Practical,regulatory and clinical considerations for development of inhalation drug products
Shuguang Hou*,Jiangyue Wu,Xu Li,Hong Shu
Sichuan Purity Pharmaceutical Technology Co.,Ltd,3-1 Jiuxing Ave,Hi-Tech Zone,Chengdu,Sichuan 610041,China
Practical,regulatory and clinical considerations for development of inhalation drug products
Shuguang Hou*,Jiangyue Wu,Xu Li,Hong Shu
Sichuan Purity Pharmaceutical Technology Co.,Ltd,3-1 Jiuxing Ave,Hi-Tech Zone,Chengdu,Sichuan 610041,China
ARTICLE INFO
Article history:
Received 4 June 2015
Received in revised form 18 August 2015
Accepted 20 August 2015
Available online 25 September 2015
Pressurized metered dose inhaler
(pMDI)
Dry powder inhaler(DPI)
Nebulizer
Formulation
Device
Clinical application
The formulation and device collectively constitute an inhalation drug product.Development of inhaled drugs must consider the compatibility between formulation and device in order to achieve the intended pharmaceutical performance and usability of the product to improve patient compliance with treatment instruction.From the points of formulation, device and patient use,this article summarizes the inhalation drugs,including pressurized metered dose inhaler(pMDI),dry powder inhaler(DPI),and nebulizer that are currently available in the US and UK markets.It also discusses the practical considerations for the development of inhalers and provides an update on the corresponding regulations of the FDA(U.S.Food and Drug Administration)and the EMA(European Medicines Agency).
©2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Respiratory drug delivery has strong interest in the pharmaceutical fi eld from both industries and academics,due to the fact that inhalation therapy not only has been widely accepted for localized treatment for pulmonary diseases such as asthma and chronic obstructive pulmonary disease(COPD),but also as an alternative route for systemic drug administration for diseases such as diabetes[1].For local activities,the orally inhaled drugs are delivered directly to the site of action in the lung,providing fast onset of action(within 5 minutes)[2].A sustained activity can also be achieved by utilizing long-acting drugs(e.g.,salmeterol and tiotropium)and/or by modifying formulation technology to better meet the clinical needs and patient compliance[3,4].In the case of systemic drug delivery via inhalation,the lung provides the advantage of large alveolar epithelial absorption area with low enzymatic activity,which makes it a suitable route for biological products such as insulin. Drugs absorbed through the lung can also avoid fi rst-pass metabolism and have thus shown an improved bioavailability.
Inhalation drug products are commonly classi fi ed into three categories:pressurizedmetereddoseinhaler(pMDI),drypowder inhaler(DPI)and nebulizer.All these inhalation drug products are a combination of formulation and device,with patient use as the target consideration(Fig.1).The formulation is designed
1818-0876/©2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/). to provide intended ef fi cacy for patients,and to achieve this goal,itneedstobecompatibleandworkstogetherwithadevice. Aninhalationproductshouldbeabletoprovideconsistentdose content,along with suitable aerodynamic particle size distribution,to ensure that the drugs can be ef fi ciently delivered to the target sites in the lung.A well-designed inhaler must also consider its usability for patients’use in terms of features such asrobustness,easeofuse,portability,andsuitabilityforallages in order to achieve good patient compliance with the technique instructions.
The development of an inhalation product must take into account of the following factors:(a)type of drugs being delivered to the lung,such as local or systemic action,chemical or biological drug,and dose and frequency for patient use; (b)physico-chemical properties of the drug substance,such as solubility pro fi le,particle size,morphology and density,as these determine the formulation development;(c)type of formulation(e.g.,dry powder,propellant driven liquid,or aqueous inhalation formulation)being selected to deliver the drug; (d)device design for compatibility with the formulation to be suitable for the targeted patient population.
Each type of inhaler has its speci fi c formulation,device and clinical advantages.The inhalation products currently available in the US and UK markets are summarized in Tables 1-3 for pMDIs,DPIs and nebulizers,respectively.Based on these product’s formulation and device information,this article discusses the practical strategies and clinical considerations for the development of each type of inhalers and provides an update for the regulatory guidelines issued by the FDA and EMA for these inhalers,speci fi cally for generic inhalation products.Any new technology and new inhalation drug that are still under the investigation phases will be brie fl y mentioned.
2. pMDIs
2.1. Formulation
pMDIs are either suspension or solution based formulation of drug in propellants.The traditional chloro fl uorocarbon(CFC) propellants,which have previously been used in pMDIs for decades,were banned by the Montreal Protocol(1989)due to their ozone depletion effect.The hydro fl uoroalkanes(HFA-134a and HFA 227)were selected as alternative propellants.This propellant transition for pMDIs is almost complete in western countries[5].All researches for pMDI development are referred to the landmark publication by Vervaet and Byron to further understand the drug-surfactant-propellant interactions in the HFA formulations.That article systemically presented an overview of the present state-of-the-art with respect to the physico-chemical characteristics of HFA and HFA-ethanol blends,drug substance properties that need to be considered for formulation development,and surfactant behaviors in the pMDI formulations[6].The important properties of the drug substance such as solubility pro fi le,particle size, morphology and density,need to be characterized fi rst,as these parameters will in fl uence the formulation decision(solution or suspension)and drug micronization method selection[7]. As for the surfactants in suspension formulations,such as oleic acid in Proventil and polyethylene glycol(PEG)1000 in Symbicort,they serve to lubricate the valves to improve the drug delivery ef fi ciency and dose uniformity.However,the surfactants previously soluble in the CFC formulations,especially the surfactants with low hydrophilic-lipophilic balance(HLB) values,show decreased solubility in the HFA propellants.More hydrophilic surfactants with higher HLB values,such as PEG, tend to dissolve more in HFAs[6].Co-solvents such as anhydrous ethanol may also needed to help improve the solubilization of the surfactants in the HFA formulations,but the ethanol content in pMDI suspension formulations should be minimized to avoid the dissolution of drug.This is because the partly soluble micronized drug may lead to crystal growth (Ostwald ripening:a phenomenon in suspension that describes the change of an inhomogeneous structure over time, i.e.,small crystals or particles dissolve,and redeposit onto larger crystals or particles),resulting to reduced drug delivery consistency and increased particle size distribution[6].Ethanol can also be used to increase the solubility of drug to form a solution formulation,such as Qvar(Beclomethasone dipropionate solution formulation).Such a solution formulation generates signi fi cantly smaller particle size compared to the typical suspension formulation,leading to reduced oropharyngeal deposition and improved lung deposition[8].Having said that, the ethanol content in solution formulations must be balanced,as higher ethanol concentration can lead to decreased fi ne particle fraction,mainly due to the increase in initial atomized droplet size that subsequently affects solvent evaporation[9,10].The chemical stability of drug could also be an issue for solution formulations[11,12].In Atrovent,a solution formulation of ipratropium bromide,water and citric acid are added to improve the drug chemical stability.Citric acid acts as an organic acid,provides stability against degradation or decomposition of the medicament resulting largely from interaction of the medicament with the cosolvent and/or water present in the solution formulation[13].The physical stability such as drug particle agglomeration and adsorption is a challenge to formulate a suspension formulation.Suspending agents,such as povidone K25 in Symbicort,are used to improve the drug particle suspension[14].Suspending agents can increase suspension viscosity and/or provide steric hindrance to aid the stabilization of microparticles in the pMDIformulations[15,16].Moisture ingress could also damage the stability of suspension formulations[17].In summary,to formulate a stable pMDI formulation,the physico-chemical property,such as solubility,particle size,morphology and density of drug substance,type of surfactants and/or suspending agents for stabilizing the formulation,use of ethanol as cosolvent for improving the solubility of surfactants and drug, should be systemically studied[6,18].

