Apoptosis is an obstacle to the differentiation of adipose-derived stromal cells into astrocytes
Xiaodong Yuan, Qiaoyu Sun, Ya Ou, Shujuan Wang, Wenli Zhang, Hongliang Deng, Xiaoying Wu, Lili Zhang
1 Department of Neurology, Kailuan General Hospital, Hebei United University, Tangshan, Hebei Province, China
2 Department of Electron Microscopy, Hebei United University, Tangshan, Hebei Province, China
Apoptosis is an obstacle to the differentiation of adipose-derived stromal cells into astrocytes
Xiaodong Yuan1, Qiaoyu Sun1, Ya Ou1, Shujuan Wang1, Wenli Zhang2, Hongliang Deng1, Xiaoying Wu1, Lili Zhang1
1 Department of Neurology, Kailuan General Hospital, Hebei United University, Tangshan, Hebei Province, China
2 Department of Electron Microscopy, Hebei United University, Tangshan, Hebei Province, China
Xiaodong Yuan, Department of
Neurology, Kailuan General Hospital, Hebei United University, Tangshan
063000, Hebei Province, China,
yxd68@sohu.com.
Previous studies have demonstrated that nerve cells differentiated from adipose-derived stromal cells after chemical induction have reduced viability; however, the underlying mechanisms remained unclear. In this study, we induced the differentiation of adult adipose-derived stromal cells into astrocytes using chemical induction. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and flow cytometry showed that, with increasing induction time, the apoptotic rate gradually increased, and the number of living cells gradually decreased. Immunohistochemical staining demonstrated that the number of glial fibrillary acidic protein-, caspase-3- and caspase-9-positive cells gradually increased with increasing induction time. Transmission electron microscopy revealed typical signs of apoptosis after differentiation. Taken together, our results indicate that caspase-dependent apoptosis is an obstacle to the differentiation of adipose-derived stromal cells into astrocytes. Inhibiting apoptosis may be an important strategy for increasing the ef fi ciency of induction.
nerve regeneration; adult adipose-derived stromal cells; cell apoptosis; caspase-dependent apoptosis; cell differentiation; astrocytes; caspase-9; caspase-3; neural regeneration
Yuan XD, Sun QY, Ou Y, Wang SJ, Zhang WL, Deng HL, Wu XY, Zhang LL. Apoptosis is an obstacle to the differentiation of adipose-derived stromal cells into astrocytes. Neural Regen Res. 2014;9(8):837-844.
Introduction
Adipose-derived stromal cells are stem cells with multidirectional differentiation potential, and have numerous advantages, including strong proliferative ability, low immunogenicity and no legal or ethical issues (Zuk et al., 2001, 2002; McIntosh et al., 2006; Liu et al., 2007; Wu et al., 2013; Cao et al., 2014). Previous studies have shown that adult adipose-derived stromal cells can be successfully transformed into neurons and astrocytes (Safford et al., 2002; Ashjian et al., 2003; Yoshimura et al., 2007; Liu et al., 2010; Ye et al., 2010; Ou et al., 2011a, b). Astrocytes participate in various physiological process and brain development, and help maintain a stable environment for neurons. They also play a very important role in repair and regeneration after brain injury and neurodegeneration (Pekny and Nilsson, 2005; Jacobsen et al., 2006; Di Giorgio et al., 2007; Holden, 2007; Van Den Bosch and Robberecht, 2008). The in vitro induction of these astroglia has provided a new way for the treatment of clinical nervous system damage and neurodegenerative disorder (Kang et al., 2003; Goldman, 2005; Nakagami et al., 2006). However, when 3-isobutyl-1-methylxanthine was used to induce adipose-derived stromal cell differentiation, the survival time of the resulting astrocytes was only approximately 30 days (Liu et al., 2010; Ou et al., 2011b), limiting further research and clinical applications.
Our previous experiments showed that apoptosis is a major cause of death of neurons originating from adipose-derived stromal cells (Cai et al., 2011; Lu et al., 2012). Studies have shown that there are two types of apoptosis; caspase-dependent and caspase-independent (Daniel, 2000; Vittar et al., 2010; Zhang et al., 2011). In caspase-dependent apoptosis, the caspase family of proteases mediates apoptosis (Gil and Esteban, 2000; Chen and Wang, 2002; Kavitha et al., 2012). The caspases include two types of caspases (Ren et al., 2012; Tatsuta et al., 2013); initiators, such as caspase-9, which cleave and activate other caspases (von Roretz et al., 2013), and effectors, such as caspase-3, which cleave various substrates and decompose cell structure or inactivate enzymes (Kang et al., 2010; Wirawan et al., 2010). As an “executioner”protease, caspase-3 plays an important role in apoptosis (Compton and Cidlowski, 1986; Adrain et al., 2001; Slee et al., 2001). Previous studies have shown that caspase-dependent apoptosis occurs during adipose-derived stromal cell differentiation into neurons (Lu et al., 2012). Therefore, in this study, we investigated caspase-dependent apoptosis during adipose-derived stromal cell differentiation into astrocytes.
