Neuroimaging studies in pati ents with obsessive-compulsive disorder in China
Qing FAN, Zeping XIAO*
·Review·
Neuroimaging studies in pati ents with obsessive-compulsive disorder in China
Qing FAN, Zeping XIAO*
1. Introduction
Obsessive-compulsive disorder (OCD) is a common mental disorder with a lifetime occurrence of 2 to 3% in the general populati on.[1]The main clinical presentations of OCD include uncontrollable obsessive thinking and compulsive behavior - which may be associated with symptoms of anxiety or depression -that cause significant impairment in social functioning and deteriorati on in quality of life.[2]The eti ology of OCD remains unclear but studies from other countries have found abnormalities in the prefronto-striato-thalamic circuit of individuals with OCD.[3]Neuroimaging studies of OCD in China started in the late 1990s with studies using PET and single photon emission computed tomography (SPECT). These methods have now largely been replaced by NRI studies that have focused on the pathophysiological mechanisms in OCD both with and without treatment. This review will summarize the results of structural and functional imaging studies of OCD in China.
2. Structural imaging studies
2.1 Structural MRI studies
NRI provides high spatial resolution and is able to image all brain structures including gray and white matt er.The three published reports by Chinese investigators of NRI studies of OCD are shown in Table 1. Using opti mized voxel-based morphometry (VBN), Li and colleagues reported greater volume of gray matter in the bilateral thalamus and the left cerebellum among individuals with OCD, which suggests that these brain structures play an important role in the development of OCD.[4]Luo and colleagues[5]reported that patients with OCD had a greater volume of white matter in the right precentral gyrus, the right postcentral gyrus, the bilateral precuneus, and the left middle occipital gyrus than control subjects; they also had a smaller volume of white matter in the bilateral superior frontal gyrus, the left postcentral gyrus, the left parahippocampal gyrus/corpus callosum, and the right inferior parietal lobule.
Using surface based morphometry (SBN), Fan and colleagues[6]found that individuals with OCD had greater cortex thickness in the right inferior parietal region and increased gyrification in dices in the left insula, left middle frontal gyrus, left lateral occipital region extending to the precuneus, and in the right supramarginal gyrus.They also found a positive correlation between the Yale-Brown Obsessive-Compulsive Scale (YBOCS) score and the local gyrification index of the left insular lobe. This suggests structural changes in the cortex among people with OCD and that the structural changes correlate with the severity of OCD symptoms.
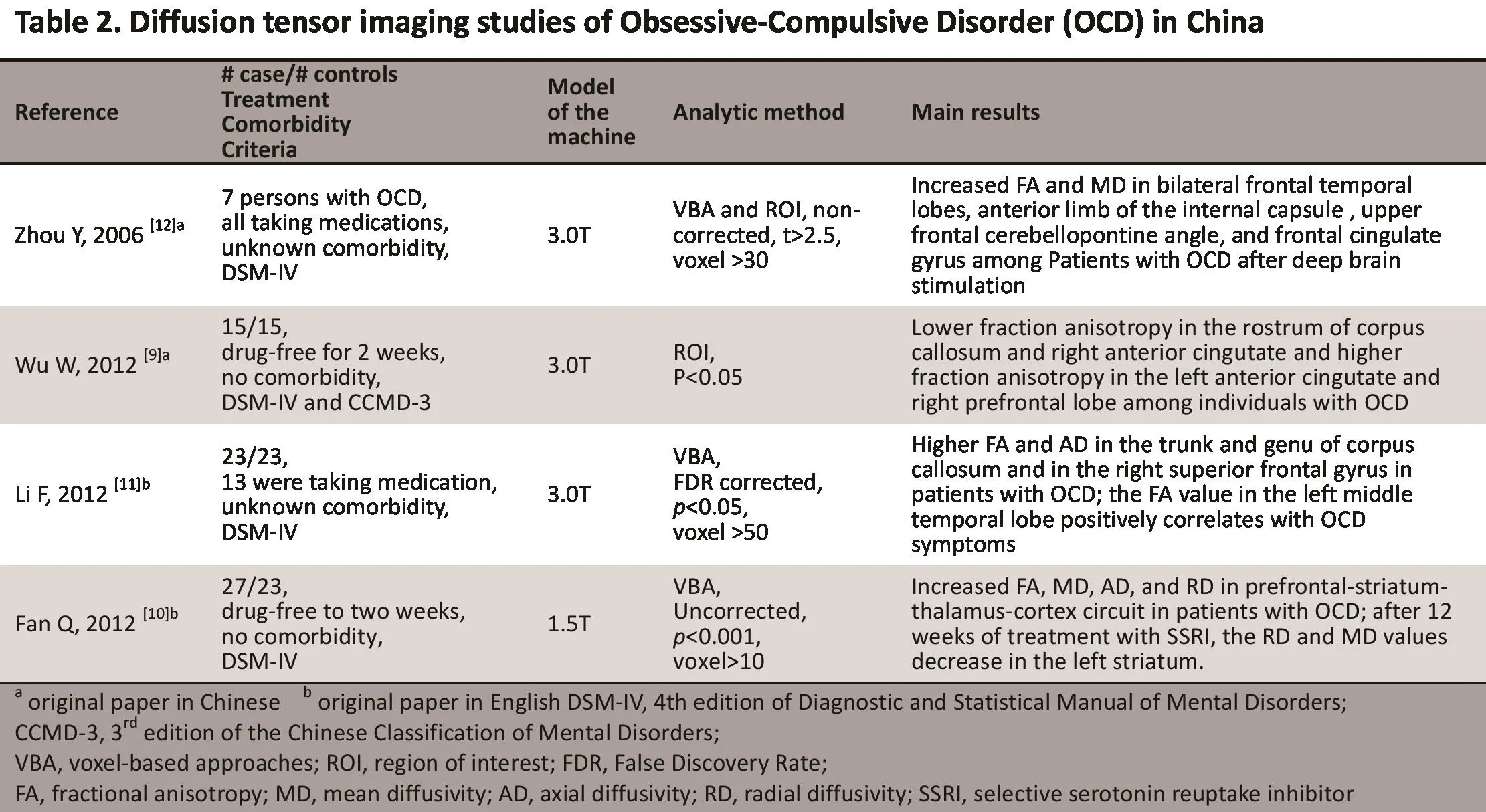
2.2 Diff usion tensor imaging (DTI】 studies
Diff usion tensor imaging (DTI) is a type of imaging technique that uti lizes the diff usion tension of watermolecules to detect detailed structural or pathological changes in organic ti ssues.[7]Anisotropic diffusion refers to the phenomenon that it is easier for water molecules to diffuse along the direction of the white matter fiber than to move vertically in the central nervous system.DTI quantifies the anisotropic diffusion of water molecules in order to observe subtle structural changes in white matter. Since myelin sheath is an organic barrier for the diffusion of water molecules, the anisotropic diffusion of the white matter is mainly aff ected by axons and myelin sheath.[8]Fractional anisotropy (FA)is a parameter that describes the degree of anisotropy of a diffusion process (from 0 to 1) which is commonly assessed in DTI studies.