Table 1-The pressurized metered dose inhalers in the US&UK markets.
2.2. Container closure system
The physical and chemical compatibility between pMDI formulation and container closure system components(including metering valve,canister and actuator)is critical for a successful development of pMDI.The target dose to be delivered is determined by the combination of drug concentration and the chamber volume of the metering valve(typically 25-100 μL). The valve materials may in fl uence the drug’s physical uptake, drug chemical degradation,moisture ingress and leak rate [17,19].The extractable and leachable pro fi les of the valve materials must also be thoroughly investigated,and the leachables from these materials into the pMDI formulations should be closely monitored during product development[7,20].The pMDI containers are normally aluminum canisters,which could cause drug loss through adsorption onto the canister and catalyze the chemical degradation[12,21,22].Inner wall coated containers may be used to replace the aluminum canisters.The common coatings are epoxy-phenolic polymer(Epoxy), polytetra fl uoroethylene(PTFE),per fl uorinated ethylene propylene copolymer(FEP),per fl uoroalkoxyalkane polymer(PFA), and polyethersulfone(PES)[22,23].Appropriate size containers should also be selected based on the actual formulation
fi ll weight.The container headspace is fi lled by the vapor of propellant;following actuations during the pMDI use,the headspace of canister increases and more propellant evaporates to fi ll the headspace,which causes the concentration of drug in the liquid phase of the formulation and thus in fl uences the delivered dose content uniformity through container life[24].The actuator atomizes the formulation,thus its ori fi ce diameter in fl uences the drug particle size distribution.The ori fi ce diameter usually ranges from 0.14 mm to 0.6 mm[25]. The fi ne particle dose and fi ne particle fraction can be increased by utilizing smaller ori fi ce diameter actuators[26].The effect of the actuator ori fi ce size on the amount of fi ne particles is more pronounced for solution pMDIs than for suspension ones,as the particle size of the drug substance is the limiting factor of the fi nal particle size of pMDIs.
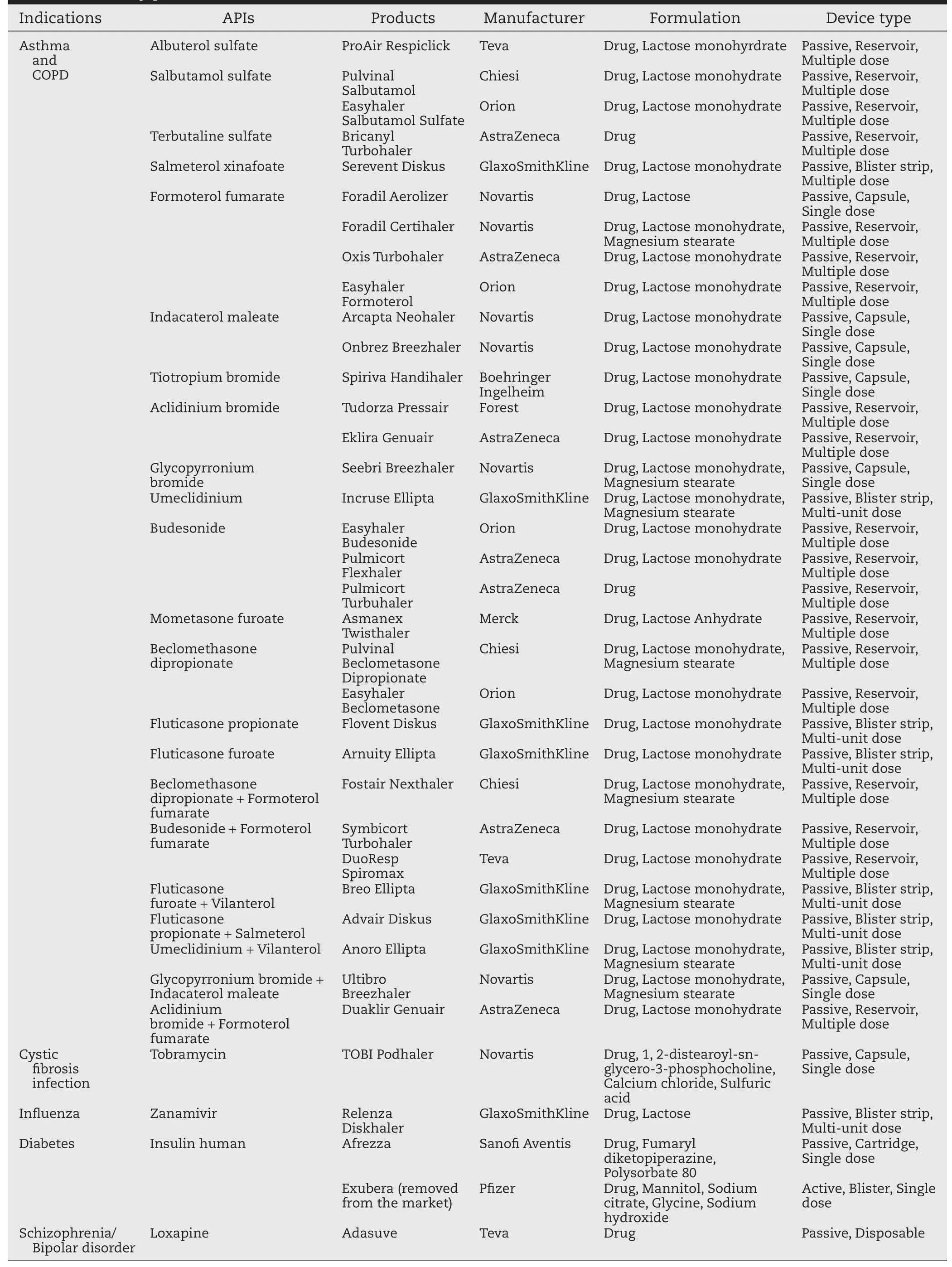
Table 2-The dry powder inhalers in the US&UK markets.
