CO2浓度升高条件下内生真菌感染对宿主植物的生理生态影响
师志冰,周 勇,李 夏,任安芝,高玉葆
(南开大学生命科学学院,天津 300071)
CO2浓度升高条件下内生真菌感染对宿主植物的生理生态影响
师志冰,周 勇,李 夏,任安芝*,高玉葆
(南开大学生命科学学院,天津 300071)
以内蒙古草原常见伴生种、感染内生真菌的天然禾草羽茅为研究对象,通过比较不同CO2浓度和不同养分供应条件下,带内生真菌和不带菌植物在种子发芽和幼苗生长等方面的差异,探讨带内生真菌的天然禾草对CO2浓度增加的响应。结果表明:CO2浓度增加对带菌种子发芽率和发芽速度均无显著影响,但CO2浓度增加显著降低了不带菌种子的发芽率和发芽速度,即CO2浓度升高加大了带菌和不带菌种子发芽率之间的差异;内生真菌感染显著提高了宿主植物的最大净光合速率和水分利用效率;羽茅的营养生长受CO2浓度和养分供应的交互影响,高CO2浓度对生长的促进作用只出现在充足养分供应条件下;CO2浓度升高和内生真菌感染对植物根系形态有显著的交互作用,在正常CO2浓度下,带菌植株根径gt;1.05 mm的根系比例显著高于不带菌植株,随着CO2浓度的升高,带菌植株上述根径根系所占比例无显著变化而不带菌植株所占比例显著升高,CO2浓度升高导致带菌和不带菌不同根径根系分配之间的差异缩小。
CO2浓度升高;羽茅;种子发芽;根系形态
产业革命以来,由于人类大量消耗石油化工燃料和对土地的不合理开发利用,使得大气CO2浓度逐步升高,从1958到2011年的50多年间,大气CO2浓度已由280增加到391 μmol/mol,若以目前的每年增加 2 μmol/mol计, 预计到2067年,全球大气CO2浓度将超过500 μmol/mol[1- 2]。由于CO2是植物光合作用的重要原料,所以大气中CO2浓度的升高,必将影响到植物光合产物的形成以及光合产物在植物及其共生的微生物之间的分配[3], 从而影响到植物和根瘤菌[4]、菌根真菌[5]以及内生真菌[6- 7]等之间的相互作用。
大量的研究表明CO2浓度升高能够提高菌根真菌的侵染率,增强菌根真菌对宿主植物的有益影响[8- 11]。与菌根真菌类似,内生真菌也是在植物中广泛存在的一类真菌,只是多生活在植物的地上部分[12],据估计,大约三分之二的冷季型禾草中有与之共生的内生真菌[13],但就已报道的研究工作来看,大量的研究集中在两个有重要经济意义的植物种即高羊茅(FestucaarundinaceaSchreb.)和黑麦草(LoliumperenneL.)上面,二者分别与内生真菌Neotyphodiumcoenophialum和N.lolii构成共生关系。内生真菌与高羊茅和黑麦草等人工禾草的互惠共生关系已被大量的实验证据所证实,具体表现在一方面植物为内生真菌提供光合产物;另一方面内生真菌的代谢物能刺激植物的生长发育[14],提高宿主植物对生物胁迫和非生物胁迫的抵抗能力,其中的生物胁迫主要包括食草动物[15]和食草昆虫[16]的取食、线虫[17]和其它真菌[18]的危害以及其它植物的竞争[19]等;非生物胁迫包括干旱[20]、低养分[21]和高温[22]等。由于内生真菌依靠宿主植物获得碳源,而对于多数C3植物而言,CO2浓度是其光合作用的限制因子,因此大气CO2浓度的增加将会通过提高植物的光合能力并增加对共生真菌的光合产物供给,从而对禾草-内生真菌的相互作用产生影响[6, 23],研究感染内生真菌的天然禾草对CO2浓度升高的响应不仅有助于预测全球气候变化条件下带菌植物的竞争力,而且有助于预测带菌植物所在草原群落的发展和演替方向,为实现草原的可持续利用提供实验数据。
1 材料与方法
1.1 种子的采集