Materials and Methods
Extraction and culture of adult adipose-derived stromal cells

Figure 1 Changes in cell morphology before and after ADSC induction (inverted phase contrast microscope).

Figure 3 Positive expression rate of glial fi brillary acidic protein (GFAP), caspase-3 and caspase-9 in adult adipose-derived stromal cells differentiated at different time points (immunocytochemistry).

Figure 4 Survival rate of adult adipose-derived stromal cells differentiated at different time points assessed with MTT.
Volunteers were 13 healthy adults, aged 20-35 years, from the Physical Check-up Center, Kailuan General Hospital, Tangshan, Hebei Province, China. Needle aspiration was used to extract abdominal subcutaneous adipose tissue of adult volunteers without endocrine or hematological diseases. 10-30 mL adipose tissue was collected each time. Written informed consent from the volunteers was obtained. The protocol was approved by the Medical Ethics Committee of Kailuan General Hospital of Hebei United University, China.

Figure 2 Expression of GFAP, caspase-3 and caspase-9 at various time points after adipose-derived stromal cells differentiation (immunocytochemical staining, inverted phase contrast microscope, × 200).
Based on the method of Ye et al. (2010) and other experimental protocols, 0.1% collagenase type I (Solarbio, Beijing, China) was added, and the tissue was placed in a 37°C water bath for digestion for 1 hour and then centrifuged at 1,000 r/min for 5 minutes. Supernatant was aspirated out. The undigested tissues and the underlying cells were stirred to mix, and then fi ltered through a 100-mesh sieve. The samples were centrifuged at 1,000 r/min for 5 minutes, and the supernatant was removed. The remaining cell pellet was seeded into culture fl asks at a density of 8 × 103/cm2and placed in a 37°C, 5% CO2humidified incubator. The culture medium was replaced after 48 hours to remove residual erythrocytes and non-adherent impurities. The culture medium was replaced every 2-3 days. About 10-14 days after the cells reached 90% con fl uence, trypsin-ethylenediaminetetraacetic acid was used for digestion, and cells were passaged at a 1:2 ratio. Morphological changes in cells were observed using an inverted phase contrast microscope (Olympus, Tokyo, Japan).
Adult adipose-derived stromal cell differentiation into astrocytes
Passage 3-6 adipose-derived stromal cells, in good condition, were digested with trypsin-ethylenediaminetetraacetic acid and slides were prepared. When the cells reached 70-80% con fl uence, the medium was removed, inducer was added, and morphological changes were observed after 48 hours and 7, 14 and 21 days under the inverted phase contrast microscope. Inducer components: 0.5 mmol/L 3-isobutyl-1-methylxanthine (Sigma, St. Louis, MO, USA), 1 μmol/L dexamethasone, 10 μmol/L insulin, 200 μmol/L indomethacin, 10 mmol/L hydroxyethyl piperazine ethanesulfonic acid (Hyclone, Logan, UT, USA), 2 mmol/L glutamine, 1% non-essential amino acids, 0.5% absolute ethyl alcohol, 10% fetal bovine serum (Hyclone), 100 U/mL penicillin and 100 μg/mL streptomycin, and 85% high-glucose Dulbecco’s modi fi ed Eagle’s medium (DMEM) (Hyclone).