2.3. Patient use
Since the first pMDI was introduced to the market,pMDIs have been evolving throughout the years to be more effective,giving it an important role in inhalation therapy because of the low price,low maintenance and convenience of use.From the point of patient use,pMDIs actively deliver drug aerosols.All pMDIs have the same drug delivery mechanism.They use highpressure lique fi ed propellants to atomize the formulation into small droplets capable of delivering drug into the deep regions of the respiratory tract[7].Thus all pMDIs show similar size andshape,andrequiresimilarinhalationtechniquewhenbeing used.The major drawback for pMDIs is the requirement for coordinationbetweenactuationandpatientinhalation,andpoor coordination may result in high drug deposition in the throat region[27].Thiscanbeovercomebyusingbreath-actuatedpMDI device such as Qvar Autohaler,a spacer or a valved holding chamber.Spacers and valved holding chambers increase the delay time between actuation and inhalation,allowing more time for propellant evaporation,and decelerate the particles, and thus they not only help the coordination,but they also increase the pulmonary deposition[28,29].In 2003,the FDA recommended(but did not mandate)the addition of dose counter or dose indicator on pMDIs to help patients ascertain the remaining dose during use[30].The dose counter has been observed in many market products,as shown in Table 1.
Clinically,all the current marketed pMDIs are for bronchospasm in asthma and COPD.The pMDI of albuterol,a short acting β2-agonist(SABA),is still the most widely used medicine for the treatment or prevention of bronchospasm.The pMDI of levabuterol(Xopenex),the R-enantiomer of albuterol,was also launched.It was reported that levabuterol can stereoselectively bind with the β2-agonist receptor,which may enhance the ef fi cacy and reduce the toxicity[31].As recommended by the Global Initiative forAsthma guidelines,the ideal maintenance treatment for asthma is a combination of long acting β2-agonists(LABA)and inhaled corticosteroids(ICS). Salmeterol and formoterol are representatives of the LABA family that can reside for an extented period of time at the receptor due to their hydrophobic property,thus providing a long duration of action[32].Among the fi ve available pMDIs of LABA/ICS combination in the market(Table 1),four products consist of formoterol,possibly due to the fact that formoterol not only has a long acting duration(>12 h),but also a fast onset of action(<3 min)[32].The pMDI available for COPD therapy is Atrovent,which contains ipratropium bromide,a shortacting muscarinic antagonist(SAMA).A combination pMDI containing ipratropium bromide and albuterol(Combivent CFC) is no longer available following the phaseout of CFC pMDIs.
3. DPIs
3.1. Formulation
The formulation compositions of DPI can be simple,mostly a blend of micronized drug powder with larger carrier particles (usually lactose monohydrate),or even pure micronized drug powder(such as Pulmicort Turbuhaler).The production of DPI formulation,however,has many particle engineering challenges from drug crystallization and micronization,mixing and blending of micronized drug(s)with carrier excipients, fi lling of dry powder formulation into capsule/blister/inhaler,storage stability(e.g.,aggregation,moisture uptake,etc.),to emptying of powder from the inhaler when being used.Such a formulation process is well reviewed byTelko and Hickey[33].Most DPI products in the market(Table 2)use a blend of micronized drug (usually<5 μm)and coarse lactose particles(30-80 μm or larger) as carriers.It is important to fi rst characterize the shape and surface morphology of drug substance,because its crystal habit in fl uences its particle aerodynamic behavior,thus further affecting the lung deposition[33].For example,elongated and pollen-shaped particles have been found to exhibit higher lung deposition ef fi ciency[34,35].Milling is currently still the mostly widely used method to micronize drug substance.Amorphous drug or excipient could be produced during the milling process, which may lead to reduced fl owability and dispersibility of the DPI formulation.Furthermore,once re-crystallization occurs in the formulation,capillary force and solid bridge may be formed due to the release of excess water,thereby impacting the physical stability of mixture[36].These amorphous materials can be induced into crystals by conditioning them in a humidity controlled or organic solvent vapor environment[37,38].Another strategy to improve drug dispersibility is to modify the particle surface morphology by producing wrinkled or hollowporous particles.It has been shown that enhanced aerosol performance of corrugated bovine serum albumin(BSA)powder than smooth spherical BSA powder,which could be explained by the lower contact area caused by the asperities of corrugated particles[39].The hollow-porous particle as a novel formulation technique has been intensively investigated.Due to characteristics such as lower density and larger size,active ingredients are readily dispersed by means of being contained [40].The lactose is used as carrier to reduce drug particle aggregation and to improve the formulation fl owability.In order to achieve the desired respiratory size(generally<5 μm),the micronized drugs are cohesive and normally form agglomerates due to their small size and high relative surface area[41]. By adding larger lactose particles,the ordered mixture could be formed,in which the fi ne drug particles will adhere to the surface of lactose particles,thereby leading to good content uniformity and fl owability[42].Fine lactose particles can also be added to promote drug release from the coarse lactose particles,thus enhancing drug dispersibility and fi ne particlefraction[43].Due to the above reasons,occupation of active sites and co-agglomeration with drug particles as two major mechanisms have been concluded by Jones and Price[44].Drug dispersibility can also be affected by the morphology of coarse lactose particles,such as the surface roughness and particle shape[45,46].Some recently developed DPI products,such as Anoro Ellipta and Ultibro Breezhaler,have magnesium stearate except for lactose in the formulations.It was reported that magnesium stearate is hydrophobic,which can provide moisture resistance,thus improving the formulation storage stability[47]. The mechanofusion with magnesium stearate also seems to increase the fi ne particle fraction(FPF)of DPIs,thereby delivering the drugs more effectively.The increased FPF might be attributed to the appropriate increase of surface energy and the homogenizing of the surface adhesiveness[48].Mannitol is an alternative to lactose for drugs such as peptides or proteins(e.g., in Exubera insulin DPI),as these drugs may interact with the reducing sugar function of the lactose through Maillard reaction[49].The formulation-excipients blend homogeneity is not only affected by the physicochemical properties of drugs and excipients,but also by the production factors such as mixer selection and mixing process control[50-52]. PulmicortTurbuhaler contains a soft spherical pellet formulation of pure drugs without any excipients,which avoids the blend problems[53].Other drug powder production techniques also include spray drying,freeze drying,solvent precipitation and others[54,55];for examples,the PulmoSphere andTechnoSphere technologies have been commercialized with the regulatory approval ofTOBI Podhaler andAfrezza,respectively.The engineered particles obtained from these technologies not only contribute to the improvement in drug dose consistency and lung target,but also facilitate the transport of active drug across the biological membranes,resulting in a rapid onset of action [56-58].These technologies can be used for drugs with large doses.
