Effect of Media on the in vitro Degradation of Biodegradable Ureteral Stent
ZOU Ting(邹 婷),WANG Lu(王 璐) ,ZHANG Ming-qing(张明庆),LI Wen-chao(李文超),WANG Fu-jun(王富军),YU Cheng-long(于成龙),WANG Wen-zu(王文祖),CHEN Fang(陈方)
1 Key Laboratory of Textile Science and Technology,Ministry of Education,Donghua University,Shanghai 201620,China
2 College of Textiles,Donghua University,Shanghai 201620,China
3 Department of Urology,Shanghai Children's Hospital,Shanghai Jiaotong University,Shanghai 200040,China
Introduction
Ureteral stent,being commonly used in urology,is a special designed tubular stent inserted into the ureter for a few days or a few weeks to drain the urine flow from the kidney[1].
A biodegradable ureteral stent,which can also retain its supporting function for defined periods, would avoid a secondary removal surgery and the relevant complications including infection,stent encrustation,and epithelialization that the commercial used biostable stent may bring[2-3].Since it was first proposed by Lumiaho et al.in 1999[4],it has received more and more attention from researchers and urology doctors.Not only the enough mechanical properties for providing essential function within a certain time but also the proper degradation behavior are necessary for the degradable stent[5-6].
Poly-glycolic acid (PGA)and the copolymer of lactic and glycolic acid (PGLA)are the degradable materials.They have been approved by Food and Drug Administration (FDA)and used in commercial degradable sutures for decades which is proved to be safe for human use.For the biodegradable ureteral stent,the degradation process should be controllable and the products of the degradation should be as few as possible[7].In this study an enhanced braid-based degradable ureteral stent which was composed of PGA and PGLA was designed.We used these two degradable materials with different degradation rates to fabricate the bicomponent stent by braiding technology so as to achieve a controllable degradation process.
The mechanism and process of the stent's degradation are very critical due to its direct impact on the stents' usage.But the in vitro degradation study of biodegradable ureteral stent is less reported,even though some media have been used and compared to investigate the in vitro degradation of biodegradable materials[8-9].Here we describe the in vitro degradation with the degradable media of human urine (HU),artificial urine(AU),and phosphate buffer solution (PBS)so as to compare the effects of media on the degradation of stents and better understand the degradation mechanism.
1 Experimental
1.1 Materials
The biodegradable stents (Fig.1,the white one)used in this experiment were manufactured from PGA and PGLA multifilaments(Shanghai Tianqing Biomaterials Co.,Ltd.).With the preparation (Chinese Invention Patents:Publication No.CN102266594A,Publication No.CN103211671A)each stent contains two areas including fiber area and membrane area(Fig.2)which provide us a stent with an enhanced mechanical property.It means that the stent has an improved supporting effect and makes the surgical implantation procedure of it much easier than that of the traditional braided stent.The control stent is commercial used 6Fr double pigtail Percuflex®Plus (Fig.1,the black one)whose main component is polyurethane and remain stable in most degradation solutions.The detailed structural parameters are as follows (Table 1).

