 133, 103, 91, 77; Analyses (%), C27H22N6OS: C, 66.12; H, 4.76; N, 16.52; O, 6.29; S, 6.31; Found: C, 66.15; H, 4.76; N, 16.51; O, 6.27; S, 6.31.
2 Results and discussion
The starting compounds 1,3-diaryl-2-propenones which were readily obtained by the reaction of aromatic aldehydes and 1-arylethanones via the Claisen-Schmidt condensation reaction in good yield, subsequently reacted with thiosemicarbazide in the presence of sodium hydroxide in ethanol, to give 3,5-diphenyl-1-thiocarbamoylpyrazolines[16](1), then underwent condensation with 2-bromo-1-(2-phenyl-1,2,3-triazol-4-yl)ethanones (2)[17]in refluxing ethanol resulted in the formation of the desired cyclized title products (3a-l) in 78~86%.
The IR spectral, mass spectra, and1H NMR spectral data gave strong evidence for the structures of 3a-l.1H NMR revealed two singlets at 7.97~7.88, and 7.16~7.12 ppm which could be attributed to the pyrazole-H and 1,2,3-triazole-H, respectively. The presence of a multiplet at 8.09~6.87 ppm was ascribed to the aromatic protons. Remarkably, three distinct double of doublets of the ABX system of the pyrazoline ring (Jab= 17.2-17.6, Jax= 5.8-6.5, Jbx= 12.0-11.6 Hz) were observed at δ 5.86-5.65, 3.95-3.88, 3.34-3.29 ppm, respectively. Their infrared spectra contained strong absorption bands for C=N double bonds at 1631-1632 cm-1. The EI mass spectra of compounds 3a-lrevealed the existence of the molecular ion peaks and anticipated fragmentation peaks, which were in good agreement with the given structures of products. For example, the mass spectrum of 3a(Fig. 1) had molecular ion peaks at m/z 448, 344 (M-C8H8), 242 (M-C15H12N1), 103 (M-C18H13N6S), 91 (C7H7), 77 (C6H5), consistent with the molecular formula.