3.2. Device
There are about 30 marketed DPIs and dozens of new devices that are being reported[5].All available DPI devices in the US and UK markets are passive inhalers,which rely on patient’s breath to activate the drug delivery;therefore it is important that the DPI device is independent of the patient’s inspiratory effect[59].DPI devices can be sorted into single dose inhaler,reservoir based multi-dose inhaler,blister or cartridge based multi-dose inhaler,and single use(disposable) inhaler.For single dose inhaler,the pre-metered formulation is packaged in a hardcapsule(e.g.Spiriva Handihaler),a blister (e.g.Exubera),or a cartridge(e.g.Afrezza).Most marketed DPIs are reservoir based devices(Table 2)in which suf fi cient formulation is stored in a chamber reservoir and a fi xed amount of powder is metered into a dosing receptacle for each dose. The most reknown DPI device is the Advair Diskus,which is a multi-unit device containing a foil blister strip fi lled with premetered formulation.Its next generation device,Ellipta,is the fi rst DPI that enables simultaneous delivery of two drugs without the need for co-formulation.The recently approved Adasuve DPI utilizes a single use device.Such disposable device can be a good option for drugs which only require a single or several doses to complete its course such as vaccine.The details of new types of DPI device have recently been reviewed by Chan et al.[1].For all these types of DPIs,the usability of the device is important in order to improve the patient compliance with the treatment instruction and adherence to the therapy[60]. The factors of the device usability include,but not are limited to,robustness,ease of use,low occurrence of errors,and ergonomics.In contrast to MDIs,each DPI has its own design with a different drug delivery mechanism,thus requiring a different inhalation instruction.Therefore,it is fair to consider“each DPI as one dosage form”,mainly based on the different designs of devices.These features could be re fl ected on the dispersion mechanisms,air fl ow resistance and fl ow rate dependence of dry powder delivery devices.
3.3. Patient use
In contrast to pMDIs,DPIs deliver the drugs by utilizing the patient’s inspiration,thus avoiding the hand and breath coordination for patient use.Such an advantage,however,may also become an issue if the activation of a DPI is fl ow rate dependent.Take Turbuhaler for example;it has been con fi rmed that the increase of the air fl ow through budesonide/ formoterolTurbuhaler from 30 to 60 l/min led to approximately double the total emitted dose and fi ne particle mass of both drugs[61].For the treatment and prevention of bronchospasm in asthma and COPD,the current DPI products have covered the drug categories of SABA(short-acting β2agonist), LABA(long-acting β2agonist),SAMA(short-acting muscarinic antagonist),LAMA(long-acting muscarinic antagonist),and ICS (inhaled corticosteroid).Multiple combination DPIs of ICS/ LABA are available and new device based combination DPIs are continuously being launched.The recently approved Breo Ellipta is the fi rst member of the ICS/LABA class to shift from twicedaily to once-daily treatment[62,63].Another category of combination DPIs that consists of LAMA/LABA are formulated for COPD treatment.The utilization of DPIs is being expanded to drugs for new indications,including antibiotics, antivirus,insulin,and vaccines.The DPI of tobramycin(TOBI Podhaler)is introduced to reduce treatment time and improve ease of use compared with tobramycin inhalation solution in cystic fi brosis(CF)patients.It is comparable to inhalation solution in ef fi cacy outcomes and safety pro fi le but has greater patient satisfaction in all the age groups[64].Although P fi zer’s inhaled insulin DPI(Exubera)was withdrawn due to poor reception in the market,its successful launch in 2006 started a new era for inhalation therapy as a route for systemic drug administration.The launch of the second insulin DPI,Afrezza, is an indication of continuous efforts from pharmaceutical industries to apply the DPI technology to systemic drugs.
4. Nebulizers
4.1. Formulation
Nebulizers are the oldest device for respiratory drug delivery. The drug is formulated in aqueous solution or suspension,which is atomized into fi ne droplets via an external nebulization source while being used for inhalation.These aqueous-based oralinhalation solutions and suspension must be sterile,and are typically packaged in single-use containers(usually 1-3 ml)[65]. Sterile water for injection is normally used as solvent,and in some cases,ethanol can also be used as the co-solvent.Sodium chloride is usually used to adjust the isotonicity.In most cases, pH values are adjusted by acid or base,such as hydrochloric acid or sodium hydroxide.For easily oxidized drugs,edetate disodium is added in formulation as a chelating agent to further remove the traced metal ions,and nitrogen is used for sparging, fi lling and pouching.Surfactants such as polysorbate 80 can be used to enhance the suspension stability(such as Pulmicort Respiles).Antimicrobial preservatives such as benzalkonium chloride are added if needed.Inhalation of nebulized solution/ suspension requires performance tests of drug delivery rate (output)and aerodynamic droplet size distribution,which are the product’s critical quality attributes(CQAs).It is important to study well and understand how the key formulation parameters,such as viscosity,surface tension and drug concentration, in fl uence these CQAs[1,66].It was reported that an increase of solution viscosity could slow down the nebulization and reduce the drug output for jet nebulizers,and the primary droplet size was proportional to the surface tension[67].For the vibrating mesh nebulizers,increasing solution viscosity decreased droplet size,but prolonged nebulization time.The electrostatic charges present in the aqueous solutions inhibited the fl ow and detachment of fl uid through the mesh,and the introduction of electrolytes could suppress the charges,thus improving particle size,drug output and nebulization time[68].The drug concentration in fl uenced the nebulizer output and such an effect was related to the type of nebulizers.When antibiotic concentration was increased,the output decreased more precipitously with the ultrasonic nebulizers than with the jet nebulizers.The drug concentration had less effect on the droplet size distribution[69].