Fig.1 Two types of stents

Fig.2 The scanning electron microscope (SEM)of biodegradable ureteral stent(×35)
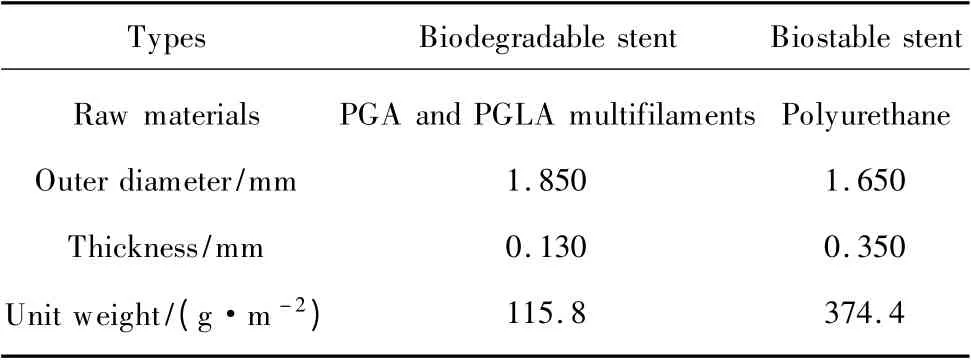
Table 1 Materials and their structural parameters
1.2 In vitro degradation
1.2.1 Degradation media
The AU has been widely used in most urology studies[10-11]whose contents and pH can be stabilized to conduct the in vitro degradation and other studies involved urine.But according to the previous preliminary study[12],the in vitro degradation rate of biodegradable ureteral stent was faster than that of in vitro degradation with the degradation medium of AU,which might be due to the difference between the two media.So the in vitro degradation with the medium of HU may simulate the in vivo degradation more accurately and the difference between the degradation with these two media need a further investigation.PBS is commonly used as a medium in most degradation study of degradable materials[8-9]for its controllable pH value.The PBS in this study had two different pH values which were just the same as those of HU and AU respectively so as to observe the influence of pH to the degradation.
So four kinds of media including HU (pH =7.4),AU(pH = 5.8),and PBS (pH = 5.8 and 7.4)were used as degradation media in this study.AU was prepared by dissolving CaCl2(0.65 g/L),MgCl2(0.65 g/L),NaCl (4.6 g/L),Na2SO4(2.3 g/L),Na3-citrate (0.65 g/L),Na2-oxalate(0.02 g/L),KH2PO4(2.8 g/L),KCl (1.6 g/L),NH4Cl(1.0 g/L),urea (25.0 g/L),and creatinine (1.1 g/L)in deionized(18.2 MΩ·cm)water.The pH was adjusted to 5.8 which was similar to that of the usual HU,and the mixture was filter sterilized through a 0.22 μm filter[10]while HU was collected from healthy male volunteers.All of the solutions were added with antimicrobial agent so as to prevent the fungal infection.
1.2.2 Degradation experiment
After being sterilized at 55℃for 2 h with ethylene oxide,several biodegradable and biostable stent samples were immersed in four kinds of degradable solutions and held at(37 ±1)℃in a constant temperature shaking incubator (speed:60 r/min).During the degradation,the solutions were changed every other day.At the same time the mechanical properties including radial compression and tensile strength tests and morphology including digital photos and SEM were observed and compared at different degradation time intervals of 0,7,14,21,28,and 35 d.
1.3 Testing methods
The tests were conducted at standard air condition ((20 ±2)℃,RH (65 ±2)%),and five trials were repeated for each stent.Because of the difference between the stent's properties in the wet and dry conditions,the stents should be immersed in deionized water for 2 h before the mechanical testing.On the contrary,they should be freeze-dried before the observation of their morphology.
1.3.1 Radial compressive test
It is important for the ureteral stent to resist compression and therefore to maintain the drainage of urine.The radial compression apparatus (LLY-06D,China) were used to measure the radial compression of stent.The experimental conditions were chosen as previously described[13]:the length of the samples:5 cm;displacement rate:10 mm/min;diameter of the presser foot:3 mm;maximum compression distance:50%of the stent's external diameter.We can get the radial compressive load (cN,compressed to 50%)results from this testing.
1.3.2 Tensile strength test
The results of tensile strength(MPa)could be got from the tensile strength testing with the universal textile mechanical tester (YG-B026H,China).The experimental conditions were as follows:tensile speed,50 mm/min;pre-tension,0 cN;gauge,30 mm.
1.3.3 Morphology
The specimens were gold sputtered and examined under a HITACHI/SU8010 SEM for the observation of the degradation.In addition,digital photos were also used to observe the overall degradation of stents.
1.3.4 Statistical analysis
Differences between the mechanical property results of stents in four kinds of medium and the Control stent were analyzed with a paired-sample student t-test.p(≤0.05)is considered statistically significant.
2 Results and Discussion
2.1 Mechanical properties
During the process of degradation, the mechanical properties including radial compressive load and tensile strength all decreased in four kinds of degradable media.