?
To date, four papers using DTI methods to assess OCD have been published by Chinese investi gators(Table 2). Using the analyti cal method of region of interest (ROI), Wu and colleagues found lower fractional anisotropy in the rostrum of corpus callosum and the right anterior cingulum and higher fractional anisotropy in the left anterior cingulum and the right prefrontal lobe among individuals with OCD.[9]
Using the analytical method of voxel-based approaches (VBA), Fan and colleagues[10]and Li and colleagues[11]compared the whole-brain FA, mean diffusivity (ND), axial diffusivity (AD), and radial diffusivity (RD) between individuals with and without OCD. Li and colleagues found higher FA and AD in the truncus and genu of the corpus callosum and in the right superior frontal gyrus among individuals with OCD, but did not find any differences in the RD; furthermore, the FA value in the left middle temporal lobe was positively correlated with the severity of OCD symptoms.[11]These results suggest the existence of structural abnormalities in axons. Fan and colleagues[10]found differences in FA, ND, AD, and RD in the prefronto-striato-thalamocorti cal circuit between individuals with and without OCD. Specifically they found increased RD values in the left medial superior frontal gyrus, temporo-parietal lobe, occipital lobe, insula, striatum, and the right midbrain, but no differences in the AD values. After 12 weeks of treatment with selective serotonin reuptake inhibitors (SSRIs), the RD and ND values of patients with OCD showed a significant decrease in the left striatum.These results suggest that the structural changes in the prefronto-striato-thalamo-corti cal circuit in OCD may be related to deterioration of the myelin sheath and that some of these abnormalities can be corrected with SSRI treatment.[10]
Using the DTI technique, Zhou and colleagues[12]found that after treatment of OCD with deep brain stimulation (DBS), the FA and ND increased in the bilateral frontal lobes, anterior limb of the internal capsule, upper frontal cerebellopontine angle, and anterior cingulate gyrus.[12]These findings provide insights into the mechanism via which DBS achieves its treatment effect.
2.3 Summary of structural imaging studies
Although there have been only a few structural imaging studies on OCD in China, they used standard imaging techniques and focused on issues that have been assessed in studies from other countries. The Chines studies included both treatment-sensitive and treatment-resistant patients. Overall the findings support the hypothesis that OCD is associated with structural changes of gray and white matter in the prefrontal lobe, striatum, and thalamus. The studies have also reported structural changes in the temporoparietal lobes, the occipital lobe, the insula lobe, the cerebellum, the anterior limb of the internal capsule,the corpus callosum, the parahippocampal gyrus, and the midbrain.
Two meta-analysis of structural imaging studies of OCD have been reported in the internati onal literature.[13,14]Rotge and colleagues[13]summarized studies using the ROI method to analyze NRI results for pati ents with OCD and found decreased volume of gray matt er in the left anterior cingulate and the bilateral orbitofrontal cortex and increased volume in the bilateral thalamus.[13]Radua and Nataix-Cols conducted a meta-analysis of NRI studies in patients with OCD analyzed using the VBN method and found increased volume in the bilateral caudate nucleus and decreased volume in the bilateral dorsomedial prefrontal lobe/anterior cingulum.[14]These meta-analyses support the role of the orbitofrontal-striato-thalamic circuit and the dorsomedial prefronto-striatal circuit in the pathophysiological mechanisms of OCD. In summary,studies from China also support these findings of the important role of the prefrontal-striato-thalamic circuit in OCD. Studies from China (and other countries) have also reported structural abnormalities in the temporoparietal lobe, occipital lobe, cerebellum, and corpus callosum, but the meta-analyses of international studies do not confirm the importance of these brain regions in the pathogenesis of OCD.[15-18]
3. Functional imaging studies
Functional changes in the brain among individuals with OCD can be studied via PET, SPECT, and functi onal magneti c resonance imaging (fNRI). And magnetic resonance spectroscopy (NRS) studies can also assess functional changes in the brain by monitoring the concentrati ons of different metabolites in the corresponding regions of the brain. Functional changes can be categorized into the following groups based on the type of experiment:a) resting-state studies which compare brain functions of individuals with and without OCD while resting; b)symptom provocati on studies which compare brain functions before and after OCD symptoms have been provoked (e.g., by making patients with OCD with a cleanliness obsession or compulsive washing touch something dirty); c) treatment studies which compare brain functions before and after medication treatment or psychotherapy; and d) cognitive studies which compare brain functions between patients with OCD and controls while executing cognitive tasks such as planning, mistake induction, reaction inhibition, reversal learning, and task conversion.
?
?