4.2. Device
Jet nebulizer,powered by compressed air,is still the most utilized apparatus for inhalation solution/suspension.But the jet nebulizer is cumbersome and somewhat noisy to use,and has a long dosing duration(10-15 minutes).The cooling effect of jet nebulizers due to the expansion of atomizing gas and evaporation of solvent also in fl uences patient’s use.Ultrasonic nebulizer generates aerosol by electronically induced vibration of a ceramic piezoelectric element.It is compact and silent, and the dosing time is shorter than that of the jet nebulizers, as the emit of ultrasonic nebulizers is higher than that of jet nebulizers[70].Heat,however,is generated during the ultrasonic nebulization process,thus it is unsuitable for heat sensitive drugs[71].Vibrating mesh nebulizers are a recent technology that uses vibrating perforated mesh to generate respirable sized droplets.They are electronic devices and have advantages over both jet and ultrasonic nebulizers,such as fast treatment time, minimal residual dose,and reduced drug waste.Clog of the tiny holes of the mesh and high cost are the drawbacks[72].A portable nebulizer device,Spiriva Respimat,has been launched by Boehringer Ingelheim.The nebulized solution is fi lled into a plastic container crimped into an aluminum cylinder(cartridge)for use with the Respimat inhaler.The Respimat inhaler is a handheld,pocket sized oral inhalation device that uses a compressed spring producing mechanical energy to generate a slow-moving aerosol cloud of medication from a metered volume of the drug solution.Due to its difference in terms of atomization mechanisms and device designs,the device is yet to be classi fi ed as nebulizer while someone prefers to consider it as propellant free MDI or metered dose liquid inhaler(MDLI)[73]. Such a portable device contains multiple doses and is able to deliver the drug in a single breath of soft mist without the need for continuous inhalations,and thus should be the future direction for new nebulizer development.
4.3. Patient useCurrently nebulizers remain widely utilized in hospitals and home settings.They allow the patients to inhale the drug aerosolswithtidalbreathingmaneuversandlittletrainingrequired. Therefore,it can be used for patients who are unable to coordinate their breathing or activate the inhalers such as MDI or DPI,especially for the elderly and children.Most inhalation solutions/suspensionsforasthmaandCOPDconsistofoneactive drug of SABA,LABA or LAMA.Most recently,the FDA approved theinhalationsprayofLAMA/LABA(Tiotropium/Olodaterol)combination,which can be given to patients once daily.
Nebulizers can continuously deliver drugs for a long dosing duration,thus are suitable for large dose drugs,such as antibiotics.Tobramycin was the fi rst antibiotics approved as an inhalation solution(TOBI)for the prevention and treatment of Pseudomonas aeruginosa infection in cystic fi brosis.A longterm therapy study indicated that inhaled tobramycin improved lung function and reduced exacerbation rate for patients with CF[74].The other clinical applications of nebulizers include pulmonary arterial hypertension and nicotine withdrawal symptoms (Table 3).
Nebulizers are not typically used for chronic-disease management because they are larger and less convenient than pMDIs and DPIs.It is critical that the inhalation solution/ suspension should be delivered using the speci fi c nebulizer recommended in drug package insert in order to obtain the expected emitted dose,particle size distribution and formulation emptying time while being used[75].Different droplet size distribution was observed with the same nebulizer while different compressors or different compressor pressures were utilized[69].With traditional jet nebulizers,only 10%of the dose may reach the lung.The majority of the drug is either remaining in the nebulizer or released into the surrounding air during expiration[67].Breath enhanced device(e.g.Pari LC Star)or breath actuated device(e.g.AeroEclipse II)have been developed to create aerosols only during inspiration,thus reducing drug wastage and improvimg delivery ef fi ciency[66,76].
5. Regulations on development of inhalation drug products
The FDA and EMA have published guidelines on the development of all these three types of inhalation drug products(pMDI, DPI and inhalation solution/suspension for nebulizer)[7,65,77]. Following the patent expiration for many inhalation products,especially the blockbuster drugs such as Advair Diskus,the switch from branded to generic inhalation medicine is a worldwide trend,and the development of generic inhalers has been a hot area for pharmaceutical industries.
The EMA in 2009 issued the guideline on the demonstration of therapeutic equivalence between two inhaled products, which described a step-wise approach for the approval of generic inhalation drug products[78].From the point of formulation,the generic and the reference products should be the identical dosage form with the same active substance(s).Any differences in crystalline structure and/or polymorphic form of the active substance and any qualitative and/or quantitative differences in excipients should not in fl uence the pharmaceutical performance and safety pro fi le of the product. Regarding the device,the handling and resistance to air fl ow of the generic and reference products should be the same.The critical quality attributes to assess the in vitro equivalence between the generic and reference products are the dose delivery uniformity and particle size distribution pro fi le.The delivered dose should be similar(within 15%).The comparison of particle size distribution,tested by a validated multistage impactor,should be performed per impactor stage or justifi ed group of stages with suitable equivalence criteria(e.g.15% may be justi fi able).If a generic product satis fi es all of the above pharmaceutical criteria for equivalence,the use of only in vitro data may be considered acceptable for product approval.Otherwise,in vivo studies(pharmacokinetics or pharmacodynamics) should be performed to substantiate equivalence.