Glial fi brillary acidic protein, caspase-3 and caspase-9 expression in the induced cells
At 48 hours, and 7, 14 and 21 days after induction, cells were fi xed in 4% paraformaldehyde for 30 minutes, permeabilized using 0.1% TritonX-100 for 8 minutes, and incubated with 3% H2O2for 10 minutes. Cells were then incubated in working solutions of primary antibodies-rabbit anti-human glial fi brillary acidic protein (1:100; Beijing Biosynthesis Biotechnology, Beijing, China), caspase-9 (1:100; Beijing Biosynthesis Biotechnology) and rabbit anti-human caspase-3 monoclonal (1:100; Boster, Wuhan, Hubei Province, China) at 4°C overnight, and then with goat anti-rabbit IgG-horseradish peroxidase (1:50; Beijing Zhongshan Goldbridge, Beijing, China) for 30 minutes at 37°C. Labeling was visualized with 3,3′-diaminobenzidine (Beijing Zhongshan Golden BridgeBiotechnology), and cells were then stained with hematoxylin. Immunoreactive cells were counted at high magni fi cation (× 100). Five fields were quantified per sample. Three samples in each group were observed under the inverted phase contrast microscope.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay for cell viability after induction
Adipose-derived stromal cells at passages 3-6 were digested and seeded onto 12-well culture plates, and inoculated at a density of 1 × 105cells/well. At 48 hours, and 7, 14 and 21 days after induction, cells in each well were incubated with 5 mg/mL MTT (Sigma), 100 μL, at 37°C for 4 hours. The liquid in the well was discarded, and 1,000 μL dimethyl sulfoxide was added to each well. The plates were then agitated at a low speed of 1,000 r/min for 15 minutes. 100 μL aliquots of the solutions were transferred to a 96-well plate and the absorbance at 490 nm was measured using a microplate reader (Thermo Scientific, Pittsburgh, PA, USA). The absorbance is directly proportional to the number of living cells (Wyllie, 1980). Each experiment was repeated fi ve times.
Quanti fi cation of early apoptotic cells by fl ow cytometry
The cells induced for 48 hours, and 7, 14 and 21 days were digested and collected. A single cell suspension was prepared at a concentration of 1 × 106/mL and centrifuged at 1,000 r/min for 5 minutes. The supernatant was discarded, and then 190 μL buffer (Invitrogen, Carlsbad, CA, USA) and 10 μL PI dye solution (Invitrogen) were added. The samples were protected from light and quanti fi ed by fl ow cytometry (BD FACSCalibur, San Jose, CA, USA) within 1 hour. Each experiment was repeated three times.
Ultrastructural characteristics of astrocytes and apoptotic cells after induction
The cells induced for 14 days were digested, centrifuged, and fi xed in 3% glutaraldehyde and 1% osmic acid, followed by propionaldehyde dehydration and epoxy resin embedding. Cells were sliced using a microtome (Abnova, Walnut, CA, USA) and stained with 2% uranyl acetate and lead citrate. The ultrastructure of cells was observed and photographed with a transmission electron microscope (H7650, Hitachi, Tokyo, Japan).
Statistical analysis
All experimental data were analyzed using SPSS 13.0 (SPSS, Chicago, IL, USA). Measurement data were expressed as mean ± SD. Intragroup differences were compared using one-way analysis of variance followed by Student-Newman-Keuls test. Values of P < 0.05 were considered statistically signi fi cant.
Results
Morphological changes in cells differentiated from adipose-derived stromal cells
Primary cultured adipose-derived stromal cells adhered at 24 hours, and were observed under the inverted phase contrast microscope. Cells had a triangular and short fusiform appearance (Figure 1A). After 48 hours of culture, the cells had a long fusiform shape, similar to that of the fi broblasts. At 7-10 days, a large number of long spindle-shaped cells were arranged in a whorl pattern (Figure 1B). When passaged to the third generation, adipose-derived stromal cells were almost fusiform. At 24 hours after induction, the cytoplasm retracted to the nucleus (Figure 1C). At 48 hours after induction, round, polygonal or irregular-shaped cells were surrounded by a halo. Parts of cell bodies stretched out slender processes and multiple branches. The cytoplasm was uniform, the nucleus was oval or round, and nucleoli were visible. At 7 days after induction, some of the cells showed the shape of typical astrocytes. Simultaneously, cell protrusions were more extensive, slender branches increased in number and displayed a reticular appearance. The cytoplasm was uniform, the nucleus was large, oval or round, and more biased towards one side of the cell body. Nucleoli were clearly visible (Figure 1D). At 14 days after induction (Figure 1E), cell morphology showed no signi fi cant changes from that at 7 days. At 21 days after induction, the number of cells was significantly reduced (Figure 1F). Cells were triangular or irregularly shaped, and cell protrusions were shorter and less numerous than at 14 days of induction. The nucleus showed no signi fi cant changes compared with day 14.