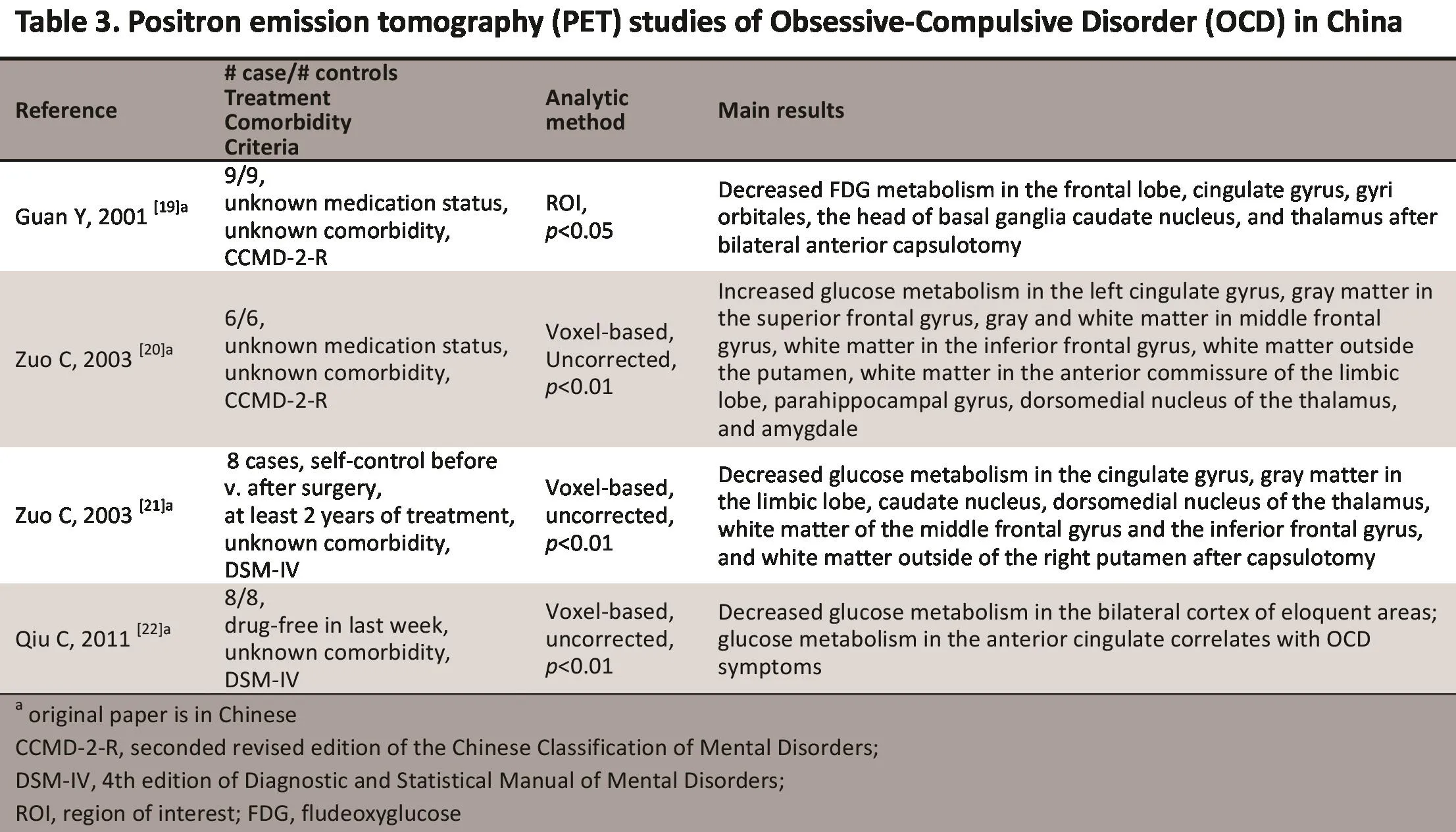
3.1 PET studies
In PET the fl udeoxyglucose (FDG) marker is used to monitor changes in brain functi ons.[18]Since 2001, PET has been used in China to compare glucose metabolism in the brain between Patients with OCD and healthy controls, and between Pati ents with OCD who do and do not receive surgery for OCD. The four studies by Chinese investigators using PET to assess patients with OCD are shown in Table 3. Using the ROI method in pati ents with intractable OCD, Guan and colleagues reported high FDG intake in the frontal lobe, the cingulate gyrus, the head of the basal ganglia caudate nucleus, and the thalamus. They also found that after bilateral anterior capsulotomy using a Radionis radiofrequency generator FDG metabolism decreased in the frontal lobe, cingulate gyrus, gyri orbitales, head of basal ganglia caudate nucleus, and thalamus. These fi ndings were used as evidence for the existence of an abnormal circuit in OCD, helped locate abnormal brain regions prior to surgery for OCD, and identi fi ed outcome measures that could be used to assess the eff ecti veness of surgery.[19]In a subsequent study,[21]the same research team assessed a sample of patients with intractable OCD and found increased glucose metabolism in several brain regions: left cingulate gyrus, gray matter in the superior frontal gyrus, gray and white matt er in the middle frontal gyrus, white matt er in the inferior frontal gyrus, white matter outside the putamen, white matter in the anterior commissure of the limbic lobe, parahippocampal gyrus, dorsomedial nucleus of the thalamus, and amygdale.[20]They also found decreased glucose metabolism after capsulotomy in several brain regions: cingulate gyrus, gray matter in the limbic lobe, caudate nucleus, dorsomedial nucleus of the thalamus, white matter of the middle frontal gyrus and the inferior frontal gyrus, and white matter outside of the right putamen.[21]Recently, this research team also found decreased glucose metabolism in the bilateral motor areas of the frontal and parietal lobes;moreover, glucose metabolism in the anterior cingulum was correlated with the severity of OCD symptoms.[22]Based on these findings, the authors suggest that the glucose metabolism in the anterior cingulate could be used as a marker of OCD severity.[22]
3.2 SPECT studies

?