Rather than issuing a general guideline as the EMA did,the FDA has published fi ve separate draft guidance for each speci fi c inhalation product:pMDIs of albuterol sulfate,ipratropium bromide,levalbuterol tartrate,budesonide/formoterol fumarate,and DPI of fl uticasone propionate/salmeterol[79-83], starting from 2013.Based on the FDA guidance,it will be a very dif fi cult process to get FDA approval in the US for generic product as it requires that the in vitro tests,pharmacokinetics and pharmacodynamics all substantiate equivalence[84]. The in vitro tests include single actuation content(SAC)and aerodynamic particle size distribution(APSD).Equivalence in spray pattern,plume geometry,priming and repriming studies are also required for generic pMDIs,apparently for supporting the similarity of valves and actuators.The similarity requirements of DPI device include the device mechanism,premetered multi-dose format,doses,operating procedures,size, shape,device resistance and dose counter.For the formulation,the generic product should use the same inactive ingredient(s)as the reference product(i.e.qualitative sameness;Q1),and the concentration of the inactive ingredient(s) used in the generic product should be within 5%of those used in the reference product(i.e.quantitative sameness;Q2).The draft guidance has been challenged by industries.Rather than the Q2requirement,it is proposed to utilize the quality-bydesign(QbD)approach to study the control space of the excipient concentration for the generic product[85].
6. Conclusions
pMDI,DPI and nebulizer each has its speci fi c advantages and limits.The increasing interest in the pulmonary route for both local and systemic acting drugs continues to promote the development of new inhalation drug products for new indications, together with new formulation and device technologies.The development of inhalation drugs should practically consider factors such as patient preference,convenience of use,and cost, as the fi nal target goal is to improve patient adherence and therapeutic outcomes.
REFERENCES
[1]Chan JGY,Wong J,Zhou QT,et al.Advances in device and formulation technologies for pulmonary drug delivery.AAPS PharmSciTech 2014;15:882-897.
[2]Lavorini F,Fontana GA,Usmani OS.New inhaler devices -the good,the bad and the ugly.Respiration 2014;88:3-15.
[3]Leach CL,Hameister WM,Tomai MA,et al.Oligolactic acid (OLA)biometrics for sustained release of asthma therapeutics.RDD 2000;VII:75-81.
[4]Beck-Broichsitter M,Merkel OM,Kissel T.Controlled pulmonary drug and gene delivery using polymeric nano-carriers.J Control Release 2012;161:214-224.
[5]Newman SP.Platforms for aerosol drug delivery:ensuring therapeutic success.RDDAsia 2014;1:1-11.
[6]Vervaet C,Byron PR.Drug-surfactant-propellant interactions in HFA-formulations.Int J Pharm 1999;186:13-30.
[7]FDA Guidance for Industry.Metered dose inhaler(MDI) and dry powder inhaler(DPI)drug products,chemistry, manufacturing,and controls documentation,<http://www.fda.gov/downloads/drugs/ guidancecomplianceregulatoryinformation/guidances/ ucm070573.pdf>;1998.[accessed 16.07.15].
[8]Leach CL,Davidson PJ,Boudreau RJ.Improved airway targeting with the CFC-free HFA-beclomethasone metered dose inhaler compared with CFC-beclomethasone.Eur Respir J 1998;12:1346-1353.
[9]Stein SW,Myrdal PB.A theoretical and experimental analysis of formulation and device parameters affecting solution MDI size distributions.J Pharm Sci 2004;93:2158-2175.
[10]Hou S,Alband T,Kriesel K,et al.The in fl uence of formulation factors on the pharmaceutical performance of a solution metered dose inhaler(MDI)product.Respir Drug Deliv Proc 2006;365-368.
[11]Purewal TS,Grant DJW.Metered dose inhaler technology, vol.37.Interpham Press;1998.
[12]Wu ZZ,Thatcher ML,Lundberg JK,et al.Forced degradation studies of corticosteroids with an alumina-steroid-ethanol model for predicting chemical stability and degradation products of pressurized metered-dose inhaler formulations. J Pharm Sci 2012;101:2109-2122.
[13]Jager PD,Kontny MJ,Nagel JH.Stabilized medicinal aerosol solution formulations,US patent 5676930,1997.
[14]Govind N,Marlow M.Composition for inhalation,WO patent 03/063842 A1,2003.
[15]Lewis DA,Keeble CA,Whit fi eld NK,et al.Suspension formulations,US patent 2011/0182997 A1,2011.
[16]Jones SA,Martin GP,Brown MB.Stabilisation of deoxyribonuclease in hydro fl uoroalkane using miscible vinyl polymers.J Control Release 2006;115:1-8.
[17]Wei N,Hou S,Jin F.Moisture ingress and its in fl uence on metered dose inhalers.Chin J Pharm 2012;43:949-953.
[18]Smyth HDC.The in fl uence of the other formulation variables on the performance of alternative propellantdriven metered dose inhalers.Adv Drug Deliv Rev 2003;55:807-828.
[19]Schultz RK,Dupont RL,Ledoux KA.Issues surrounding metered dose valve technology:past,present,and future perspectives.RDD 1994;IV.
[20]Howlett D,Colwell J,Goldsmith S,et al.Correlation of extractables and leachables from marketed pMDIs.RDD 2002;VIII:129-136.
[21]Wu ZZ,Govind N,Johnson PR.C-17/21 OH 20-ketosteroid solution aerosol products with enhanced chemical stability. U.S.Patent No.6315985,2001.
[22]Ashurst IC,Herman CS,Li L,et al.Metered dose inhaler for salmeterol.US Patent No.6143277,2000.
[23]Traini D,Young PM,Rogueda P,et al.The use of AFM and surface energy measurements to investigate drug-canister material interactions in a model pressurized metered dose inhaler formulation.Aerosol Sci Technol 2006;40:227-236.
[24]Hou S,Kriesel K,Alband T,et al.The in fl uence of canister headspace on the pharmaceutical performance of a solution metered dose inhaler(MDI)product.Respir Drug Deliv Proc 2006;369-371.
[25]Lewis D,Ganderton D,Meakin B,et al.Theory and practice with solution systems.Respir Drug Deliv 2004;IX:109-115.
[26]Hou S,Anderson RN,Kriesel K,et al.Comparison of Andersen and next generation impactors on aerodynamic sizing of a solution metered dose inhaler(MDI)tested with different ori fi ce diameter actuators.Respir Drug Deliv Eur Proc 2007;217-220.