Expression of glial fi brillary acidic protein, caspase-3 and caspase-9 during adipose-derived stromal cell differentiation
At 48 hours of induction, glial fi brillary acidic protein expression was observed in a part of the cytoplasm by immunocytochemical staining. The number of glial fi brillary acidic protein-positive cells gradually increased (P < 0.05), and peaked at 14 days. At 21 days, there was no signi fi cant difference in the number of glial fi brillary acidic protein-positive cells compared with 14 days (P > 0.05). Expression of caspase-3 and caspase-9 was mainly observed in the cytoplasm. The number of caspase-3- and caspase-9-positive cells gradually increased over the induction period (P < 0.05; Figures 2, 3).
Cell survival after adult adipose-derived stromal cell differentiation
MTT assay showed that the survival rate of cells gradually decreased over time: at 48 hours, and 7, 14 and 21 days of induction (P < 0.05; Figure 4).
Early apoptosis after adult adipose-derived stromal cell differentiation
Flow cytometry revealed that at 48 hours, and 7, 14 and 21 days after induction, the apoptotic rate was 4.43 ± 0.26%, 7.80 ± 0.53%, 11.93 ± 0.34% and 14.72 ± 0.43%, respectively. The apoptotic rate gradually increased over time: at 48 hours, and 7, 14 and 21 days (P < 0.05; Figure 5).
Apoptosis and ultrastructural features of astrocytes differentiated from adipose-derived stromal cells
Under the transmission electron microscope, at 14 days after differentiation, the cell bodies of astrocytes were roundor oval. The membrane surface was not smooth, and there were numerous processes. There were varying amounts of bundles of fibers, endoplasmic reticulum, lysosomes and mitochondria in the cytoplasm. Mitochondrial volume was large, and nuclei were oval or irregularly shaped, having 1 or 2 nucleoli, more euchromatin, and reduced and dispersed heterochromatin. The cell bodies of apoptotic astrocytes narrowed, processes from the cell membrane were noticeably decreased or missing, and the surface was smooth. In the cytoplasm, mitochondria exhibited swelling, volume was increased, and the cristae were ruptured and vacuolated. Nuclei were irregular; the nuclear membranes were wrinkled and retracted or even showed karyolysis. Chromatin was reduced, but the amount of heterochromatin was increased. The nuclear chromatin was condensed, and often accumulated near the edges of the nuclear membrane, with blocky or crescent-shaped bodies (Figure 6).
Discussion
Numerous studies have shown that adipose-derived stromal cells can successfully differentiate into astrocytes in vitro (Safford et al., 2002; Ashjian et al., 2003; Liu et al., 2010; Ou et al., 2011b). The induced cells have the typical morphology of astrocytes and express specific markers, and have a characteristic ultrastructure and electrophysiological properties (Ou et al., 2011a). However, our previous experiments showed that many cells died during adipose-derived stromal cell differentiation, and the number of viable cells decreased with increasing length of induction (Liu et al., 2010; Ou et al., 2011b), thereby limiting further research and hindering the application of astrocytes differentiated from adipose-derived stromal cells. Indeed, in the present study, although the expression rate of glial fi brillary acidic protein, the speci fi c marker of astrocytes, reached a peak of 79.2% at 14 days, the number of viable cells had decreased. Therefore, it is necessary to perform further research on the mechanisms of cell death during the differentiation of adipose-derived stromal cells into astrocytes, and to optimize cell differentiation protocols to improve the survival rate of astrocytes differentiated from adipose-derived stromal cells.
Apoptosis is a process of programmed cell death (Wyllie, 1980; Johansson et al., 2010; Giansanti et al., 2011; Freire, 2012; Igder et al., 2013; Tognon et al., 2013). Our previous studies showed that apoptosis is a major cause of the death of neurons differentiated from adipose-derived stromal cells (Cai et al., 2011; Lu et al., 2012). As the preferred method for early detection of apoptotic cells, Annexin V/PI double-staining and fl ow cytometry can be used to analyze early apoptotic cells ef fi ciently (Nicoletti et al., 1991; Grebeňová et al., 2004; Samsel et al., 2004; Suárez et al., 2004; Guo et al., 2011; Wlodkowic et al., 2013). Therefore, we studied cell death rate in the early stage by fl ow cytometry. The results demonstrated that the death rate increased over time, peaking at 21 days of induction. This further con fi rmed that apoptosis is a major cause of cell death.