SPECT uses a radionuclide tracer to monitor changes in the blood fl ow of different brain regions as a method of assessing functional changes in the brain. Four research teams in China have published a total of 11 papers(the main papers are shown in Table 4) that use the 99mTc-ECD tracer with SPECT to evaluate patients with OCD. Earlier studies[23]using the statistical parametric mapping (SPN) soft ware for data analysis found that compared to the cerebellum (the reference), patients with OCD showed changes in blood fl ow in the precuneus, left superior temporal gyrus, right superior gyriorbitales, bilateral superior frontal gyrus, and leftsuperior parietal lobule; when compared to the whole brain region, these patients showed changes in blood perfusion in the bilateral precuneus, right cuneus, right temporal gyrus, left superior temporal gyrus, bilateral superior fontal gyrus, superior gyri orbitales, leftsuperior parietal lobule, left frontal cingulate, bilateral putamina, right angular gyrus, and right cerebellum.These findings suggest blood fl ow changes in the cerebellum among patients with OCD; thus, changes of blood fl ow in the cerebellum itself or other brain regions related to the cerebellum may be missed when using the cerebellum as the reference region.[23]Later, this research team[24]found that compared to the whole brain region, patients with OCD have decreased blood fl ow in the bilateral putamen, superior temporal lobe, precuneus, right gyri orbitales, right superior frontal gyrus, right middle frontal gyrus, lefttemporal and occipital lobe, superior parietal lobule,and the cerebellar vermis. These findings support the hypothesized changes in the prefronto-striatal circuit among individuals with OCD.[24]Recently, this research team[25]used an automated extraction method (which is more efficient that the ROI method) in patients with OCD and found decreased blood fl ow in the leftinferior temporal lobe, supramarginal gyrus, transverse temporal gyrus, outer dorsal nuclei, outer posterior nucleus, intercalated nucleus, and increased blood fl ow in the left superior parietal lobule, and the dorsal thalamus. Overall these results indicate that Pati ents with OCD have decreased blood fl ow in multiple regions in the bilateral temporal lobes and increased blood fl ow in the dorsal thalamus.
Using the ROI method and employing the optic region as the reference, Chen and colleagues[26]found that blood fl ow in pati ents with OCD was lower in the left temporal and occipital lobes than in controls.Among patients with OCD, blood fl ow was lower in the left prefrontal, temporal and occipital lobes than in the corresponding lobes on the right. Differences in the rates of blood fl ow in the bilateral caudate between mild and severe OCD support suggesti ons about the role for the temporal lobe and the caudate nucleus in the pathophysiology of OCD.[26]The same research team compared patients with OCD, anxiety disorders,and depressive disorders using SPECT[27]and found significant differences in the blood fl ow of Patients with OCD compared to that in pati ents with anxiety disorders but no significant differences between Patients with OCD and patients with depressive disorders.
Using the ROI method, Lin and colleagues[28]compared Patients with OCD with healthy controls and subdivided the OCD group into those whose symptoms were limited to intrusive thoughts (n=8), those with intrusive suspicion and repetitive behavior (n=8), and those with intrusive fears, excessive hand washing and avoidance (n=12). They found increased blood fl ow in the bilateral thalamus, parietal lobe, and basal ganglia and decreased blood fl ow in the right temporal lobe.There was a positive correlation between the blood fl ow in the right basal ganglia and the obsessive-compulsive behavior score among patients in the intrusive fears,excessive hand washing and avoidance group. These results suggest that patients with OCD have hyperfunctioning of the bilateral thalamus, parietal lobes,and basal ganglia and hypo-functioning of the right temporal lobe. Obsessive-compulsive behavior in Patients with OCD with intrusive fears, excessive washing and avoidance is related to hyper-functioning of the basal ganglia.[28]
Using the ROI method and the cerebellum as the reference region, Li and colleagues studied the brain blood fl ow among pati ents with OCD and correlated the results with scores on the Nodified version of Wisconsin Card Sorting Test (WCST) and with clinical severity. They found increased blood fl ow in bilateral prefrontal lobes and in the anterior temporal lobe. There was also a negative correlation between blood fl ow in the right prefrontal lobe and the number of correct answers on the WCST, a positive correlation between blood fl ow in the right anterior temporal lobe and the number of wrong answers, a positive correlation between the blood fl ow in the right prefrontal lobe and the number of consecutive wrong answers, and a positive correlation between blood fl ow in bilateral prefrontal lobes and right temporal lobe and intrusive thoughts.These results suggest a correlation between cognitive impairment and blood fl ow in the right prefrontal lobe and in the left thalamus; the results also suggest that abnormal functioning of the prefrontal lobe and of the right anterior temporal lobe may be the biological origin of intrusive thoughts among pati ents with OCD.[29]This research team also compared the SPECT results of Patients with OCD with those of patients with depressive disorders;[30]but in contrast to the findings of Chen’s group[26]they found increased blood fl ow in the prefrontal lobe and anterior temporal lobe among patients with OCD, and decreased blood fl ow in these regions among patients with depressive disorders.
3.3 fMRI studies
The fNRI technique uses blood oxygen level dependent(BOLD) signals to ref l ect changes of brain function.Statistical methods to analyze resting state fNRI data include regional homogeneity (ReFo), amplitude of low frequency fl uctuati on (ALFF), functional connectivity,and independent component analysis (ICA).[31]The 5 fNRI studies on OCD conducted in China are presented in Table 5.
Tian and colleagues[32]reported increased ReFo in patients with OCD in the right frontal cortex, right parietal cortex, right insular, left cingulum and leftparietal lobe. Yang and colleagues[33,34]assessed nevermedicated Pati ents with OCD and found increased ReFo and ALFF in the left anterior cingulum, increased ALFF in the left middle cingulate gyrus, and lower ReFo in the left inferior temporal gyrus. Zhang and colleagues[35]found abnormal functional connectivity in the neural control networks of pati ents with OCD,including decreased functional connectivity in the posterior temporal area; increased functional binding in the cingulum, precuneus, thalamus and cerebellum;and a significantly higher level of local clustering(compared to the small-world architecture of health control subjects).