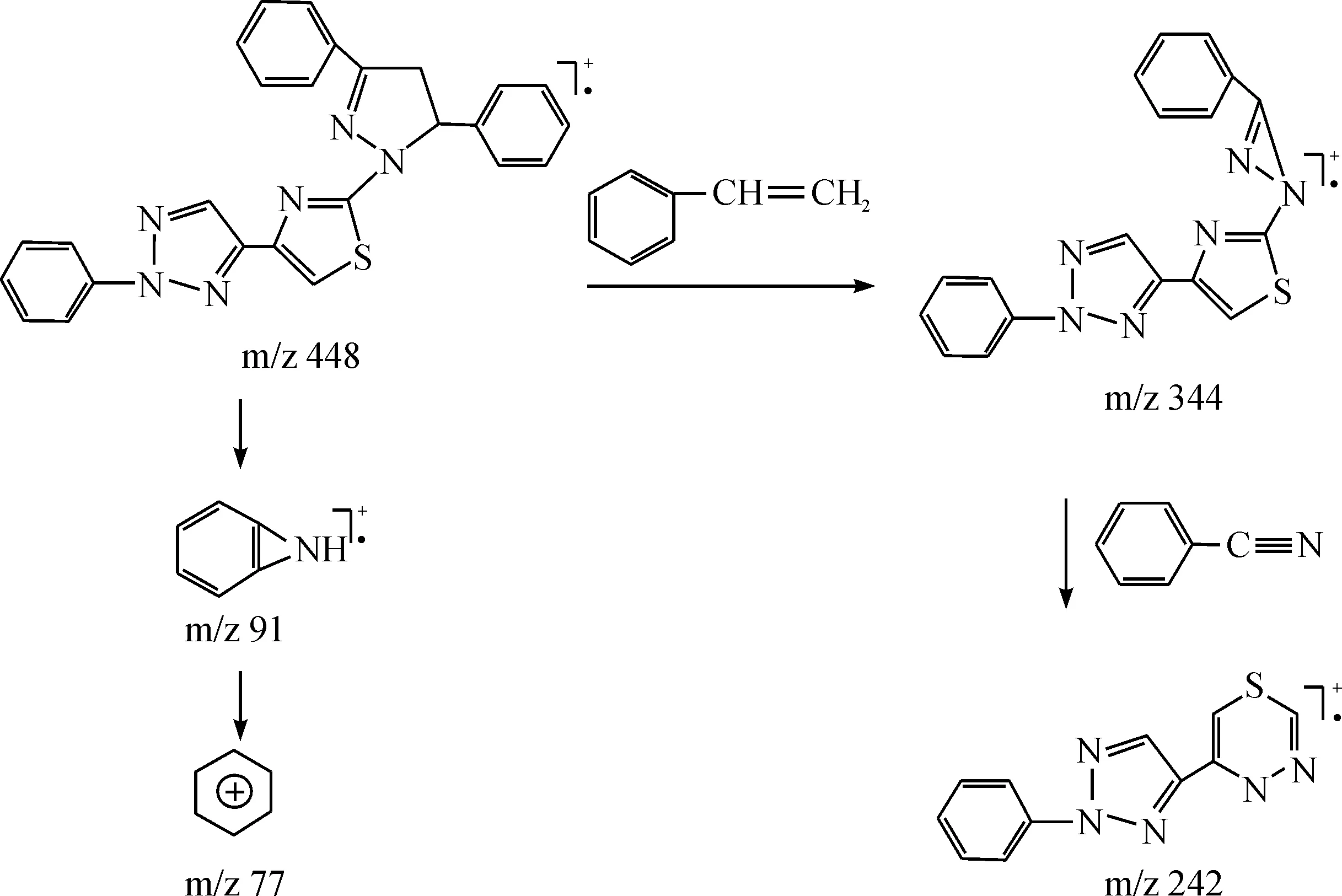
Fig. 1 Proposed fragmentation patterns of 3a
[1] Zhao Peiliang, Wang fu, Zhang Mingzhi,etal. Synthesis, Fungicidal, and Insecticidal Activities of β-Methoxyacrylate-Containing N-Acetyl Pyrazoline Derivatives[J]. Agric Food Chem,2008,56(22):10767-10773.
[2] Frank E, Mucsi Z, Zupkó I,etal. Efficient Approach to Androstene-Fused Arylpyrazolines as Potent Antiproliferative Agents. Experimental and Theoretica Studies of Substituent Effects on BF3-Catalyzed Intramolecular [3+2] Cycloadditions of Olefinic Phenylhydrazones[J]. J Am Chem Soc,2009,131(11):3894-3904.
[3] Du Xiaohui, Guo Chun, Hansell E,etal. Synthesis and Structure -Activity Relationship Study of Potent Trypanocidal Thio Semicarbazone Inhibitors of the Trypanosomal Cysteine Protease Cruzain[J]. J Med Chem,2002,45(13):2695-2707.
[4] Lange J H M, Coolen H A C, Stuivenberg H H,etal. Synthesis, Biological Properties, and Molecular Modeling Investigations of Novel 3,4-Diarylpyrazolines as Potent and Selective CB 1 Cannabinoid Receptor Antagonists[J]. J Med Chem,2004,47(3):627-643.
[5] Cheng Huiming, Wan Junting, Lin Meng-i,etal. Design, Synthesis, and in Vitro Biological Evaluation of 1H-1,2,3-Triazole-4-carboxamide Derivatives as New Anti-influenza A Agents Targeting Virus Nucleoprotein[J]. J Med Chem,2012,55(5):2144-2153.
[6] Genin M J, Allwine D A, Anderson D J,etal. Substituent Effects on the Antibacterial Activity of Nitrogen-Carbon-Linked (Azolylphenyl)oxazolidinones with Expanded Activity Against the Fastidious Gram-Negative Organisms Haemophilus Influenzae and Moraxella catarrhalis[J]. J Med Chem,2000,43(5):953-970.