[27]Vanderman AJ,Mos JM,Bailey JC.Inhaler misuse in an older adult population.Consult Pharm 2015;30:92-100.
[28]Newman SP,Newhouse MT.Effect of add-on devices for aerosol drug delivery:deposition studies and clinical aspects.J Aerosol Med 1996;9:55-70.
[29]Aggarwal B,Gogtay J.Use of pressurized metered dose inhalers in patients with chronic obstructive pulmonary disease:review of evidence.Expert Rev Respir Med 2014;8:349-356.
[30]FDA Guidance for industry.Integration of dose-counting mechanisms into MDI drug products,<http://www.fda.gov/downloads/drugs/ guidancecomplianceregulatoryinformation/guidances/ ucm071731.pdf>;2003.[accessed 16.07.15].
[31]McCullough JR,Handley DA,Jerrussi TP,et al.Development of enantiomerically pure levabuterol.RDD 1998;VI:113-118.
[32]Zheng X,Righton L,Chen Y,et al.The progress of formoterol-containing pMDIs.Int Pharm News China 2012;12:28-30.
[33]Telko MJ,Hickey AJ.Dry powder inhaler formulation.Respir Care 2005;50:1209-1227.
[34]Fults KA,Miller IF,Hickey AJ.Effect of particle morphology on emitted dose of fatty acid-treated disodium cromoglycate powder aerosols.Pharm Dev Technol 2008;2:67-79.
[35]Crowder TM,Rosati JA,Schroeter JD,et al.Fundamental effects of particle morphology on lung delivery:predictions of Stokes’law and the particular relevance to dry powder inhaler formulation and development.Pharm Res 2002;19:239-245.
[36]De Boer AH,Chan HK,Price R.A critical view on lactosebased drug formulation and device studies for dry powder inhalation:which are relevant and what interactions to expect?Adv Drug Deliv Rev 2012;64:257-274.
[37]Briggner LE,Bystrom K,Jakupovic E,et al.Pharmaceutical formulation.US Patent 5874063,1999.
[38]Kusssendrager KD,Ellison MJH.Carrier material for dry powder inhalation.WO 0207705,2002.
[39]Chew NYK,Chan HK.Use of solid corrugated particles to enhance powder aerosol performance.Pharm Res 2001;18:1570-1577.
[40]Bot AI,Tarara TE,Smith DJ,et al.Novel lipid-based hollow-porous microparticles as a platform for immunoglobulin delivery to the respiratory tract.Pharm Res 2000;17:275-283.
[41]Kendall K,Stainton C.Adhesion and aggregation of fi ne particles.Powder Technol 2001;121:223-229.
[42]Kassem NM,Ganderton D.Dry powder inhalers,Dans: advances in pharmaceutical sciences.Academic Press;1992. p.165-191.
[43]Lucas P,Clarke MJ,Anderson K,et al.The role of fi ne particle excipients in pharmaceutical dry powder aerosols. Proceedings of Respiratory Drug Delivery VI,vol.IL. Interpharm Press;1996.p.243-250.
[44]Jones MD,Price R.The in fl uence of fi ne excipient particles on the performance of carrier-based dry powder inhalation formulations.Pharm Res 2006;23:1665-1674.
[45]Zeng XM,Martin GP,Marriott C,et al.The in fl uence of carrier morphology on drug delivery by dry powder inhalers. Int J Pharm 2000;200:93-106.
[46]Flament MP,Leterme P,Gayot A.The in fl uence of carrier roughness on adhesion,content uniformity and the in vitro deposition of terbutaline sulphate from dry powder inhalers. Int J Pharm 2004;275:201-209.
[47]Guchardi R,Frei M,John E,et al.In fl uence of fi ne lactose and magnesium stearate on low dose dry powder inhaler formulations.Int J Pharm 2008;348:10-17.
[48]Kumon M,Machida S,Suzuki M,et al.Application and mechanism of inhalation pro fi le improvement of DPI formulations by mechanofusion with magnesium stearate. Chem Pharm Bull 2008;56:617-625.
[49]Steckel H,Bolzen N.Alternative sugars as potential carriers for dry powder inhalations.Int J Pharm 2004;270:297-306.
[50]Sudah OS,Cof fi n-Beach D,Muzzio FJ.Effects of blender rotational speed and discharge on the homogeneity of cohesive and free- fl owing mixtures.Int J Pharm 2002;247:57-68.
[51]Alexander A,Shinbrot T,Johnson B,et al.V-blender segregation patterns for free- fl owing materials:effects of blender capacity and fi ll level.Int J Pharm 2004;269:19-28.
[52]Staniforth JN,Rees JE,Lai FK,et al.Interparticle forces in binary and ternary ordered powder mixes.J Pharm Pharmacol 1982;34:141-145.
[53]Wetterlin K.Turbuhaler:a new powder inhaler for administration of drugs to the airways.Pharm Res 1988;5:506-508.
[54]Chan HK,Chew NYK.Novel alternative methods for the delivery of drugs for the treatment of asthma.Adv Drug Deliv Rev 2003;55:793-803.
[55]Hoppentocht M,Hagedoorn P,Frijlink HW,et al. Technological and practical challenges of dry powder inhalers and formulations.Adv Drug Deliv Rev 2014;75:18-31.
[56]Weers J,Tarara T.The PulmoSphereTMplatform for pulmonary drug delivery.Ther Deliv 2014;5:277-295.
[57]Steiner SS,Kisco NYM,Woods RJ.Puri fi cation and stabilization of peptide and protein pharmaceutical agents. Patent US 2004/0077528 A1,2004.
[58]Pfützner A,Forst T.Pulmonary insulin delivery by means of the Technosphere64 drug carrier mechanism.Expert Opin Drug Deliv 2005;2:1097-1106.
[59]Muralidharan P,Hayes D Jr,Mansour HM.Dry powder inhalers in COPD,lung in fl ammation and pulmonary infections.Expert Opin Drug Deliv 2014;12:947-954.
[60]Azouz W,Chetcuti P,Hosker HS.The inhalation characteristics of patients when they use different dry powder inhalers.J Aerosol Med Pulm Drug Deliv 2015;28:35-42.