Fou and colleagues[36]conducted fNRI while Patients with OCD and control subjects completed the Chinese version of the Stroop task and a block design test. They found that several brain regions of Patients with OCD showed above normal levels of acti vati on during the relatively easy non-interference secti on of the color and word matching task (including the leftparahippocampal gyrus, paracentral lobule, thalamus,and calcarine gyrus) and only a few regions showed lower levels of acti vati on than controls (the anterior cingulate and left caudate nucleus); however, during the more difficult matching task when interference was present there were no brain regions in Pati entswith OCD with higher than normal activation and some brain regions showed lower than normal activation(e.g., bilateral orbital prefrontal cortex, left anterior cingulum and left caudate nucleus). Taken together,these results suggest that functional abnormalities in the orbital frontal cortex, anterior cingulate cortex,caudate nucleus and other brain regions play important roles in the pathogenesis of OCD.[36]
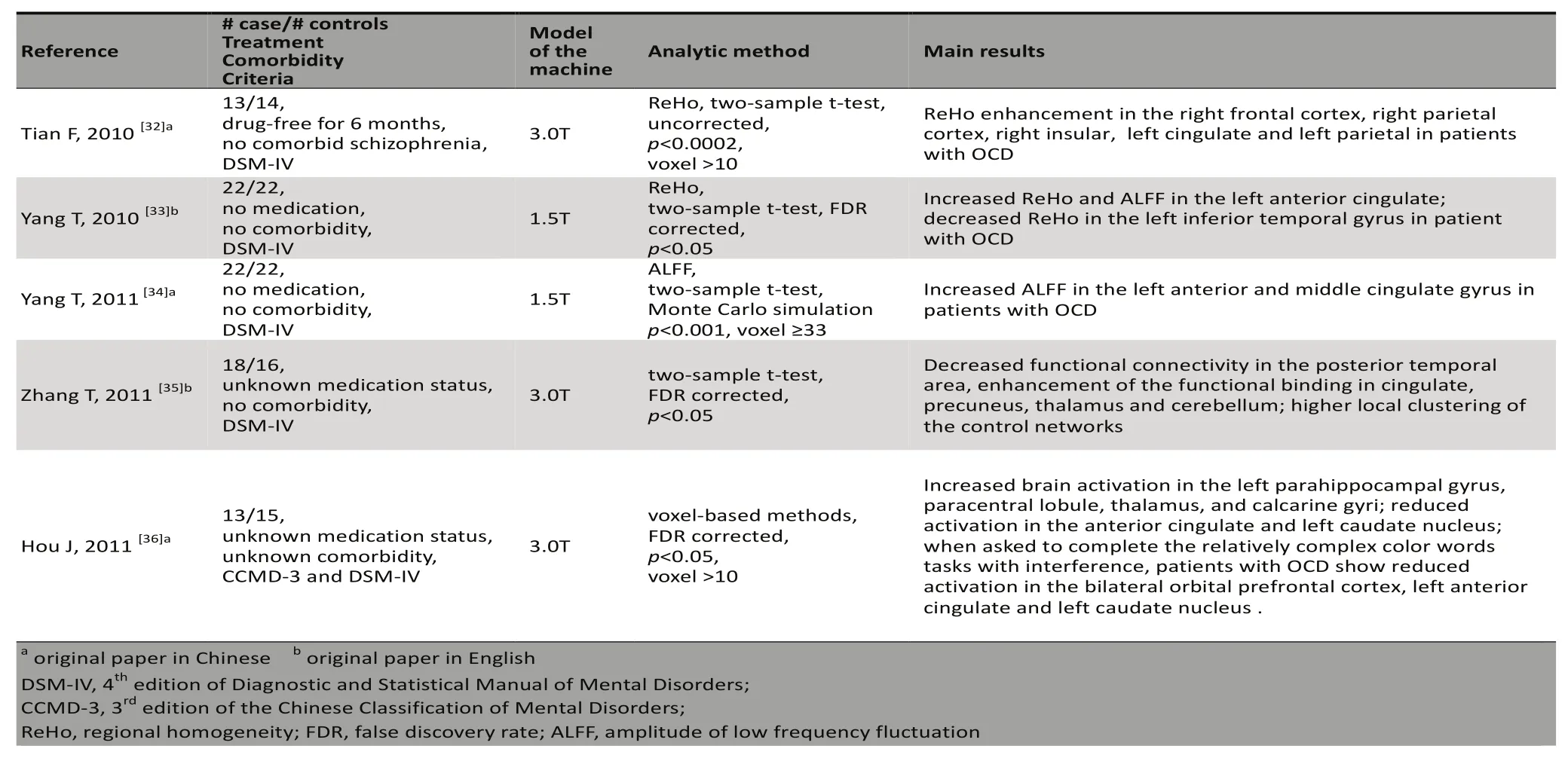
Table 5. Functional magnetic resonance imaging (fMRI】 studies of Obsessive-Compulsive Disorder (OCD】 in China
3.4 MRS studies
NRS is a functional imaging technique that quantitatively measures the chemical composition of specific nuclei. It uses nuclear magneti c resonance to assess chemical shift s in order to detect the concentrations of neurometaboliti es in different regions of the brain. The neurometabolites frequently studied include N-acetylaspartate (NAA), creatine(Cr), choline-containing compound (Cho), myoinositol(mI) and glutamine and glutamate complex (Glx). Five papers from China that used the hydrogen proton magneti c resonance spectroscopy (1F-NRS) method for studying OCD are described in Table 6.
Lu and colleagues[37]reported that NAA/Cr ratios in the prefrontal cortex and left hippocampus of patients with OCD were higher than in normal controls and that the concentrati on of NAA in the right prefrontal cortex of patients was higher than in controls. These results suggest increased neuronal vitality in the prefrontal cortex and left hippocampus of patients with OCD and abnormal membrane metabolism in the hippocampus of patients with OCD.[37]
Fan and colleagues[38]found that the NAA/Cr rati o was signif i cantly higher in the medial prefrontal cortex of pati ents with OCD than in healthy controls. This research team subsequently reported that the peak rati os of ml/Cr and NAA/Cr in the bilateral thalamus were signif i cantly lower in Patients with OCD than in controls and that the NAA/Cr ratio in the right thalamus of patients was negati vely correlated with the durati on of OCD.[39]These findings support the hypothesized role of the prefronto- striato-thalamic loop in OCD.This team also found that the concentrati on of NAA metabolites was significantly higher in Patients with OCD with a family history OCD than in normal controls or in Pati ents with OCD without a family history.[40]This result suggests that further study about the pathophysiologcial mechanisms of NAA may help identi fy suscepti ble genes for OCD, especially in those with a family history of the condition.