Apoptosis includes caspase-dependent apoptosis and caspase-independent apoptosis (Liu et al., 2013). In caspase-dependent apoptosis, the caspase family is a key mediator of cell death. As the preferred method for detecting apoptosis (Zhou et al., 2012), transmission electron microscopy plays a crucial role in its detection (Ji et al., 2011; Würstle et al., 2012). Our fi ndings revealed that caspase-dependent apoptosis is a major apoptotic pathway during cell differentiation. Furthermore, the numbers of caspase-9- and caspase-3-positive cells increased over time and were associated with apoptosis of astrocytes differentiated from adipose-derived stromal cells. The expression rate of caspase-9, the main initiation factor of apoptosis (Fombonne et al., 2012; White et al., 2012; Brentnall et al., 2013; Qin et al., 2013), reached 30.27% at 21 days of induction. However, for caspase-3, the rate was 46.47%, signi fi cantly higher than that for caspase-9, suggesting a significant amplification effect. For both caspase-3 and caspase-9, expression is mainly in the cytoplasm, especially around the nucleus; however, there is no signi fi cant expression in the protrusions of the induced cells. The expression of caspase-9 was less than the expression of executioner caspase-3, suggesting an amplification effect in the process of induction.
During the differentiation of adipose-derived stromal cells into astrocytes, expression of glial fi brillary acidic protein, an important marker of astrocytes (Bernal and Peterson, 2011; Sokolowski et al., 2011; Yeh et al., 2011; Sukumari-Ramesh et al., 2012; Hoppe et al., 2013), is evenly detected in the cytoplasm as well as in the protrusions. Glial fi brillary acidic protein bundles are evenly distributed in the cytoplasm as well as in the cell processes of normal astrocytes differentiated from adipose-derived stromal cells. More organelles are observed, including mitochondria, endoplasmic reticulum and lysosomes. There is more euchromatin and less heterochromatin in nuclei, and the heterochromatin is dispersed. In contrast, in the cytoplasm of apoptotic cells, the mitochondria, concentrated around the nuclei, were signi fi cantly swollen, the cristae were ruptured, and vacuolization was observed. The membranes of nuclei were wrinkled, and some were fragmented. The chromatin was condensed and distributed at the membrane, with a block or crescent shape, typical of apoptosis. Finally, cell morphological changes, including shrinkage, and a decrease or even disappearance of protrusions and microvilli occur.
In summary, adipose-derived stromal cells can differentiate into astrocytes using a chemical inducer mainly consisting of 3-isobutyl-1-methylxanthine. The number of living cells decreases over the duration of induction. Caspase-dependent apoptosis is the main pathway of apoptosis during induction.
Acknowledgments:The author would like to thank Yang XL and Bo Y of Cosmetic Plastic Surgery Center from Kailuan General Hospital in China. Constant supports and technical assistance have been offered generously by teachers from the Experimental Center of Hebei United University in China.
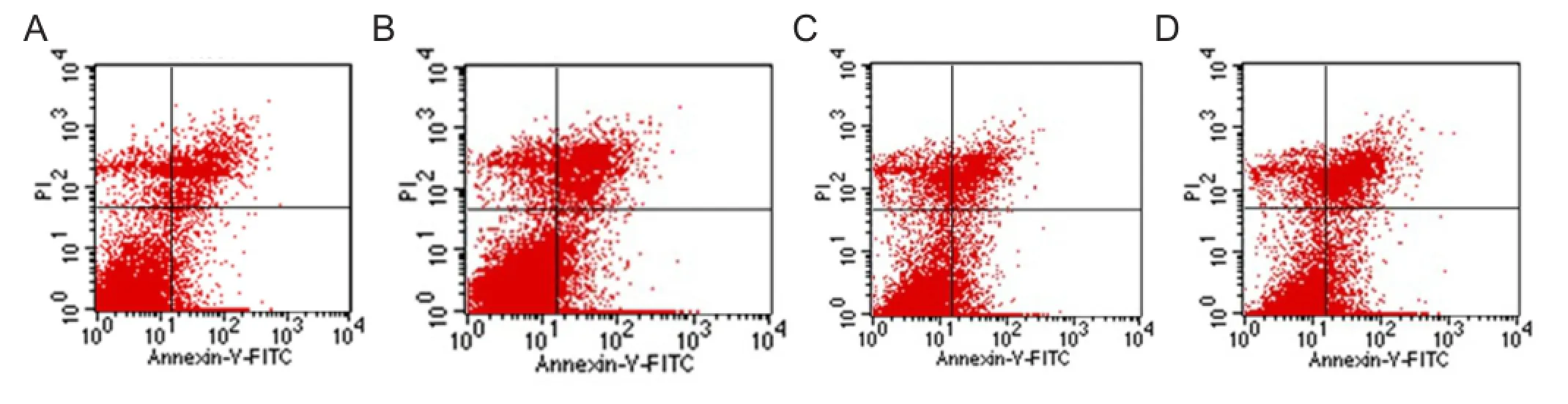
Figure 5 Quantitative distribution of early apoptosis of Adipose-derived stromal cells at various time points after differentiation, as detected by fl ow cytometry.