For the radial compression results (Fig.3),before the degradation,the radial compressive load of biodegradable stent(471.16 cN)was about thrice of that of commercial biostable stent (160.38 cN) which could resist the mechanical compression better (p <0.05).There was small difference between the stents in four media at the day of 7.Between the days of 7 and 14,the compressive load of stents in HU showed a dramatic fall from 354.37 to 101.40 cN,which decreased by over 70.0%,while the stents in other three media had the similar loads with that of biostable stent (160 cN).
At the day of 21,the stents in HU had no compressive load because of their complete degradation.In addition, the compressive loads of biodegradable stents in AU,PBS5.8,and PBS7.4 were 85.90,58.42,and 40.85 cN respectively,which were all lower than that of biostable stent (p <0.05).In summary,the stents in AU had the highest radial compressive load during the first three weeks.Although the stents in HU owned a favorable compressive load during the 1st week,it then declined rapidly and became lower to the biostable stent after 10 days of degradation.
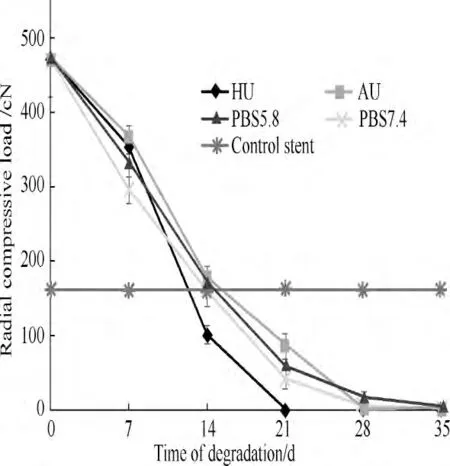
Fig.3 Changes of radial compressive load during degradation
Similarly,the tensile strength of biodegradable stents during degradation saw a decreasing trend (Fig.4).The tensile strength of degradable stents (75.06 MPa)were much higher than that of biostable stent (12.38 MPa)(p <0.05).There was also no significant difference between the tensile strength of stents in four kinds of degradation media at the day of 7.
Between the days of 7 and 14,the tensile strength of stents in HU declined by 56.0% from 65.74 to 28.90 MPa while that of stents in AU declined by 36.6% from 69.56 to 44.07 MPa.In comparison,the tensile strengths of stents in PBS5.8 and PBS7.4 were similar to each other and decreased much slightly than the other two.All of the degradable stents still had a higher tensile strength than biostable stents (p <0.05)after 14 d of degradation.The tensile strength of stents in PBS5.8 became the highest at the day of 21.This was then followed by AU and PBS7.4 (p <0.05).Likewise,during the degradation the tensile strength of stents in HU decreased the fastest which became lower than that of biostable stent at the day of 17.

Fig.4 Changes of tensile strength during degradation
2.2 Morphology
There were a number of stents in the petri dish for degradation (Fig.5 (a)).Each of them consisted of the membrane area and fiber area which had a uniform distribution on the stents (Fig.5(b)).No obvious difference could be seen from the morphology at the day of 7.
At the day of 14 (Fig.6),some sediment appeared only on the stents from the AU.There was a small amount of cracks on the membrane of stents in AU,PBS5.8,and PBS7.4,while the cracks on the stents in HU were more than the others'.Some of them were even going to fall off.
After 18 d of degradation (Fig.7),every stent in HU began to break into sections with fibers and membranes'complete fracture.

Fig.5 The biodegradable stents before degradation
At the day of 21 (Fig.8),all broken fragment in HU had already become small powder while the other three kinds of stents still remained the intact tubular shape.The number of membrane's cracks on the stents which immersed in AU and PBS7.4 were more than that in PBS5.8.There was still some sediments on the stents in AU.