羽茅(Achnatherumsibiricum(L.) Keng)是禾本科芨芨草属的一种多年生草本植物,在内蒙古的各类草场中较为常见,具有很高的内生真菌侵染率(86%—100%)[24],共生的内生真菌为Neotyphodium属内生真菌[25],种子于2012年采集自中国农业科学院呼伦贝尔草原生态系统国家野外实验站,种子采集样地位于119.40°E,49.06°N,海拔629 m,年降雨量367 mm,年均温-2.0 ℃,土壤为暗栗钙土,植被为草甸草原。对采回的种子进行内生真菌的检测,发现带菌率为100%,种子于4 ℃冰箱中保存。
1.2 羽茅带菌(E+)和不带菌(E-)种群的构建
带菌的种子由野外采集,存放于4 ℃冰箱中备用,不带菌的种子则通过将野外采集的种子置于60 ℃温箱中处理30 d后获得,前期的研究表明,60 ℃高温处理羽茅种子30 d能完全杀灭种子中的内生真菌,同时高温处理对种子发芽率、发芽势和发芽指数均无显著影响[26]。选取带菌和不带菌的、饱满成熟的种子分别播种于装满蛭石的塑料花盆中,置于温室中培养,待幼苗生长1个月后,每盆分别选取3株长势良好的分蘖进行内生真菌的检测,检测方法参考Latch等的苯胺兰染色法[27]。检测结果显示一直在4 ℃冰箱存放的种子所获得的植株的带菌率为100%,高温处理过的种子所获得的植株带菌率为0。此时,进行间苗,保证每盆有8棵健壮的幼苗,备用。
1.3 种子发芽实验
本实验为两因素实验,因素一为CO2浓度,包括400 μmol/mol (C-)和800 μmol/mol (C+)两个水平;因素二为内生真菌感染状态,包括带菌(E+)和不带菌(E-),每个处理5个重复。实验开始时,分别在每个培养皿中均匀摆放浸泡好的种子50枚,将培养皿分别放入不同CO2浓度的智能人工培养箱中,两个培养箱除CO2浓度不同外,其余的实验参数均完全相同,即温度25 ℃,湿度60%。种子开始发芽后,每天观察并记录发芽种子数目,直至连续两天无种子萌发为止,共历时14 d,计算种子发芽率及发芽速度指数。种子发芽率及发芽速度指数计算公式[28]如下:
发芽率=(发芽种子数/供试种子数)×100%
发芽速度指数=(N1/1)+(N2-N1)/2 +(N3-N2)/3+…+(Nn-Nn-1)/n
式中,N为第1天、第2天…第n天的发芽种子数目。
1.4 幼苗生长实验
本实验为三因素实验,因素一为CO2浓度,包括400 μmol/mol (C-)和800 μmol/mol (C+)两个浓度;因素二为养分处理:包括充足养分供应(N+P+)、缺氮(N-P+)和缺磷(N+P-)3个水平;因素三为内生真菌感染状态,包括带菌(E+)和不带菌(E-),每个处理5个重复。将花盆分别放入不同CO2浓度的培养箱中,两个培养箱的其它参数为:温度25 ℃,湿度50%,光强40%,光照12 h。养分处理通过浇灌Hoagland营养液控制,充足养分供应组浇完全营养液;缺氮处理组使用CaCl2和KCl分别替代Hoagland营养液中的Ca(NO3)2和KNO3,缺磷处理组使用KCl代替KH2PO4,每周定期浇营养液1次,期间保证水分充足供应。实验期间每周随机调换培养箱内花盆的位置,以使花盆位置对实验的影响效应最小。
1.5 各项指标的测定
在幼苗进入智能人工培养箱之前,测量每盆的总分蘖数、叶片数、株高,分析其差异性,结果发现这些指标在带菌和不带菌植株之间均无显著差异。在人工培养箱培养6周后,用LI-6400便携式光合作用测定仪(LI-COR,Lincoln,USA)测定植株的最大净光合速率、气孔导度和蒸腾速率,测定时选取植株刚刚完全伸展开的叶片,光强由LI-6400-02BLED红蓝光源自动控制到1200 μmol·m-2·s-1,叶片水分利用效率由光合速率/蒸腾速率计算获得[29],收获前统计每盆中的叶片数、分蘖数和株高。在收获时,将地上部分和地下部分分开,取一定量的根系,洗净后放入装有蒸馏水的无色透明塑料盘中,用EPSON 1680扫描仪(Epson,Long Beach,USA)以400 dpi分辨率扫描获取根系图像,并以WinRHIZO软件分析得到相关数据。将地上部和地下部烘干称重,计算生物量和根冠比。所得数据采用SPSS 19.0软件进行统计处理。