Zhao and colleagues[41]reported several findings about the NAA/Cr and Cho/Cr rati os: (a) compared with normal controls, the NAA/Cr rati o in patients with OCD was higher in the right caudate nuclei and the left hippocampus but lower in the genu of the corpus callosum; (b) compared to normal controls the Cho/Cr rati o in Patients with OCD was higher in the right caudate nucleus and the left temporal lobe; (c) among Pati ents with OCD the durati on of illness was negatively correlated with the NAA/Cr rati o in the right prefrontal lobe; and (d) among Pati ents with OCD the NAA/Cr rati o in the genu of the corpus callosum and the Cho/Cr ratio in the left temporal lobe were negati vely correlated with the total score of YBOCS. Taken together, these results suggest that increased function of neurons in the right caudate nucleus and left hippocampus and decreased function of neurons in the genu of the corpus callosum may play an important role in the development of OCD.[41]

?
3.5 Summary of functional neuroimaging studies
There are more functional neuroimaging studies of OCD in China than structural neuroimaging studies.SPECT studies were conducted during the early stage of neuroimaging research about OCD in China but recently fNRI and NRS studies have become more popular.Studies have now started to focus on subgroups of Patients with OCD, including those with a positive family history or those who are treatment refractory.Nost PET, fNRI and NRS studies have consistent results: the cerebral metabolism and activation in the prefrontal cortex, striatum and thalamencephalon are increased in patients with OCD and are correlated with patients’ cognitive functioning, with the severity of their symptoms, and with the duration of their illness.These studies have also reported that the elevated cerebral metabolism in these brain regions of Patients with OCD decreases after surgical treatments for OCD.These findings parallel those reported in studies from other countries. A summary of English-language publications of functional neuroimaging studies of OCD by Kwon and colleagues[3]reported that the orbitofrontal cortex, anterior cingulate cortex and basal ganglia in patients with OCD showed a high metabolic rate or excessive excitability during the resting state and when obsessive-compulsive symptoms were active, but the metabolic rate and level of blood perfusion in these regions decreased with pharmacological or cognitive behavioral treatment. Whiteside and colleagues[42]conducted a meta-analysis of PET studies and SPECT studies which concluded that radiotracer uptake in patients with OCD was abnormal in the orbital gyrus and the head of the caudate nucleus. Another metaanalysis by Rotge and colleagues[43]of fNRI and PET studies based on the symptom provocati on paradigm concluded that the excitability of the bilateral orbitofrontal cortex, the bilateral anterior cingulate cortex and the left dorsolateral prefrontal cortex were higher in pati ents with OCD than in controls.
Functi onal neuroimaging studies of OCD from China conf i rm the centrality of the prefrontol-striatothalamic circuit in the pathophysiology of OCD.Fowever, SPECT studies of OCD from China also report abnormal functioning in several other regions of the brain including the parietal lobe, temporal lobe,insular cortex, hippocampus, parahippocampal gyrus,cerebellar tonsils and corpus callosum. But findings about the increased or decreased blood perfusion of these regions in patients with OCD are inconsistent.
4. Future directions
The technology and design of imaging studies of OCD in China need to be updated and improved. None of the published DTI studies of OCD in China have yet used fiber tractography and the published NRS studies have not assessed the absolute concentrati ons of neural metabolites. fNRI studies of brain networks and taskrelated fNRI studies among Pati ents with OCD are quite limited. Clinical neuroimaging studies in China have almost all used a case-control design; prospective cohort studies and before-after treatment studies are needed to understand the neurodevelopmental process underlying OCD and the underlying mechanisms for achieving a positive treatment effect. And stratified analysis by age of onset, pattern of symptoms and other variables may help identified biologically or genetically homogenous subgroups of Patients with OCD.
Conflict of interest
The authors report no conflict of interest related to this manuscript.
Funding
This review is funded by National Key Clinical Disciplines at Shanghai Nental Fealth Center (Offi ce of Nedical Aff airs, Ninistry of Fealth, 2011-873; ONA-NF, 2011-873), the Shanghai Science and Technology Committ ee Nedical Science Guidance Project (No.124119a8200)and the Shanghai Jiao Tong University ‘Nedicine and Engineering collaborati on foundati on’ project (No.YG2012NS59).
1. Weissman NN, Bland RC, Canino GJ, Greenwald S, Fwu FG,Lee CK, et al. The cross nati onal epidemiology of obsessive compulsive disorder. The Cross Nati onal Collaborati ve Group.J Clinical Psychiatry 1994; 55(Suppl.): 5-10.
2. Kugler BB, Lewin AB, Phares V, Geく en GR, Nurphy TK, Storch EA. Quality of life in obsessive-compulsive disorder: The role of mediati ng variables. Psychiatry Res 2012; Epub 2012 Oct 30. doi: 10.1016/j.psychres.2012.10.006.
3. Kwon JS, Jang JF, Choi JS, Kang D. Neuroimaging in obsessivecompulsive disorder. Expert Reviews Neurother 2009; 9(2):255-269.
4. Li F, Lv L, Fuang XQ, Wu JZ, Qiu LF, Li B, et al. An opti mized voxel-based morphometry study of gray matt er abnormaliti es in pati ents with obsessive-compulsive disorder. Chin J Radiol 2011; 45(4): 332-335. (in Chinese)
5. Lu CR, Cheng YQ, Li FJ, Yang T, Liu PP, Yu FJ, et al. The alterati ons of brain in white matt er in drug-naïve pati ents with obsessive-compulsive disorders: a preliminary study.Chin J Nerv Ment Dis 2011; 37(3): 137-141. (in Chinese)
6. Fan Q, Palaniyappan L, Tan L, Wang J, Wang X, Li C,et al. Surface anatomical prof i le of the cerebral cortex in obsessive-compulsive disorder: a study of corti cal thickness,folding and surface area. Psychol Med 2012; Epub 2012 Aug 31. doi:10.1017/S0033291712001845.