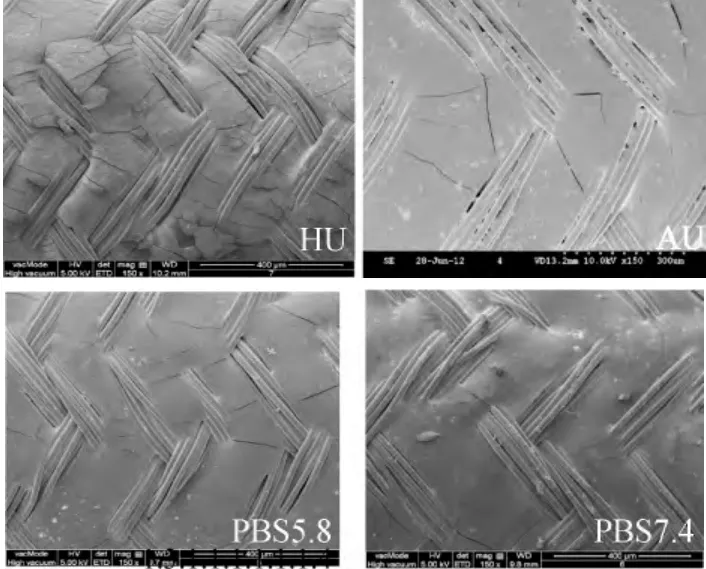
Fig.6 Morphology of degradable stents at the day of 14

Fig.7 Morphology of degradable stents in HU at the day of 18

Fig.8 Morphology of degradable stents at the day of 21
By the day of 28 (Fig.9),most stents have degraded greatly especially for the stents in AU and PBS7.4.Most of the membranes on the stents in AU had dropped off and were replaced with much sediment while the stents in PBS7.4 showed obvious transverse cracks on membrane.Some of the fibers'breakages appeared on the stents in AU and PBS7.4.
Ultimately,after 35 d of degradation (Fig.10),the stents in PBS7.4 disintegrated while the stents in PBS5.8 appeared to have the network structure of fiber with most of the membrane's fragments falling off.

Fig.9 Morphology of degradable stents at the day of 28

Fig.10 Morphology of degradable stents at the day of 35
In general,comparing the four kinds of media,the degradation rate of stents in HU was the highest which maintained 30% of their radial compression load before the day of 14,and began to break into fragments at the day of 18,finally became powder at the day of 21.The degradation rate of stents in AU was much slower than those in HU.The degradation behavior of stents in PBS with the pH of 7.4 and 5.8 were similar to each other with the result of becoming softer just as that in AU rather than breaking into fragments before the day of 35.
Comparing the stents in the media of PBS with the pH of 7.4 and 5.8,the degradation rate was a little bit faster with the degradable solution that was slightly alkaline.This result is just consistent with previous study[8]that it is the acid which gradually retards the hydrolysis that slowing the degradation rate,so that the degradation rate of stents in HU is influenced by its pH of about 7 which makes it faster than that of stents in AU with a pH of 5.8.But the difference between the degradation in these two media is so significant that it is not only influenced by pH but also some kinds of components in the urine which might be the trace elements and urine enzyme[14].Much sediment could be seen on the stents in the medium of AU while seldom in the media of HU and PBS.This is probably due to the deposition of calcium in AU.
For the two areas of the stents,the membrane area tends to degrade faster than the fibers with several obvious cracks.This may be due to the difference of crystalline structure of the two areas.Although the fibers look intact from the SEM,they have already fully degraded which can be seen from the results of mechanical testing.This is mainly because of the bulk degradation of the ester-based absorbable polymers including PGA and PGLA[15].
3 Conclusions
In summary,the in vitro degradation rate and process of stents in four kinds of media is extremely different from both the results of mechanical properties and morphological observations.The degradation rate of stents in HU was the highest.The impact of media's pH to the degradation is not obvious while the components in the media contribute much to the difference.Further work will be required to identify the actual influencing factors that cause the difference of degradation which will provide a guide for the future research on the in vitro degradation of biodegradable ureteral stent.
[1]Chew B H,Paterson R F,Clinkscales K W,et al.In vivo Evaluation of the Third Generation Biodegradable Stent:a Novel Approach to Avoiding the Forgotten Stent Syndrome[J].Journal of Urology,2013,189(2):719-725.