2 结果与分析
2.1 种子萌发
所有种子均在第3天开始发芽,直至第12天后发芽数量不再增加。随着CO2浓度的增加,带菌和不带菌种子的发芽率和发芽速度的变化不同(图1),表现在正常CO2浓度下,带菌种子的发芽率和发芽速度均显著高于不带菌种子;而在高浓度CO2处理下,与正常CO2浓度相比,带菌种子的发芽率和发芽速度无显著变化,而不带菌种子的发芽率和发芽速度均显著下降,即CO2浓度增加使得带菌和不带菌种子的发芽率和发芽速度之间的差异变得更大。
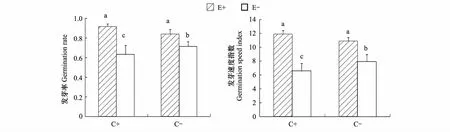
图1 不同CO2浓度下内生真菌感染对羽茅种子发芽率和发芽速度的影响Fig.1 Effects of endophyte infection on seed germination rate and germination speed of Achnatherum sibiricum under different CO2 concentrations字母不相同表示差异显著(Plt;0.05)
2.2 幼苗生长
羽茅的营养生长受CO2浓度和养分供应的交互影响(表1,图2)。在正常CO2浓度下,羽茅的叶片数和地上部分生物量均以充足养分供应组最优,缺氮组最差,缺磷组居中;在高浓度CO2处理下,羽茅的生长状况与养分供应的关系虽然与正常CO2处理组相似,但与正常CO2处理组相比,充足养分供应显著促进了羽茅的营养生长,而缺氮和缺磷组羽茅的生长并没有随CO2浓度的提高而增加。内生真菌感染显著提高了宿主植物的最大净光合速率和水分利用效率(图3),且内生真菌对宿主植物的这一有益作用不受CO2浓度和养分供应的影响。
表1不同CO2浓度和养分处理下内生真菌感染对羽茅影响的三因素方差分析
Table1Three-wayANOVAforgrowthcharactersofendophyte-infected(E+)orendophyte-free(E-)AchnatherumsibiricumundervariousconditionsofCO2andnutrientsavailability

叶片数Leafnumber分蘖数Tillernumber株高Height地上干重Shootdryweight地下干重Rootdryweight总干重Totaldryweight根冠比Root∶shoot净光合速率Netphotosyntheticrate气孔导度Stomatalconductance蒸腾速率Evaporationrate水分利用效率WateruseefficiencyCO2(C)***NS**NS******NS**养分(N)****************NSNS**内生真菌(E)NSNSNSNSNSNSNS**NSNS**C×N*NSNS**NS*NSNSNSNSNSC×ENSNSNSNSNSNSNSNSNSNSNSN×ENSNSNSNSNSNSNSNSNSNSNSC×N×ENSNSNSNSNSNSNSNSNSNSNS
*,**分别表示Plt;0.05,0.01,NS表示差异不显著
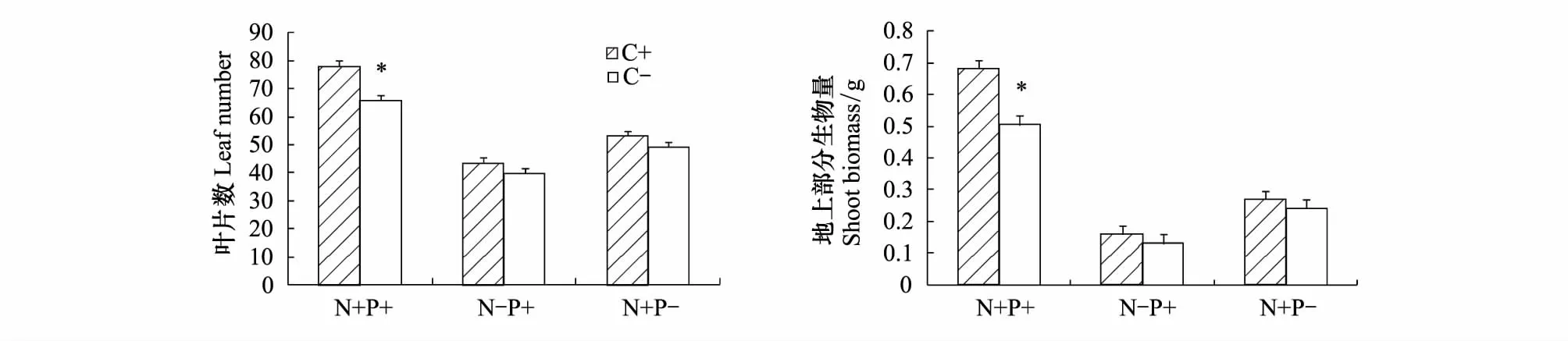
图2 不同CO2浓度和养分处理对羽茅叶片数和地上生物量的影响Fig.2 Effects of CO2 and nutrients availability on leaf number and shoot biomass of Achnatherum sibiricum*表示差异显著(Plt;0.05)

