阳离子表面活性剂和有机酸混合水溶液的热响应特性
韩传红 耿培培 郭 严 陈肖肖 郭晓冬 张军红 刘 杰 魏西莲(聊城大学化学与化工学院,山东省化学储能与新型电池技术重点实验室,山东聊城252059)
阳离子表面活性剂和有机酸混合水溶液的热响应特性
韩传红耿培培郭严陈肖肖郭晓冬张军红刘杰魏西莲*
(聊城大学化学与化工学院,山东省化学储能与新型电池技术重点实验室,山东聊城252059)
用稳态和动态流变学方法研究了阳离子表面活性剂十六烷基三甲基溴化铵(CTAB)和有机酸3-甲基水杨酸(3MS)的混合水溶液随浓度和温度变化的流变特性。在加热过程中混合溶液呈现三种不同类型的温度响应。其中最有趣的是,当3MS的浓度在80与100 mmol∙kg-1之间时,有浅蓝色的稀溶液出现。随着温度的升高,样品由浅蓝色溶液转化成透明的粘弹性溶液,同时聚集态从囊泡转变成长的蠕虫状胶束,且开始转化的温度随溶液中3MS浓度的增加而升高。利用流变温度扫描和电导率测定对此转变进行了验证。定性解释这个转化是因为在高温下吸附的3MS分子从囊泡上解吸被溶解到水相中。
表面活性剂;胶束;囊泡;热敏性;流变性能
[Article]
www.whxb.pku.edu.cn
In aqueous solutions of cationic surfactants,organic acids containing unsaturated hydrocarbon rings,such as salicylic acid, hydroxynaphthalene carboxylic acid,and their carboxylate were generally selected to co-construct variable self-assembled structures.When such hydrotropes are added to aqueous surfactant solutions,various self-assembled structures such as micelles (spherical,rod-like,wormlike,and disklike),vesicles,liquid crystals,gels,and lamellar structures can form10-14.Among these self-assembled structures,vesicles and micelles are considered to be two of the most useful structures for prospective applications. Vesicles are hollow spheres enclosed by a single bilayer or multilayers of amphiphilic molecules and are commonly used as encapsulating agents for the controlled release of drugs15.Wormlike micelles are of interest because of their complex flow behaviors and viscoelastic properties16,17,and have universal applications in drag reduction and the thickening of chemical formulations.
Nuclear magnetic resonance(NMR)studies on a cetyltrimethylammonium(CTA+)-salicylate system indicate that the salicylate anion binds between the adjacent surfactant head groups.The―OH and COO-functional groups protrude out and away from the micellar surface18,19.This binding mode means that the anion can interact strongly with the cationic surfactant,both electrostatically and hydrophobically,and promote micellar growth1.Davis20and Raghavan3et al.studied the mixtures of CTAB and 5-methylsalicylic acid(5MS).On increasing the temperature,the structures of CTAB/5MS are transformed into worm-like micelles from the initial spherical or unilamellar vesicles,depending on the molar ratio.In addition,Davis et al.21also reported that in dilute aqueous cetyltrimethylammonium chloride (CTAC)/sodium 3-methylsalicylate(CH3Sal-)solution,the selfassembled morphology can transform from vesicles to cylindrical micelles upon shearing.In view of this,what about the mixed system of CTAB and 3-methylsalicylic acid(3MS)?Here,we explored the mixed CTAB/3MS system in details.Major differences in CTAB/3MS,CTAB/4MS,and CTAB/5MS solutions were compared and studied.Surprisingly,we found that transitions from spherical micelles to worm-like micelles and then to vesicles could also occur in the CTAB/3MS solutions by changing the system composition.Furthermore,altering the temperature induces the transition of vesicles to long micelles.The arguments based on the adsorption equilibrium of the anions and the packing parameter of the aggregates were used to explain these results.
2 Materials and methods
2.1Materials
Analytical grade cetyltrimethylammonium bromide(99% CTAB,Shanghai Ziyi Reagent Co.,Ltd.),and 3,4,and 5-methylsalicylic acid(98%3MS,99%4MS and 98%5MS,Aladdin Chemistry Co.,Ltd.)were used without further purification.The water used in solution preparation was redistilled from alkaline potassium permanganate.