Figure 6 Ultrastructural characteristics of adipose-derived stromal cells-differentiated astrocytes and apoptotic cells at 14 days (transmission electron microscopy).
Author contributions:Yuan XD was responsible for writing the manuscript, study design, concept, implementation and data arrangement. Sun QY, Ou Y and Zhang LL performed the whole experiment. Wang SJ and Deng HL assisted in data collection and analysis. Zhang WL was responsible for images analysis of electron microscopy. Wu XY provided instruments, equipment and reagents. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Peer review:This study found that caspase-dependent apoptosis is a reason for the death of astrocytes differentiated from adipose-derived stromal cells. The inhibition of this kind of apoptosis may be an important measure to promote the induction effects of astrocytes. This provides a substance basis for treatment and repair of brain injury and neurodegenerative disease using astrocytes.
Adrain C, Creagh EM, Martin SJ (2001) Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J 20:6627-6636.
Ashjian PH, Elbarbary AS, Edmonds B, DeUgarte D, Zhu M, Zuk PA, Lorenz HP, Benhaim P, Hedrick MH (2003) In vitro di ff erentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg 111:1922-1931.
Bernal GM, Peterson DA (2011) Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells. Aging cell 10:466-482.
Brentnall M, Rodriguez-Menacol L, De Guevara RL, Cepero E, Boise LH (2013) Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 14:32.
Cai YN, Yuan XD, Ou Y, Lu YH (2011) Apoptosis during β-mercaptoethanol-induced di ff erentiation of adult adipose-derived stromal cells into neurons. Neural Regen Res 6:750-755.
Chen M, Wang J (2002) Initiator caspases in apoptosis signaling pathways. Apoptosis 7:313-319.
Compton MM, Cidlowski JA (1986) Rapid in vivo e ff ects of glucocorticoids on the integrity of rat lymphocyte genomic deoxyribonucleic acid. Endocrinology 118:38-45.
Daniel P (2000) Dissecting the pathways to death. Leukemia 14:2035-2044.
Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K (2007) Non-cell autonomous e ff ect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci 10:608-614.
Fombonne J, Bissey PA, Guix C, Sadoul R, Tibert C, Mehlen P (2012) Patched dependence receptor triggers apoptosis through ubiquitination of caspase-9. Proc Natl Acad Sci U S A 109:10510-10515.
Freire MA (2012) Pathophysiology of neurodegeneration following traumatic brain injury. West Indian Med J 61:751-755.
Giansanti V, Torriglia A, Scovassi AI (2011) Conversation between apoptosis and autophagy:“is it your turn or mine?”. Apoptosis 16: 321-333.
Gil J, Esteban M (2000) Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis 5:107-114.
Goldman S (2005) Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol 23:862-871.
Grebeňová D, Kuželová K, Fuchs O, Halada P, Havlı́ček V, Marinov I, Hrkal Z (2004) Interferon-alpha suppresses proliferation of chronic myelogenous leukemia cells K562 by extending cell cycle S-phase without inducing apoptosis. Blood Cells Mol Dis 32:262-269.
Guo FH, Chen DM, Liu Z, Wen K, Du J (2011) Progress of apoptosis imaging using Annexin V labeled with positron radionuclides. Yuanzineng Kexue Jishu 45:908-914.
Holden C (2007) Neuroscience. Astrocytes secrete substance that kills motor neurons in ALS. Science 316:353.
Hoppe JB, Rattray M, Tu H, Salbego CG, Cimarosti H (2013) SUMO-1 conjugation blocks beta-amyloid-induced astrocyte reactivity. Neurosci Lett 546:51-56.
Igder S, Asadikaram GR, Sheykholeslam F, Sayadi AR, Mahmoodi M, Kazemi Arababadi M, Rasaee MJ (2013) Opium induces apoptosis in Jurkat cells. Addict Health 5:27-34.
Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, Martone R, Morrison JH, Pangalos MN, Reinhart PH, Bloom FE (2006) Early-onset behavioral and synaptic de fi cits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 103:5161-5166.
Ji YB, Qu ZY, Zou X (2011) Juglone-induced apoptosis in human gastric cancer SGC-7901 cells via the mitochondrial pathway. Exp Toxicol Pathol 63:69-78.
Johansson AC, Appelqvist H, Nilsson C, Kågedal K, Roberg K, Öllinger K (2010) Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis 15:527-540.