图3 内生真菌感染对羽茅叶片最大净光合速率和水分利用效率的影响Fig.3 Effects of endophyte infection on maximum net photosynthetic rate and water use efficiency of Achnatherum sibiricum*表示差异显著(Plt;0.05)
2.3 根系形态
根系表面积的大小与植物吸收能力密切相关,CO2浓度增加和充足的养分供应都显著增加了羽茅的根系表面积,而内生真菌感染对这一参数无显著影响(图4)。为了明确粗细不同的各级根系对表面积变化的贡献,本文按照根径大小将根系划分为如下3类:即根径 lt;0.45 mm、0.45—1.05 mm、gt;1.05 mm,计算了各根径下根系长度的百分比,结果发现(表2,图5),前两个级别根系的比例只受到养分供应的显著影响,而后一个级别根系的比例除与养分供应有关外,也与CO2浓度和内生真菌状态有关,在正常CO2浓度下,带菌植株根径gt;1.05 mm根系的比例显著高于不带菌植株,随着CO2浓度的升高,带菌植株根径gt;1.05 mm根系所占比例无显著变化而不带菌植株该级别根系所占比例显著升高,即CO2浓度升高导致带菌和不带菌不同根径根系分配之间的差异缩小。

图4 不同二氧化碳浓度、养分处理和内生真菌感染状态对羽茅根系表面积的影响Fig.4 Effects of CO2 concentration, nutrients availability and endophyte infection on root surface area of Achnatherum sibiricum字母不相同表示差异显著(Plt;0.05)
Table2Three-wayANOVAforrootmorphologyofendophyte-infected(E+)orendophyte-free(E-)AchnatherumsibiricumundervariousconditionsofCO2andnutrientsavailability

总长度Totallength总表面积Totalarea平均直径Averagediameterlt;0.45mm0.45—1.05mmgt;1.05mmCO2(C)NS*NSNSNSNS养分(N)***********内生真菌(E)NSNSNSNSNS**C×NNSNSNSNSNSNSC×ENSNSNSNSNS*N×ENSNSNSNSNSNSC×N×ENSNSNSNSNS**

图5 不同CO2浓度下内生真菌感染对羽茅根径gt;1.05 mm根系比例的影响Fig.5 Effects of endophyte infection on the proportion of root length with a diameter of gt;1.05 mm of Achnatherum sibiricum under different CO2 concentrations*表示差异显著(Plt;0.05)
3 讨论
关于CO2浓度增加对种子发芽时间和发芽率影响的报道存在明显的差异,有的报道为无直接联系[30],有的报道为有明显的促进作用[31]。内生真菌是靠宿主的种子进行传播的,关于内生真菌感染对宿主种子发芽率的影响,Clay[32]发现,带菌的黑麦草和高羊茅种子,其发芽率均比相应不带菌种子高10%左右,在对黑麦草的研究中也发现,内生真菌感染显著提高了宿主种子的发芽势和发芽率[33],而在天然禾草中,彭清青等[34]发现,内生真菌感染只是提高了宿主植物的发芽势,而对宿主植物的发芽率无显著影响,本研究中虽然在两种CO2浓度处理下,带菌种子的发芽率和发芽速度均显著高于不带菌种子,然而,我们的不带菌种子是通过高温杀菌的方法获得的,因此内生真菌对宿主发芽的促进作用还需进一步证实。值得注意的是,带菌和不带菌种子对于CO2浓度增加的反应不同,CO2浓度增加对带菌种子发芽率和发芽速度均无显著影响,但CO2浓度增加显著降低了不带菌种子的发芽率和发芽速度,即CO2浓度升高加大了带菌和不带菌种子发芽率之间的差异。