2.2Rheological measurements
Samples were prepared by mixing CTAB,3MS/4MS/5MS,and water at the given molality.The samples were homogenized using a magnetic stirrer,and were stored in a constant-temperature water bath at 25°C to allow temperature equilibration.A stress-controlled rheometer(AR2000ex,TAinstruments,USA)with a coneplate geometry of 2°cone angle and 20 mm diameter was used for rheological measurements.The gap between the center of the cone and plate is 50µm.The measuring unit was equipped with a temperature unit(Peltier plate),which provides both rapid change and accurate control of the temperature(uncertainty:±0.05°C) over extended time periods.All measurements were repeated twice to ensure reproducibility.
2.3Electrical conductivity
The electric conductivity of the mixed solutions was measured over the 15-55°C temperature range using a Model DDSJ-308A conductivity meter(Shanghai Leici instruments,China).An ultrathermostat was used to raise and maintain the temperatures of systems.Sampleswithcertaincombinationwerestirred throughout to ensure homogeneity.
2.4Polarizing optical microscopy
Optical analyses were carried out with a polarizing optical microscope(BK-POL,OPTEC instruments,China).The samples were spread on a microscope slide and a cover slip placed over it. Images were recorded at a magnification of 100×as movies with a digital camera.
2.5Cryogenic transmission electron microscopy (cryo-TEM)
Samples for cryo-TEM measurement were prepared in a controlled environment vitrification system(CEVS,Tecnai 20,FEI, Holland).Adrop of solution(1-5 μL)was placed on a TEM grid covered with a perforated carbon film and blotted with a filter paper to form a thin solution film on the grid.Samples were quenched rapidly in liquid ethane to form a vitrified sample and then transferred to liquid nitrogen until examination.The cryogenic temperature is below-166°C.Images were recorded on a high-resolution cooled charge-coupled device(CCD)camera.
3 Results and discussion
3.1Studies at room temperature for CTAB/3MS solution
At a fixed CTAB concentration(CCTAB)of 100 mmol∙kg-1,the mixed aqueous solutions of CTAB/3MS showed interesting physicochemical properties as a function of 3MS concentration(C3MS).When C3MS/CCTAB⩽0.7,the samples formed a transparent homogeneous phase,but the solutions became slightly blue and turbid when this ratio reached 0.8.The difference in the appearance suggests that the system is sensitive to counterion concentration.
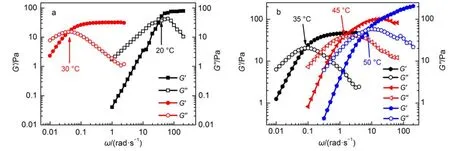
To comprehend the microstructure of the system,the rheological properties of the aqueous solutions were systematically studied using steady-state and frequency sweep measurements. Fig.1a shows the zero-shear viscosity,η0,over a range of 3MS concentrations with a constant CTAB concentration of 100 mmol∙kg-1.The viscosity of the sample is very low in 3MS concentrations of less than 30 mmol∙kg-1.When the concentration of 3MS becomes 40 mmol∙kg-1,the viscosity exhibits a dramatic increase of several orders of magnitude and achieves a maximum when the C3MS/CCTABratio is 0.5.The increased viscosity is observed within a narrow range of concentrations,which illustrates the sensitivity of the system to changes of the counterion concentration1.The zero-shear viscosity of a solution containing 3MS(30 mmol∙kg-1) is approximately 4 Pa∙s,and the steady viscosity of the solution (Fig.2a)exhibits shear thinning behavior above the critical shear rate,γc.Long micelles begin to form when the concentration of 3MS is 30 mmol∙kg-122,23.The presence of a viscosity maximum, which is usually considered to signify a transition from linear to branched micelles,is ubiquitous in the literature on worm-like micelles.In the CTAB/3MS system,nevertheless,unusual results with increasing C3MSare observed.The aqueous solutions of CTAB (100 mmol∙kg-1)and 3MS(30 mmol∙kg-1)have the appearance of a sticky,flowing solution.The dynamic rheological spectra (Fig.2b)show the typical characteristics of viscoelastic worm-like micelle solutions24.At higher 3MS concentrations,the appearanceand rheological properties of the solutions resemble those of gel. The samples can support their own weight for a sustained period when inverted(see the inset