[7] Maurya S K, Gollapalli D R, Kirubakaran S,etal. Triazole Inhibitors of Cryptosporidium parvumInosine 5'-Monophosphate Dehydrogenase[J]. J Med Chem,2009,52(15):4623-4630.
[8] Dìaz L, Bujons J, Casas J,etal. Click Chemistry Approach to New N-Substituted Aminocyclitols as Potential Pharmacological Chaperones for Gaucher Disease[J]. J Med Chem,2010,53(14):5248-5255.
[9] Katsura Y, Tomishi T, Inoue Y,etal. Anti-Helicobacter pylori Agents. 4. 2-(Substituted guanidino)-4-phenylthiazoles and Some Structurally Rigid Derivatives[J]. J Med Chem,2000,43(17):3315-3321.
[10] Yu Haibo, Qin Zhenfeng, Dai Hong,etal. Synthesis and Insecticidal Activity of N-Substituted (1,3-Thiazole)alkyl Sulfoximine Derivatives[J]. Agric Food Chem,2008,56(23):11356-11360.
[11] Romagnoli R, Baraldi P G, Brancale A,etal. Convergent Synthesis and Biological Evaluation of 2-Amino-4-(3',4',5'-trimethoxyphenyl)-5-aryl Thiazoles as Microtubule Targeting Agents[J]. J Med Chem,2011,54(14):5144-5153.
[12] Sharma S K, Tandon M, Lown J W. Design and Synthesis of Novel Thiazole-Containing Cross-Linked Polyamides Related to the Antiviral Antibiotic Distamycin[J]. Org Chem,2000,65(4):1102-1107.
[13] Shi Hai, Liu Fangming, Shen Songwei. Synthesis and Spectral Characterization of Some Novel Thiazolyl-Pyrazoline Derivatives Containing 1,2,3-Triazole Moiety[J]. Phosphorus Sulfur and Silicon,2011,186(2):263-270.
[14] Chen Shengqin, Zhang Yichao, Liu Fangming. Synthesis and Spectral Characterization of Some New Thiazolyl-Pyrazolines Bearing 1,2,4-Triazole Moiety[J]. Phosphorus Sulfur and Silicon,2011,186(2):319-325.
[15] Gresser R, Hartmann H, Wrackmeyer M,etal. Synthesis of thiophene-substituted aza-BODIPYs and their optical and electroche mical properties[J]. Tetrahedron,2011,67:7148-7155.
[16] Liu Xiangfeng, Shi Daohua. Research progress in the synthesis of chalcone[J]. Applied Chemical Industry,2009,38(8):1210-1219.
[17] Wu Xiaolong, Ye Jiawei, Yang Debao,etal. Synthesis and characterization of 1,4-diaryl-3-(ethoxycarbonyl)-5-[2-phenyl-1,2,3-triazolyl)carbonyl]-4,5-dihydropyrazole derivatives[J].Chin J Org Chem,2010,30(10):1502-1507.
新型含1,2,3-三唑基的噻唑-吡唑啉衍生物的合成
陈 菲,刘方明,陈森林,董志强
(杭州师范大学材料与化学化工学院,浙江 杭州 310036)
1-氨基硫代甲酰基-3,5-二芳基-4,5-二氢吡唑(1)和1-(2-苯基-1,2,3-三唑-4-基)-2-溴乙酮(2)在乙醇中回流环化, 制备一系列新型含噻唑基、1,2,3-三唑基的吡唑啉衍生物, 其结构经红外、核磁氢谱、质谱和元素分析确认.
1,2,3-三唑;吡唑啉;噻唑
date:2012-03-16
LIU Fang-ming(1966—),male, professor, engaged in synthesis of heterocyclic compound. E-mail: fmilu859@sohu.com
11.3969/j.issn.1674-232X.2012.05.008
O626.25ArticlecharacterA
1674-232X(2012)05-0420-06