关于CO2浓度增加对内生真菌-禾草相互作用影响的研究目前还很少,Compant等[35]报道CO2浓度增加会提高高羊茅的内生真菌感染率,与之相对照,Marks和Clay[36]和Chen等[23]发现CO2浓度增加对禾草-内生真菌共生体的影响不大,本研究中内生真菌感染显著提高了宿主植物的净光合速率和水分利用效率,但内生真菌的这一有益影响不受CO2浓度的影响。内生真菌对根系形态的影响与CO2浓度有关,在正常CO2浓度下,带菌植株根径gt;1.05 mm根系的比例显著高于不带菌植株,随着CO2浓度的升高,带菌植株上述根径根系所占比例无显著变化而不带菌植株所占比例显著升高,CO2浓度升高导致带菌和不带菌植株不同根径根系分配之间的差异缩小,在他人的研究中,Compant等[35]报道CO2浓度升高使得宿主体内的生物碱浓度下降,Hunt等[6]发现带菌和不带菌植物体内碳水化合物浓度的差异也随着CO2浓度的增加而减小,即CO2浓度升高缩小了带菌和不带菌幼苗生长和代谢物之间的差异。
与菌根真菌-植物共生体相比,内生真菌-禾草共生体对CO2浓度增加较为不敏感[23],Hunt等[6]发现与正常CO2浓度相比,不带菌植株中的可溶性蛋白浓度在高浓度CO2下下降约40%,而带菌植株的可溶性蛋白浓度随CO2浓度增加无显著变化,本研究中也发现随着CO2浓度的升高,带菌种子的发芽率和根系分配均无显著变化,而不带菌植株的上述指标均发生了显著变化,内生真菌感染有可能弱化CO2浓度增加对宿主植物的影响。当然,本文只在种子发芽和幼苗生长方面研究了感染内生真菌的植物对CO2浓度增加的相应,要阐明感染内生真菌的植物对CO2浓度增加的综合反应及其反应机制,还需要大量的研究工作。
[1] Peters G P, Marland G, Le Quéré C, Boden T, Canadell J, Raupach M R. Rapid growth in CO2emissions after the 2008—2009 global financial crisis. Nature Climate Change, 2012, 2(1): 2- 4.
[2] Franks P J, Adams M A, Amthor J S, Barbour M M, Berry J A, Ellsworth D S, Farquhar G D, Ghannoum O, Lloyd J, McDowell N, Norby R J, Tissue D T, von Caemmerer S. Sensitivity of plants to changing atmospheric CO2concentration: from the geological past to the next century. New Phytologist, 2013, 197(4): 1077- 1094.
[3] Hu S J, Firestone M K, Chapin F S. Soil microbial feedbacks to atmospheric CO2enrichment. Trends in Ecology and Evolution, 1999, 14(11): 433- 437.