Kang JW, Kim JH, Song K, Kim SH, Yoon JH, Kim KS (2010) Kaempferol and quercetin, components of Ginkgo biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytother Res 24:S77-82.
Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS (2003) Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol 183:355-366.
Kavitha K, Vidya Priyadarsini R, Anitha P, Ramalingam K, Sakthivel R, Purushothaman G, Singh AK, Karunagaran D, Nagini S (2012) Nimbolide, a neem limonoid abrogates canonical NF-κB and Wnt signaling to induce caspase-dependent apoptosis in human hepatocarcinoma (HepG2) cells. Eur J Pharmacol 681:6-14.
Liu TM, Martina M, Hutmacher DW, Hui JHP, Lee EH, Lim B (2007) Identi fi cation of common pathways mediating di ff erentiation of bone marrow-and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 25:750-760.
Liu YH, Yuan XD, Ye CQ, Ou Y, Cai YN (2010) Adult adipose-derived stem cells di ff erentiation into a satrocyte cells morphology and ultrastructure in vitro. Zhonghua Xingwei Kexue yu Nao Kexue Zazhi 19: 617-620.
Lu YH, Yuan XD, Ou Y, Cai YN, Wang SJ, Sun QY, Zhang WL (2012) Autophagy and apoptosis during adult adipose-derived stromal cells di ff erentiation into neuron-like cells in vitro. Neural Regen Res 7:1205-1212.
McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Di Halvorsen Y, Ting JP, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM (2006) The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells 24:1246-1253.
Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y (2006) Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Tromb 13:77.
Nicoletti I, Migliorati G, Pagliacci M, Grignani F, Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and fl ow cytometry. J Immunol Methods 139:271-279.
Ou Y, Yuan XD, Cai YN, Lu YH (2011a) Ultrastructure and electrophysiology of astrocytes differentiated from adult adipose-derived stromal cells. Chin Med J (Engl) 124:2656-2660.
Ou Y, Yuan XD, Cai YN, Lu YH (2011b) A novel ethanol-based method to induce di ff erentiation of adipose-derived stromal cells into astrocytes. Neural Regen Res 6:738-743.
Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50:427-434.
Qin S, Yang C, Wang X, Xu C, Li S, Zhang B, Ren H (2013) Overexpression of Smac promotes cisplatin-induced apoptosis by activating caspase-3 and caspase-9 in lung cancer A549 cells. Cancer Biother Radiopharm 28:177-182.
Ren SX, Cheng AS, To KF, Tong JH, Li MS, Shen J, Wong CC, Zhang L, Chan RL, Wang XJ, Ng SS, Chiu LC, Marquez VE, Gallo RL, Chan FK, Yu J, Sung JJ, Wu WK, Cho CH (2012) Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res 72:6512-6523.
Safford KM, Hicok KC, Safford SD, Halvorsen Y-DC, Wilkison WO, Gimble JM, Rice HE (2002) Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 294:371-379.
Samsel L, Zaidel G, Drumgoole HM, Jelovac D, Drachenberg C, Rhee JG, Brodie AM, Bielawska A, Smyth MJ (2004) Te ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate 58:382-393.
Slee EA, Adrain C, Martin SJ (2001) Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276:7320-7326.
Sokolowski JD, Nobles SL, He ff ron DS, Park D, Ravichandran KS, Mandell JW (2011) Brain-speci fi c angiogenesis inhibitor-1 expression in astrocytes and neurons: implications for its dual function as an apoptotic engulfment receptor. Brain Behav Immun 25:915-921.
Suárez L, Vidriales M-B, García-Laraña J, Sanz G, Moreno M-J, López A, Barrena S, Martínez R, Tormo M, Palomera L, Lavilla E, López-Berges MC, de Santiago M, de Equiza ME, Miguel JF, Orfao A (2004) CD34+cells from acute myeloid leukemia, myelodysplastic syndromes, and normal bone marrow display different apoptosis and drug resistance-associated phenotypes. Clin Cancer Res 10:7599-7606.
Sukumari-Ramesh S, Alleyne CH, Dhandapani KM (2012) Astrocytespecific expression of survivin after intracerebral hemorrhage in mice: a possible role in reactive gliosis? J Neurotrauma 29:2798-2804.
Tatsuta T, Hosono M, Sugawara S, Kariya Y, Ogawa Y, Hakomori S, Nitta K (2013) Sialic acid-binding lectin (leczyme) induces caspase-dependent apoptosis-mediated mitochondrial perturbation in Jurkat cells. Int J Oncol 43:1402.