[61]Tarsin W,Assi KH,Chrystyn H.In-vitro intra-and interinhaler fl ow rate-dependent dosage emission from acombination of budesonide and eformoterol in a dry powder inhaler.J Aerosol Med 2004;17:25-32.
[62]Matera MG,Capuano A,Cazzola M.Fluticasone furoate and vilanterol inhalation powder for the treatment of chronic obstructive pulmonary disease.Expert Rev Respir Med 2015;9:5-12.
[63]Syed YY.Fluticasone Furoate/Vilanterol:a review of its use in patients with asthma.Drugs 2015;75:407-418.
[64]Geller D,Nasr SZ,Piggott S,et al.Tobramycin inhalation powder in cystic fi brosis patients:response by age group. Respir Care 2014;59:388-398.
[65]FDA,Guidance for Industry.Nasal spray and inhalation solution,suspension,and spray drug products,chemistry, manufacturing,and controls documentation,<http://www.fda.gov/downloads/drugs/ guidancecomplianceregulatoryinformation/guidances/ ucm070575.pdf>;2002.[accessed 16.07.15].
[66]Labiris NR,Dolovich MB.Pulmonary drug delivery.Part II:the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications.Br J Clin Pharmacol 2003;56:600-612.
[67]O’Callaghan C,Barry PW.The science of nebulised drug delivery.Thorax 1997;52:31-44.
[68]Chan JGY,Traini D,Chan HK,et al.Delivery of high solubility polyols by vibrating mesh nebulizer to enhance mucociliary clearance.J Aerosol Med Pulm Drug Deliv 2012;25:297-305.
[69]Weber A,Morlin G,Cohen M,et al.Effect of nebulizer type and antibiotic concentration on device performance.Pediatr Pulmonol 1997;23:249-260.
[70]Sterk PJ,Plomp A,van der Vate JF,et al.Physical properties of aerosols produced by several jet-and ultrasonic nebulizers.Bull Eur Physiopathol Respir 1984;20:65-72.
[71]Dalby R,Suman J.Inhalation therapy:technology milestones in asthma treatment.Adv Drug Deliv Rev 2003;55:779-791.
[72]Kesser KC,Geller DE.New aerosol delivery devices for cystic fi brosis.Respir Care 2009;54:54-67.
[73]Watts AB,McConville JT,Williams RO III.Current therapies and technological advances in aqueous aerosol drug delivery.Drug Dev Ind Pharm 2008;34:913-922.
[74]Ryan G,Singh M,Dwan K.Inhaled antibiotics for long-term therapy in cystic fi brosis.Cochrane Database Syst Rev 2011;(3):CD001021.
[75]Martin AR,Finlay WH.Nebulizers for drug delivery to the lungs.Expert Opin Drug Deliv 2015;12:889-900.
[76]Arunthari V,Bruinsma RS,Lee AS,et al.A prospective, comparative trial of standard and breath-actuated nebulizer: ef fi cacy,safety,and satisfaction.Respir Care 2012;8:1242-1247.
[77]EMA.Guideline on the pharmaceutical products quality of inhalation and nasal products,<http://www.ema.europa .eu/docs/en_GB/document_library/Scienti fi c_guideline/2009/ 09/WC500003568.pdf>;2006.[accessed 16.07.15].
[78]EMA.Guideline on the requirements for clinical documentation for orally inhaled products(OIP)including the requirements for demonstration of the therapeutic equivalence between two inhaled products for use in the treatment of asthma and chronic obstructive pulmonary disease(COPD)in adults and for use in the treatment of asthma in children and adolescents,<http://www.ema .europa.eu/docs/en_GB/document_library/ Scienti fi c_guideline/2009/09/WC500003508.pdf>;2009. [accessed 16.07.15].
[79]FDA.Draft guidance on albuterol sulfate,<http://www.fda.gov/downloads/drugs/ guidancecomplianceregulatoryinformation/guidances/ ucm082419.pdf>;2013.[accessed 16.07.15].
[80]FDA.Draft guidance on fl uticasone propionate;salmeterol xinafoate,<http://www.fda.gov/downloads/drugs/ guidancecomplianceregulatoryinformation/guidances/ ucm367643.pdf>;2013.[accessed 16.07.15].
[81]FDA.Draft guidance on ipratropium bromide,<http://www.fda.gov/downloads/drugs/ guidancecomplianceregulatoryinformation/guidances/ ucm436831.pdf>;2015.[accessed 16.07.15].
[82]FDA.Draft guidance on levalbuterol tartrate,<http://www.fda.gov/downloads/drugs/ guidancecomplianceregulatoryinformation/guidances/ ucm452780.pdf>;2015.[accessed 16.07.15].
[83]FDA.Draft guidance on budesonide;formoterol fumarate dihydrate,<http://www.fda.gov/downloads/drugs/ guidancecomplianceregulatoryinformation/guidances/ ucm452690.pdf>;2015.
[84]Fuglsang A.Approval of generic fl uticasone propionate/ salmeterol xinafoate dry powder inhalers in the US:a dif fi cult exercise in regulatory science.Pharmaceut Med 2014;28:169-173.
[85]Holt J,Hickey A,Sandell D.From Q2 to QbD:the in fl uence of formulation changes on MDI performance.RDDAsia 2014;IL:33-43.
*Corresponding author.Sichuan Purity PharmaceuticalTechnology Co.,Ltd,3-1 Jiuxing Ave,Hi-Tech Zone,Chengdu,Sichuan 610041,China. Tel.:+86 28 85172367;fax:+86 28 85177164.
E-mail address:shuguang.hou@scpurity.com(S.Hou).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.08.008
 Asian Journal of Pharmacentical Sciences2015年6期
Asian Journal of Pharmacentical Sciences2015年6期
- Asian Journal of Pharmacentical Sciences的其它文章
- Rethinking bioequivalence and equivalence requirements of orally inhaled drug products
- Inhaled nicotine replacement therapy
- Inhalation of nanoparticle-based drug for lung cancer treatment:Advantages and challenges
- Mathematical approach for understandingdeagglomeration behaviour of drug powder in formulations with coarse carrier
- The effects of surface morphology on the aerosol performance of spray-dried particles within HFA 134a based metered dose formulations
- Delivery of theophylline as dry powder for inhalation