[4] Zanetti S, Hartwig U A, Luscher A, Hebeisen T, Frehner M, Fischer BU, Hendrey G R, Blum H, Nosberger J. Stimulation of symbiotic N2fixation inTrifoliumrepensL. under elevated atmosphericpCO2in a grassland ecosystem. Plant Physiology, 1996, 112(2): 575- 583.
[5] Johnson N C, Wolf J, Koch G W. Interactions among mycorrhizae, atmospheric CO2and soil N impact plant community composition. Ecology Letters, 2003, 6(6): 532- 540.
[6] Hunt M G, Rasmussen S, Newton P C D, Parsons A J, Newman J A. Near-term impacts of elevated CO2, nitrogen and fungal endophyte-infection onLoliumperenneL. growth, chemical composition and alkaloid production. Plant, Cell and Environment, 2005, 28(11): 1345- 1354.
[7] Newman J A, Abner M L, Dado R G, Gibson D J, Brookings A, Parsons A J. Effects of elevated CO2, nitrogen and fungal endophyte-infection on tall fescue: growth, photosynthesis, chemical composition and digestibility. Global Change Biology, 2003, 9(3): 425- 437.
[8] Rillig M C, Field C B, Allen M F. Fungal root colonization responses in natural grasslands after long-term exposure to elevated atmospheric CO2. Global Change Biology, 1999, 5(5): 577- 585.
[9] Hartwig U A, Wittmann P, Raun R B. Arbuscular mycorrhiza infection enhances the growth response ofLoliumperenneto elevated atmosphericpCO2. Journal of Experimental Botany, 2002, 53(371): 1207- 1213.
[10] Treseder K K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2in field studies. New Phytologist, 2004, 164(2): 347- 355.
[11] Hu S J, Wu J S, Burkey K O, Firestone M K. Plant and microbial N acquisition under elevated atmospheric CO2in two mesocosm experiments with annual grasses. Global Change Biology, 2005, 11(2): 223- 233.
[12] Arnold A E, Meijia L C, Kyllo D, Rojas E I, Maynard Z, Robbins N, Herre E A. Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(26): 15649- 15654.
[13] Müller C B, Krauss J. Symbiosis between grasses and asexual fungal endophytes. Current Opinion in Plant Biology, 2005, 8(4): 450- 456.
[14] Rahman M H, Saiga S. Endophytic fungi (Neotyphodiumcoenophialum) affect the growth and mineral uptake, transport and efficiency ratios in tall fescue (Festucaarundinacea). Plant and Soil, 2005, 272(1/2): 163- 171.
[15] Burns J C, Fisher D S. Intake and digestion of ′Jesup′ tall fescue hays with a novel fungal endophyte, without an endophyte, or with a wild-type endophyte. Crop Science, 2006, 46(1): 216- 223.
[16] Züst T, Härri S A, Müller C B. Endophytic fungi decrease available resources for the aphidRhopalosiphumpadiand impair their ability to induce defences against predators. Ecological Entomology, 2008, 33(1): 80- 85.
[17] Eerens J P J, Visker M H P W, Lucas R J, Easton H S, White J G H. Influence of the ryegrass endophyte on phyto-nematodes // Neotyphodium/Grass Interactions. New York: Plenum Press, 1997: 153- 156.
[18] Van Hecke M M, Treonis A M, Kaufman J R. How does the fungal endophyteNeotyphodiumcoenophialumaffect tall fescue (Festucaarundinacea) rhizodeposition and soil microorganisms? Plant and Soil, 2005, 275(1/2): 101- 109.
[19] Quigley P E. Effects ofNeotyphodiumloliiinfection and sowing rate of perennial ryegrass (Loliumperenne) on the dynamics of ryegrass/subterranean clover (Trifoliumsubterraneum) swards. Australian Journal of Agricultural Research, 2000, 50(1): 47- 56.
[20] Hesse U, Schoberlein W, Wittenmayer L, Forster K, Warnstorff K, Diepenbrock W, Merbach W. Influence of water supply and endophyte infection (Neotyphodiumspp.) on vegetative and reproductive growth of twoLoliumperenneL. genotypes. European Journal of Agronomy, 2005, 22(1): 45- 54.