Tognon R, Nunes ND, Castro FA (2013) Apoptosis deregulation in myeloproliferative neoplasms. Einstein (Sao Paulo) 11:540-544.
Van Den Bosch L, Robberecht W (2008) Crosstalk between astrocytes and motor neurons: what is the message? Exp Neurol 211:1-6.
Vittar NB, Awruch J, Azizuddin K, Rivarola V (2010) Caspase-independent apoptosis, in human MCF-7c3 breast cancer cells, following photodynamic therapy, with a novel water-soluble phthalocyanine. Int J Biochem Cell Biol 42:1123-1131.
von Roretz C, Lian XJ, Macri AM, Punjani N, Clair E, Drouin O, Dormoy-Raclet V, Ma JF, Gallouzi IE (2013) Apoptotic-induced cleavage shifts HuR from being a promoter of survival to an activator of caspase-mediated apoptosis. Cell Death Di ff er 20:154-168.
Würstle ML, Laussmann MA, Rehm M (2012) Te central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res 318:1213-1220.
White MJ, Schoenwaelder SM, Josefsson EC, Jarman KE, Henley KJ, James C, Debrincat MA, Jackson SP, Huang DC, Kile BT (2012) Caspase-9 mediates the apoptotic death of megakaryocytes and platelets, but is dispensable for their generation and function. Blood 119:4283-4290.
Wirawan E, Walle LV, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, Roelandt R, De Rycke R, Verspurten J, Declercq W, Agostinis P, Vanden Berghe T, Lippens S, Vandenabeele P (2010) Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis 1:e18.
Wlodkowic D, Skommer J, Akagi J, Fujimura Y, Takeda K (2013) Multiparameter analysis of apoptosis using lab-on-a-chip fl ow cytometry. Curr Protoc Cytom 66:9.42.41-49.42.15.
Wyllie A (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284:555-556. Ye CQ, Yuan XD, Liu H, Cai YN, Ou Y (2010) Ultrastructure of neuronal-like cells di ff erentiated from adult adipose-derived stromal cells. Neural Regen Res 5:1456-1463.
Yeh CY, Vadhwana B, Verkhratsky A, Rodriguez JJ (2011) Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer’s disease. ASN Neuro 3:271-279.
Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I (2007) Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 327:449-462.
Zhang N, Chen Y, Jiang R, Li E, Chen X, Xi Z, Guo Y, Liu X, Zhou Y, Che Y, Jiang X (2011) PARP and RIP 1 are required for autophagy induced by 11′-deoxyverticillin A, which precedes caspase-dependent apoptosis. Autophagy 7:598-612.
Zhou A, Wang H, Lan K, Zhang X, Xu W, Yin Y, Li D, Yuan J, He Y (2012) Apoptosis induced by pneumolysin in human endothelial cells involves mitogen-activated protein kinase phosphorylation. Int J Mol Med 29:1025-1030.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211-228.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279-4295.
Copyedited by Patel B, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
Retraction Notice
The following article from Neural Regeneration Research, ‘Brain-derived neurotrophic factor inhibits glucose intolerance after cerebral ischemia’ by Shu et al., published on Volume 8, Issue 25, Pages 2370-2378 has been retracted by agreement between the authors and the journal. The retraction has been agreed because the figures and corresponding descriptions included in the article is a duplication of the paper by Harada et al. that had already appeared in J Pharmacol Sci, 118, 109-116; 2012.
One of the conditions of submission of a paper for publication is that authors declare explicitly that their work is original and has not been submitted for or appeared in a publication elsewhere. As such this article represents a violation of the journal’s publishing policies.
Reference
Shu XL, Zhang YS, Xu H, Kang K, Cai DL (2013) Brain-derived neurotrophic factor inhibits glucose intolerance after cerebral ischemia. Neural Regen Res 8:2370-2378.
10.4103/1673-5374.131600
http://www.nrronline.org/
Accepted: 2014-03-28
- 中国神经再生研究(英文版)的其它文章
- The synthetic thyroid hormone, levothyroxine, protects cholinergic neurons in the hippocampus of naturally aged mice
- Citalopram increases the differentiation ef fi cacy of bone marrow mesenchymal stem cells into neuronal-like cells
- Fusion protein of single-chain variable domain fragments for treatment of myasthenia gravis
- Similar effects of substance P on learning and memory function between hippocampus and striatal marginal division
- Regulatory effects of anandamide on intracellular Ca2+concentration increase in trigeminal ganglion neurons
- Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases

