A Study on the Contract Research Organization
LIANG Yu,TIAN Li-juan*
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
A Study on the Contract Research Organization
LIANG Yu,TIAN Li-juan*
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Objective To provide a reference for the development of contract research organizations. Methods Literature was studied to summarize the development background,current status of the contract research organizations and the problems they were facing. Results and Conclusion Contract research organizations should take measures from the following aspects,such as international cooperation,contract research organization union,industry chain,quality authentication system and talent training,etc. to accelerate its development.
contract research organization; development status; trend; problem; suggestion
As a product of enterprises drug R&D and innovation,clinical contract research organization (CRO) develops fast in China,bringing many good results to our pharmaceutical company. Yet compared with foreign CRO,there is a large gap. As the clinical trials shift from the developed countries to the Asia-Pacific region,foreign CRO entered China’s market,and our CRO faces more challenges. This paper studied China’s CRO development process combined with future trends to offer some suggestions.
1 The definition of CRO and its rising background
The definition of CRO in GCP: CRO is an academic or commercial scientific institution and the applicant can entrust it to perform some tasks in clinical trials,but they must make a written trust[1].
According to the statistics,pharmaceutical companies can save about 2 years by outsourcing clinical trials to CRO,which is 30% faster than the pharmaceutical companies. Through the collaboration with CRO,it will shorten 3−5 years to develop a new drug[2].
The birth of CRO in China is due to the following reasons such as a large number of pharmaceutical companies want to change the R&D model,the guidance of national policy and the entry of foreign CRO company into China’s market.
2 The development status of CRO
2.1 The birth of CRO
The birth of CRO in China is late compared with America. The first CRO company,Beijing KendleWits Medical Consulting Co.,Ltd. was set up in 1997. In 2000,a large number of CRO companies emerged in China. At the same time,some large foreign CRO companies like Quintiles,Covance,ICON,and PPD also set up their offices in China.
This emerging industry quickly attracted widespread attention and it was easy to establish a CRO company due to its low barriers,a little capital investment and few staffs. Therefore,a large number of CRO companies quickly appeared in the market. Many overseas Chinese saw the bright future of CRO industry and they chose to set up their CRO companies in China.
2.2 CRO services
As the market is expanding,CRO provides more services. At the initial stage,CRO provides a limited number of services. Now the company offers a wide range of services like chemistry preclinical pharmacology,toxicology preclinical experiments,new drug development,application for approval of new drugs,other clinical trials and related business content[3].
2.3 The current situation of China’s CRO
Due to their disadvantage that they are unfamiliar with Chinese law,regulation and organization for drug clinical trial,foreign CRO has encouraged mergers and reorganizations of China’s CRO companies. Among the 3 famous Chinese CRO companies,Excel PharmaStudies,Inc. was merged by PPD,and Icon merged with KendleWits,only Tigermed Consulting Ltd. is still independent. In 2008,due to its outstanding performance,Qiming invested 500 million in it,making Tigerme the leader of the industry.
According to the data from Frost & Sullivan research,the growth of China’s CRO market in 2007−2009 was 27.1 percent,and of the growth was 20.7% in 2009−2013[4]. In 2010 there were about 916 clinical trials approved by the State Council,and CRO undertook about 40% of the projects. CRO played an important role in the drug development. In 2012,China had nearly 200 CRO companies and the market size was nearly billion[5]. As to the scales of CRO companies and their rankings,we do not have accurate data. Based on the Tigermed’s prospectus,we made Table 1 to introduce several large CRO companies in China.

Table 1 Introduction of some large CRO companies
3 The development trend of CRO
By retrieving documents and the latest articles on the industry,we could make the conclusion that the development trend of CRO included internationalization,professional diversification and standardization etc.
3.1 CRO develops rapidly and it shifts to China
Global pharmaceutical R&D outsourcing industry grows rapidly. Data showed that contract research organization had a share of 19 billion dollars in the global investment in biopharmaceutical drugs development in 2013. And it is expected to reach 23 billion dollars. The annual growth rate of global clinical research outsourcing would be 6%−8%[6]during the period of 2013−2016. With the advantages of low labor costs,shorter time to recruit patients (large population,many diseases) and professional personnel reserves,China is attracting clinical trials increasingly.
3.2 Strengthening CRO industry regulation
As the clinical trials become important,CFDA has issued some guidelines to solve the problems in clinical trials. “Data Management Plan for Drug Clinical Trial” was released in 2013 and it was a long-term plan to improve data management for drug clinical trials. To strengthen the guidance of international multi-center clinical trials and protect the interests and safety of patients,CFDA issued global multi-center clinical trial draft in November 2014. Some normative documents on clinical trials by CFDA are indicated in Table 2.

Table 2 Normative documents on clinical trials by CFDA
To save time and the cost of medical R&D for pharmaceutical companies,more and more CRO companies choose to provide a full range of services. At the same time,some big CRO companies want to strengthen its position in the market,they offer more specialized services to improves their business. For example,Tigermed Consulting Ltd. made good use of its own resources to set up some related companies to offer services involving in medical imaging,drug safety,risk management of clinical trial and quality management.
4 The analysis of the obstacles faced by domestic CRO companies
4.1 Competition from foreign CRO companies
When foreign CRO companies entered China’s market,some experts thought the local CRO companies would not be affected in that for these foreign CROcompanies charged high and China’s pharmaceutical companies had few drug R&D. Now the situation is different. Quintiles have pointed out their target customers were China’s pharmaceutical companies ranking top 10 or top 20. In addition,they also established their own CRO companies to solve the problem that they were not familiar with China policies,a case in point is Kentia in China.
As a talent-intensive industry,Quintiles launched a project called CRA trainee to attract more talents. They recruited medical graduates and offered them about 4 months professional training before they started their work. The project has attracted a large number of talents. Due to the high salary,broad development platform and friendly culture in foreign CRO companies,many talented people joined them. As a result,the domestic CRO companies do not have qualified talents to do the clinical trials.
4.2 Small-scale and less competitive
China’s CRO industry is developing rapidly and it is still at the initial stage. There are a number of small CRO companies,but the strong ones are few. Most of the local CRO companies maintain their operations by providing simple services. Those big foreign pharmaceutical companies who want to do the clinical trials in China could not get the ideal local CRO companies to collaborate with. Though the global clinical trials shift to China which can not bring big share to the local CRO companies and it is monopolized by foreign CRO companies. Nowadays,the competition in the CRO companies is in a pyramid form,shown in Figure 1.
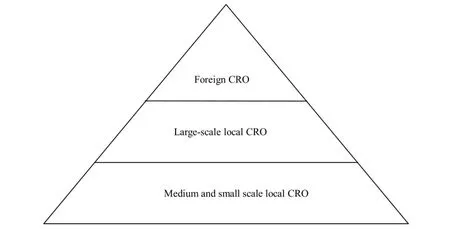
Figure 1 The competition pattern of contract research organization
The above pyramid illustrates the competitive situation of China’s CRO:
①Numbers of company: Foreign CRO<<large-scale local CRO<<small-scale local CRO;
经过数天精心的实验,结果仍然与迈克尔逊在1881年做的实验结果一样,测不到任何“以太”漂移的速度,1887年12月,他们发表论文“地球运动和传光的‘以太’”,宣布了得到否定结论的实验结果,由此说明地球和“以太”之间不存在相对运动。这就是物理学史上有名的“以太”漂移的“零结果”。
②Quality of service: foreign CRO>>large-scale local CRO>>small-scale local CRO;
③Service object (from top to bottom): large-scale foreign pharmaceutical companies,the top 10 domestic pharmaceutical companies; few foreign pharmaceutical companies,large-scale domestic pharmaceutical companies; small and medium-sized domestic pharmaceutical companies.
4.3 Lacking qualification
China’s CRO industry does no have an official quality certification system. That is to say,this industry has no standard at all. In 2010 the Contract Research Organization Union (CROU) and other certification companies had a certification examination for some large CRO companies in China and it was the first qualification activities in CRO industry. As the certification is based on ISO9000,some of the details from ISO9000 cannot meet the need of CRO outsourcing. Therefore,it is necessary to establish a China’s certification system based on the industry characteristics[7].
5 Suggestions
Nowadays,CRO companies face both opportunities and challenges. The obstacles and the changing trend of the industry bring much stress to China’s CRO companies. At the same time,the huge market share and the shift ofclinical trials to China have brought opportunities to our companies as well. Therefore,we provide some suggestions to China’s CRO companies for their future development.
5.1 Strengthening cooperation with international CRO
Foreign CRO companies who entered China’s market stand for the word’s highest standards. By cooperating with international CRO companies to participate in a global clinical trial,the local CRO companies can learn the international regulations,the high-quality clinical trial data and the mature project management methods. The local CRO companies can improve their competitiveness by shortening the gap with the foreign CRO companies.
5.2 Joining in industry alliance
With the cooperation among pharmaceutical companies and industry associations,Zhongguancun CRO alliance,CROU and Asia-Pacific Union have been established in China. As to the small and medium-sized companies,the alliance is a good platform to realize complementary resources,breaking the bottleneck in the capital,technology and market. Thus these companies can improve their competitiveness and gain more market share[8].
5.3 Enhancing the core competitiveness and improving the industry chain
At the initial stage of CRO industry,some companies undertook the projects with low prices to have the market share. Now with the increasing number of companies,this method does not work any more. The small and mediumsized companies should focus on their core business. For example,WuXi App Tec took advantage of its own strong chemical synthesis and preclinical research skill to become the leader in this industry. The size of firm grows from small to large,and it becomes a strong company. As to those large-scale companies,they can offer a one-stop service to win the favor of pharmaceutical companies.
5.4 Establishing a quality certification system
Studies showed that big pharmaceutical companies were more willing to collaborate with CRO companies who had been certified by CAP[9]. Therefore,China should develop a certification system as soon as possible to catch up with the international quality certification standard. By doing so,China’s CRO industry can improve its competitiveness and gain more market share.
5.5 Attaching importance to absorbing and training talents
Talent is the core resource for CRO companies. CRO companies can further broaden recruitment channels such as campus recruitment and online recruitment. They can offer some internship for graduates to know more about the industry. For the new staffs,company should provide a good career path and training system for them. For the old staffs,a satisfying salary and self-worth are the things that company should pay attention to so that they can develop a sense of belonging to the company.
[1]CFDA. Good clinical pratice [EB/OL]. http://www.sfda.gov.cn,2003-09-01.
[2]CHEN Ning. Overview and enlightenment of american biomedical industry contract research organization [J]. Global Science,Technology and Economy Outlook,2007 (5): 45-49.
[3]FAN Miao-xuan,HU Hao,ZHAO Hai-yu,et al. Research on the competitive advantages of China’s pharmaceutical R&D outsourcing industry [J]. Forum on Science and Technology in China,2010,5 (19): 36-46.
[4]The Frost & Sullivan. Clinical research companies explore potential market in APAC [EB/OL]. http://www.frost.com,2010-08-06.
[5]Food and Drug Safety Net. National pharmaceutical CRO market association technical training center inaugurated the commonwealth [EB/OL]. http://www.sda.gov.cn,2010-05-03.
[6]Hangzhou Tiger Pharmaceutical Technology Co. Annual report 2013 [EB/OL]. http://data.eastmoney.com,2014-03-18.
[7]BIAN Yun,QIU Jia-xue. Analysis on the quality status certification of contract research organization [J]. China Pharmaceutical,2012,21 (14): 7-8.
[8]MO Qian-ning,ZHU Chang-hui,XU Yi,et al. Thinking about contract research organization development status [J]. Modern Preventive Medicine,2009 (36): 267-268.
[9]XU Jin,LI ping. Introduction about American association of pathologists laboratory accreditation program [J]. Journal of Modern Laboratory Medicine,2008,2 (1): 69-70.
* Corresponding author: TIAN Li-juan,associate professor. Major research area: rational drug use,new drug development,registry. Tel: 024-23986543,E-mail: tianlijuan_8@126.com
- 亚洲社会药学杂志的其它文章
- Toxic TCM Supervision: Current Situations and Countermeasures
- WHO Prequalification of Medicines Program: Technical Assistance Effect
- Drug Registration and Approval System in China: Current Status,Controversies and Future Perspectives
- Analysis of TCM Patented Technology Based on the IPC Classification System
- Detection and Analysis on Outlier of the Average Medical Expense in China
- An Empirical Study on Model of Consumers’ Initial Trust in Online Pharmacies