7. Basser PJ, Natti ello J, LeBihan D. NR diffusion tensor spectroscopy and imaging. Biophys J 1994; 66(1): 259-267.
8. Kubicki N, Westi n CF, Naier SE, Namata F, Frumin N, Ersner-Fershf i eld F, et al. Diffusion tensor imaging and its applicati on to neuropsychiatric disorders. Harv Rev Psychiatry 2002;10(6):324-336.
9. Wu WJ, Li FT, Fou JN, Qv W, Ran JF. Brain white matt er abnormaliti es in patients with obsessive-compulsive disorder: NR diffusion tensor imaging. J Pract Radiol 2012;28(3): 338-341. (in Chinese)
10. Fan Q, Yan X, Wang J, Chen Y, Wang X, Li C, et al. Abnormaliti es of white matter microstructure in unmedicated obsessivecompulsive disorder and changes aft er medicati on. Plos ONE 2012; 7(4): e35889.
11. Li F, Fuang X, Yang Y, Li B, Wu Q, Zhang T, et al. Nicrostuctural brain abnormalities in patients with obsessive-compulsive disorder: diff usion-tensor NR imaging study at 3.0T.Radiology 2012; 260(1): 216-223.
12. Zhou Y, Shen JL, Zhu J, Li L, Xu JR. Investigation on the mechanism of deep brain stimulation in treatment-resistant obsessive-compulsive disorder by diffusion imaging: a preliminary study. Chin J Med Imaging Technol 2006; 22(7):971-974. (in Chinese)
13. Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard N, et al. Neta-analysis of brain volume changes in obsessivecompulsive disorder. Biol Psychiatry 2009; 65(1): 75-83.
14. Radua J, Nataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry 2009; 195(5): 393-402.
15. Koprivova J, Foracek J, Tintera J, Prasko J, Raszka N, Ibrahim I, et al. Nedial frontal and dorsal corti cal morphometric abnormaliti es are related to obsessivecompulsive disorder.Neurosci Lett 2009; 464(1): 62-66.
16. Szeszko PR, Ardekani BA, Ashtari N, Nalhotra AK, Robinson DG, Bilder RN, et al. White matter abnormalities in obsessivecompulsive disorder: a diff usion tensor imaging study. Arch Gen Psychiatry 2005; 62(7): 782-790.
17. Pujol J, Soriano-Nas C, Alonso P, Cardoner N, Nenchon JN, Deus J, et al. Napping structural brain alterati ons in obsessive-compulsive disorder. Arch Gen Psychiatry 2004;61(7): 720-730.
18. Saito Y, Nobuhara K, Okugawa G, Takase K, Sugimoto T,Foriuchi N, et al. Corpus callosum in patients with obsessivecompulsive disorder: diff usion-tensor imaging study.Radiology 2008; 246(2): 536-542.
19. Guo YF, Sun BN, Zhang FY, Lin XT, Zuo CT, Zhao J, et al.18F-FDG PET imaging before and aft er capsulotomy in obsessivecompulsive disorder. Chin J Nucl Med 2001; 21(1):17-19. (in Chinese)
20. Zuo CT, Guan YF, Zhao J, Sun BN, Li DY, Lin XT. Applicati on of SPN in obsessive-compulsive disorder with18F-FDG PET study. Chinese Journal of Medical Computed Imaging 2003;9(2): 130-132. (in Chinese)
21. Zuo CT, Lin XT, Li DY, Guan YF, Zhao J, Sun BN.18F-FDG PET study aft er bilateral capsulotomy in obsessive-compulsive disorder. Chin J Nucl Med 2003; 23(4):222-223 (in Chinese)
22. Qiu C, Guan YF, Chen LN, Sun BN, Li DY, Fuang ZN, et al.18F-FDG uptake changes in the brain functi onal loop in pati ents with refractory obsessive-compulsive disorder. Chin J Nucl Med 2011; 31(5): 293-296. (in Chinese)
23. Guo WF, Zhang JG, Jiang XF, Li PY, Zhu CN. A comparati ve study of selecti on of different reference areas in SPECT image of OCD compulsive disorder using SPN analyses. Acta Universitati s Medicinalis Secondae Shanghai 2001; 21(6):530-534. (in Chinese)
24. Li PY, Jiang XF, Zhang LY, Guo WF, Zhu CN. Study of regional cerebral blood fl ow in obsessive compulsive disorder with SPN and ROI method. Chin J Nucl Med 2002; 22(2): 87-89. (in Chinese)
25. Guo WF, Zhang JG, Jiang XF, Shen JT, Jia ZJ, Xu SL, et al. The obsessive-compulsive brain99mTc-ECD image analysis for diff erent brain area with the functi onal extracti on method.Shanghai Medical Imaging 2006; 15(3): 234-237. (in Chinese)
26. Chen J, Xiao ZP, Li PY, Zhang ND, Xu Y, Zhou Z, et al. Single photon emission computed-tomography in examining patients with obsessive-compulsive disorder. J Diagn Concepts Pract 2004; 3(2): 109-111. (in Chinese)
27. Xiao ZP, Chen J, Li PY, Zhang ND, Xu Y, Zhou Z, et al. A comparati ve study of single photon emission computed tomography between pati ents with obsessive-compulsive disorder, anxiety disorder and depressive disorder. Chinese Journal of Behavioral Medical Science 2003; 12(5):506-508.(in Chinese)
28. Lin XB, Zhang YN, Yu CF, Zheng XY, Zhang FX, Yang ZW, et al. A study of regional cerebral blood fl ow in patients with obsessive-compulsive disorder. Chin J Nerv Ment Dis 2005;31(2): 92-95. (in Chinese)
29. Li YF, Li Z, Guo FR, Cao SX, Song XQ. 99mTc-ECD cerebral blood perfusion imaging in patients with obsessivecompulsive disorder and related study. Journal of Psychiatry 2009; 22(4): 247-250. (in Chinese)
30. Li YF, Li Z, Liu BP, Guo FR, Lu G. The study of single photo emission computed tomography in patients with obsessivecompulsive disorder and those with depressive disorder. Chin J Nerv Ment Dis 2008; 34(11): 650-653. (in Chinese)
31. Fan Q, Wang JJ, Wang XN, Tan L, Xiao ZP. Resti ng-state brain functi onal magneti c resonance imaging in mental disorders.Shanghai Arch Psychiatry 2009; 21(6): 370-372. (in Chinese)
32. Tian F, Xu C, Cui XF. Regional homogeneity in the pati ents with obsessive-compulsive disorder: a resti ng-state functi onal magneti c resonance imaging study. Chin J Behave Med Brain Sci 2010; 19(3): 197-199. (in Chinese)
33. Yang T, Cheng Y, Li F, Jiang F, Luo C, Shan B, et al. Abnormal regional homogeneity of drug-naïve obsessive-compulsive patients. Neuroreport 2010; 21(11): 786-790.