[21] Lewis G C. Effects of biotic and abiotic stress on the growth of three genotypes ofLoliumperennewith and without infection by the fungal endophyteNeotyphodiumlolii. Annals of Applied Biology, 2004, 144(1): 53- 63.
[22] Marks S, Clay K. Physiological responses ofFestucaarundinaceato fungal endophyte infection. New Phytologist, 1996, 133(4): 727- 733.
[23] Chen X, Tu C, Burtonm G, Watsond M, Burkeyk O, Hu S J. Plant nitrogen acquisition and interactions under elevated carbon dioxide: impact of endophytes and mycorrhizae. Global Change Biology, 2007, 13(6): 1238- 1249.
[24] Wei Y K, Gao Y B, Xu H, Su D, Zhang X, Wang Y H, Chen L, Nie L Y, Ren A Z.Neotyphodiumin native grasses in China and observations on endophyte/host interactions. Grass and Forage Science, 2006, 61(4): 422- 429.
[25] Zhang X, Ren A Z, Wei Y K, Lin F, Li C, Liu Z J, Gao Y B. Taxonomy, diversity and origins of symbiotic endophytes ofAchnatherumsibiricuminthe Inner Mongolia Steppe of China. FEMS Microbiology Letters, 2009, 301(1): 12- 20.
[26] Li X, Han R, Ren A Z, Gao Y B. Using high-temperature treatment to construct endophyte-freeAchnatherumsibiricum. Microbiology China, 2010, 37(9): 1395- 1400.
[27] Latch G C M, Christensen M J, Samuels G J. Five endophytes ofLoliumandFestucain New Zealand. Mycotaxon, 1984, 20(2): 535- 550.
[28] Chiapuso G, Sanchez A M, Reigosa M J, Gonzalez L, Pellissier F. Do germination indices adequately reflect allelochemical effects on the germination process?. Journal of Chemical Ecology, 1997, 23(11): 2445- 2453.
[29] Farquhar G D, Richards R A. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology, 1984, 11(6): 539- 552.
[30] Garbutt K, Williams W E, Bazzaz F A. Analysis of the differential response of five annuals to elevated CO2during growth. Ecology, 1990, 71(3): 1185- 1194.
[31] Heichel G H, Jaynes R A. Stimulating emergence and growth of Kalmia genotypes with CO2. Horticulture Science, 1974, 9(1): 60- 62.
[32] Clay K. Effects of fungal endophytes on the seed and seedling biology ofLoliumperenneandFestucaarundinacea. Oecologia, 1987, 73(3): 358- 362.
[33] Ren A Z, Gao Y B, Gao W S. Effects of endophyte infection on seed germination, seedling growth and osmotic stress resistance of perennial ryegrass (LoliumperenneL.). Acta Phytoecologica Sinica, 2002, 26(4) 420- 426.
[34] Peng Q Q, Li C J, Song M L, Liang Y, Nan Z B. Effects ofNeotyphodiumendophytes on seed germination of three grass species under different pH conditions. Acta Prataculturae Sinica, 2011, 20(5): 72- 78.
[35] Compant S, van der Heijden M G A, Sessitsch A. Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiology Ecology, 2010, 73(2): 197- 214.
[36] Marks S, Clay K. Effects of CO2enrichment, nutrient addition, and fungal endophyte-infection on the growth of two grasses. Oecologia, 1990, 84(2): 207- 214.
参考文献:
[26] 李夏, 韩荣, 任安芝, 高玉葆. 高温处理构建不感染内生真菌羽茅种群的方法探讨. 微生物学通报, 2010, 37(9): 1395- 1400.
[33] 任安芝, 高玉葆, 高文生. 内生真菌侵染对黑麦草种子萌发、幼苗生长及渗透胁迫抗性的影响. 植物生态学报, 2002, 26(4): 420- 426.
[34] 彭清青, 李春杰, 宋梅玲, 梁莹, 南志标. 不同酸碱条件下内生真菌对三种禾草种子萌发的影响. 草业学报, 2011, 20(5): 72- 78.