[2]El-Faqih S R, Shamsuddin A B, Chakrabarti A, et al.Polyurethane Internal Ureteral Stents in Treatment of Stone Patients:Morbidity Related to Indwelling Times[J].Journal of Urology,1991,146(6):1487-1491.
[3]Cevik I,Dillioglugil O,Akdas A,et al.Is Stent Placement Necessary after Uncomplicated Ureteroscopy for Removal of Impacted Ureteral Stones?[J].Journal of Endourology,2010,24(8):1263-1267.
[4]Lumiaho J,Heino A,Tunninen V,et al.New Bioabsorbable Polylactide Ureteral Stent in the Treatment of Ureteral Lesions:an Experimental Study[J].Journal of Endourology,1999,13(2):107-112.
[5]Li G,Wang Z X,Fu W J,et al.Introduction to Biodegradable Polylactic Acid Ureteral Stent Application for Treatment of Ureteral War Injury [J].BJU International,2011,108(6):901-906.
[6]Al-Aown A,Kyriazis L,Kallidonis P,et al.Ureteral Stents:New Ideas,New Designs[J].Therapeutic Advances in Urology,2010,2(2):85-92.
[7]Chew B H,Lange D,Paterson R F,et al.Next Generation Biodegradable Ureteral Stent in a Yucatan Pig Model [J].Journal of Urology,2010,183(2):765-771.
[8]Chu C C.An in vitro Study of the Effect of Buffer on the Degradation of Poly (glycolic acid)Sutures [J].Journal of Biomedical Materials Research,1981,15(1):19-27.
[9]Tomihata K,Suzuki M,Kada Y.The pH Dependence of Monofilament Sutures on Hydrolytic Degradation[J].Journal of Biomedical Materials Research,2001,58(5):511-518.
[10]Domergue R,Castano I,de Las Penas A,et al.Nicotinic Acid Limitation Regulates Silencing of Candida Adhesins during UTI[J].Science,2005,308(5723):866-870.
[11]Chutipongtanate S,Thongboonkerd V.Systematic Comparisons of Artificial Urine Formulas for in vitro Cellular Study [J].Analytical Biochemistry,2010,402(1):110-112.
[12]Shang Y F.Investigation of a Novel Braided Biodegradable Ureteral Stent in vitro and in vivo [D].Shanghai:Shanghai Jiaotong University,2012:59-64.(in Chinese)
[13]Liu G H,Hu H,Zhang P H,et al.Radial Compressive Properties of the Biodegradable Braided Regeneration Tubes for Peripheral Nerve Repair [J].Journal of Industrial Textiles,2006,36(1):35-46.
[14]Menei P,Daniel V,Monteromenei C,et al.Biodegradation and Brain Tissue Reaction to Poly (D,L-lactide-co-glycolide)Microspheres[J].Biomaterials,1993,14(6):470-478.
[15]Buddy D R,Allan S H,Frederick J S,et al.Biomaterials Science:an Introduction to Materials in Medicine[M].2nd ed.Singapore:Elsevier,2004:121-125.
——于成龙
 Journal of Donghua University(English Edition)2013年5期
Journal of Donghua University(English Edition)2013年5期
- Journal of Donghua University(English Edition)的其它文章
- Electrospun Small Diameter Tubes to Mimic Mechanical Properties of Native Blood Vessels Using Poly(L-lactide-co-ε-caprolactone)and Silk Fibroin:a Preliminary Study
- Properties of Scaffold Reinforcement for Tendon Tissue Engineering in vitro Degradation
- Mineralized Composite Nanofibrous Mats for Bone Tissue Engineering
- Promoted Cytocompatibility of Silk Fibroin Fiber Vascular Graft through Chemical Grafting with Bioactive Molecules
- Fatigue Performance of Fabrics of Stent-Grafts Supported with Z-Stents vs.Ringed Stents
- Radial Force Analysis of Polydioxanone Coronary Stent by Finite Element Method