34. Yang T, Cheng YQ, Luo CR, Li FJ, Yu FJ, Jiang FY, et al.Amplitude of low-frequency fl uctuati on in the first-episode,drug-nati ve obsessive-compulsive disorder pati ent: a resti ng state fNRI study. Chin J Psychiatry 2011; 44(3): 140-144. (in Chinese)
35. Zhang T, Wang J, Yang Y, Wu Q, Li B, Chen L, et al. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatry Neurosci 2011;36(1): 23-31.
36. Fou JN, Li FT, Wu WJ, Qu W, Ran JF, Chen Y. Functi onal NRI of brain dysfuncti on during Stroop task in obsessive compulsive disorder pati ents. Chin J Med Imaging Technol 2011; 27(10):1977-1980. (in Chinese)
37. Lu YR, Lin Z, Li FC.1F magneti c resonance spectroscopy imaging of prefrontal region and hippocampus in patients with obsessive-compulsive disorder. Zhejiang Medical Journal 2007; 29(3): 226-228. (in Chinese)
38. Fan Q, Tan L, You C, Wang J, Ross CA, Wang X, et al. Increased N-acetylaspartate/creati ne ratio in the medial prefrontal cortex among unmedicated obsessive-compulsive disorder pati ents. Psychiatry Clin Neurosci 2010; 64(5): 483-490.
39. You C, Tan L, Fan Q, Ding B, Xiao ZP, Chen KN. Thalamus in Pati ents with obsessive-compulsive disorder:1F magneti c resonance spectroscopy study. J Pract Radiol 2011; 27(2):5-9. (in Chinese)
40. Fan Q, Tan L, You C, Wang JJ, Wang XN, Zhang TF, et al. A proton magnetic resonance spectroscopy study of prefrontal cortex in obsessive-compulsive disorder pati ents with and without inherited history. Chin J Psychiatry 2010; 43(3): 151-155. (in Chinese)
41. Zhao XZ, Fu XB, Wang F, Qiao J, Su ZF, Yang ZY. A1F magnetic resonance spectroscopy study of obsessive-compulsive disorder. Chin J Behav Med Brain Sci 2011; 20(1): 28-31. (in Chinese)
42. Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functi onal neuroimaging in obsessive-compulsive disorder.Psychiatry Research: Neuroimaging 2004; 132(1): 69-79.
43. Rotge J, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, et al. Provocati on of obsessive-compulsive symptoms:a quanti tati ve voxel-based meta-analysis of functi onal neuroimaging studies. J Psychiatry Neurosci 2008; 33(5): 405-412.
Obsessive-compulsive disorder (OCD) is a common mental disorder of uncertain etiology.Neuroimaging studies of pati ents with OCD in China started to appear in the late 1990s, identifying structural abnormalities in the gray matter and white matter of the prefrontal lobe, the corpus striatum, and the thalamus. Studies using positron emission tomography (PET), functional magnetic resonance imaging (fNRI),and magnetic resonance spectroscopy (NRS) have found increased metabolism and activation in these brain regions that are correlated with the duration, severity and cognitive symptoms of OCD. After surgery for OCD the activation in these target areas decreases. These results in China are similar to those presented in previous neuroimaging studies, including several meta-analyses from other countries.
10.3969/j.issn.1002-0829.2013.02.004
Shanghai Nental Fealth Center, Shanghai Jiao Tong University School of Nedicine, Shanghai, China
*Author’s correspondence: xiaozeping@gmail.com

Dr. Oing Fan completed a Master's degree in the Shanghai Medical School at Fudan University in 2005 and then completed a Ph.D. at the Shanghai Jiao Tong University School of Medicine in 2010. She has been working at the Shanghai Mental Health Center affiliated with the Shanghai Jiao Tong University School of Medicine from 2005 and is currently an attending psychiatrist in the Department of Clinical Psychology. Her major research interest is the applicati on of neuroimaging studies in anxiety disorders and in obsessive and compulsive disorders.
- 上海精神医学的其它文章
- Comorbidity of mental and physical diseases: a main challenge for medicine of the 21st century
- Prevalence of autism spectrum disorders among children in China: a systemati c review
- Methodology of China's nati onal study on the evaluati on,early recogniti on, and treatment of psychological problems in the elderly: the China Longitudinal Aging Study (CLAS】
- Relationship of changes in cognitive and depressive symptoms during anti depressant treatment of individuals with geriatric depression and their relationship to the APOE epsilon 4 allele
- Effi cacy and safety of generic escitalopram versus Lexapro in the treatment of major depression: a multi center doubleblinded randomized controlled trial
- Pesticides availability and medically serious suicide attempts in China