Physio-ecologicaleffectsofendophyteinfectiononthehostgrasswithelevatedCO2
SHI Zhibing, ZHOU Yong, LI Xia, REN Anzhi*, GAO Yubao
CollegeofLifeSciences,NankaiUniversity,Tianjin300071,China
Carbon dioxide (CO2) enrichment in the atmosphere stimulates photosynthetic activity and growth of C3plants. This may in turn alter the availability of photosynthates for plant-associated microbes, modifying the symbiosis formed such as mycorrhizae and plant-endophyte complexes. Documents are accumulating to show that elevated CO2increases hyphal growth and root colonization by arbuscular mycorrhizal fungi (AMF). Similar to AMF, endophytes are also fungi that are widely associated with plants but they mostly exist in the shoots rather than the roots of plants. Up to now, however, few studies have focused on the responses of endophyte-infected plants to elevated CO2. In the present study, we examined how elevated CO2affects endophytes and their functions, usingAchnatherumsibiricum(L.) Keng as model species.A.sibiricumis a caespitose perennial grass, widely distributed in the Inner Mongolia steppe and usually highly infected byNeotyphodiumendophytes. Seeds ofA.sibiricumwere collected from natural population in Hailar in the Northeast part of China. Detection of endophytes using the aniline blue staining method showed that endophyte infection frequency of the Hailar population was almost 100%. To eliminate the endophyte, we heat-treated a subset of randomly chosen seeds in a convection drying oven for 30 d at 60 ℃. Two experiments were performed in two growth chambers, with ambient (C-) and elevated (C+) CO2, separately. In Experiment 1, germination rates of endophyte-infected (E+) and endophyte-free (E-) seeds were compared under two different CO2concentrations. In Experiment 2, vegetative growth of E+ and E- seedlings was compared. The design of this experiment was completely randomized and a 2×2×3 factorial, with CO2concentration (C+ vs. C-), infection status (E+ vs. E-) and nutrients availability (N+P+, N-P+, N+P-, i.e. N and P supply, N deficiency P supply, N supply P deficiency) as the variables. There were five replicates per treatment group. The results showed that both the germination rate and germination speed of E+ seeds were not affected by elevated CO2while those of E- seeds were significantly decreased by elevated CO2. That is to say, elevated CO2increased the germination rate difference between E+ and E- seeds. Endophyte infection significantly improved maximum net photosynthetic rate and water use efficiency of the host grass. The vegetative growth was significantly affected by the interaction of elevated CO2and nutrients availability, but was not affected by endophyte infection. The beneficial improvement of elevated CO2on vegetative growth ofA.sibiricumoccurred only under N+P+ conditions. With N or P deficiency, the beneficial effect of elevated CO2on the growth did not exist. The root morphological characters were affected by the interaction of elevated CO2and endophyte infection. In the ambient CO2treatment, the proportion of root length with a diameter of gt;1.05 mm was significantly higher in E+ than in E- plants. With elevated CO2, no significant difference was found in the proportion of the root length stated above between E+ and E- roots. Elevated CO2decreased the difference of root morphology between E+ and E- plants. When compared with plant-AMF associations, the present study suggested that the grass-endophyte association was less sensitive to CO2enrichment. It is suggested that more experiments are needed to fully examine the potential impacts of elevated CO2on plant-endophyte associations.
elevated CO2;Achnatherumsibiricum; seed germination; root morphology
国家自然科学基金资助项目(31270463); 国家基础学科人才培养基金资助项目(J1103503)
2013- 06- 08;
2013- 07- 23
*通讯作者Corresponding author.E-mail: renanzhi@ nankai.edu.cn
10.5846/stxb201306081432
师志冰,周勇,李夏,任安芝,高玉葆.CO2浓度升高条件下内生真菌感染对宿主植物的生理生态影响.生态学报,2013,33(19):6135- 6141.
Shi Z B, Zhou Y, Li X, Ren A Z, Gao Y B.Physio-ecological effects of endophyte infection on the host grass with elevated CO2.Acta Ecologica Sinica,2013,33(19):6135- 6141.