in Fig.1a).Furthermore,the steadyshear viscosity measurements(Fig.3a)show no visible plateaus, and the viscosity of the solutions becomes quite high at a 3MS concentration of 40-50 mmol∙kg-1.For the solution that contains 50 mmol∙kg-13MS,no crossover point is detected over the experimentally accessible range of dynamic frequency indicating a very long relaxation time(tR),but it does show plateau in the elastic modulus(G′)curve.Also,G′exceeds the viscous modulus (G″)throughout the examined frequencies(Fig.3b).This is in accordance with the gel-like behavior25,26.The results for G′and G″indicate that at C3MS=40,50 mmol∙kg-1,both the CTAB/3MS solutions form entanglement networks.Whenη0reaches its peak, a strong electrostatic screening effect caused by the electrostatic interaction should occur between the cation of CTAB and the anion of 3MS.This is the expected behavior for these salicylic acid derivatives3,20,27.A possible electrostatic interaction between the molecules is shown in Fig.1b using a mole ratio of CTAB/ 3MS=0.5.In theory,the 3MS embedded in micelles should increase the effective area of the head group of the micelles and has a negative effect on the growth of the micelles,because of the methyl group positioned ortho to the hydrophilic hydroxyl group. The maximum point ofη0should be an equilibrium state for the two opposing driving forces.Further increasing the 3MS con-centration causes a precipitous drop in viscosity.The explanation for this in the literature is that some mixtures of surfactants and additives change the shapes of the aggregates from linear structures to branched worm-like micelles28,29.However,it seems clear that the sharply drop of η0owes to the damage of the network structure by the increase of the 3MS concentration.Of particular interest is the observation that when the concentration of 3MS is greater than 80 mmol∙kg-1,the samples show a bluish hue and very low viscosity like water.These bluish hued solutions are birefringent under dark field microscopy using crossed polarizer. As shown in Fig.4,photographs of the samples present distinct Maltese cross pattern.The Maltese cross texture demonstrates the existence of a lamellar phase30,31.Besides,the bluish color is a manifestation of the Tyndall effect due to the presence of large scatterers in solution,and is often seen in solutions containing vesicles32.

Fig.1 (a)Zero-shear viscosity(η0)of the CTAB(100 mmol∙kg-1) solution as a function of C3MSat 25°C;(b)schematic illustration of a possible electrostatic interaction between the molecules and of the transition from rod-like micelle to viscoelastic micelles

Fig.2 Rheological behaviors of a CTAB(100 mmol∙kg-1)and 3MS(30 mmol∙kg-1)sample at 25°C (a)apparent viscosity(η)versus shear rate(γ); (b)G′and Gʺversus shear frequency(ω)

Fig.3 Curves of apparent viscosity(η)versus shear rate(γ)(a) and variations of G′and Gʺwith the shear frequency(b),for mixed aqueous solutions of CTAB(100 mmol∙kg-1)with 3MS at 40 and 50 mmol∙kg-1at 25°C
For the sake of comparing the impact of the position that the methyl group relative to the hydrophilic hydroxyl group,the rheological properties of the CTAB/4MS and CTAB/5MS mixed solutions at several of the same concentrations were also studied using steady-state and frequency sweep measurements.Although the similar trends in the variation of zero-shear viscosity(η0)with the MS concentrations are observed in the CTAB/5MS and CTAB/ 4MS solutions,the influence of the distance between the methyl group and the hydrophilic hydroxyl group is distinct.Two rheological parameters,η0,and relaxation time(tR)for three mixed systems are reported in Table 1.It is not hard to find that in terms of the same conditions,the CTAB/3MS solutions have the greatest η0and the longest tR;the CTAB/5MS solutions take the second place and the CTAB/4MS solutions have the lowest values.This is because the methyl group on 3MS positions ortho to the hydrophilic hydroxyl group,so 3MS has the smallest stereo-hindrance that it can be more easily embedded in micelles and has a stronger electrostatic screening effect.This result is more effective promotion for the growth of micelles,thus increases the viscosity and relaxation time of the solution.

Fig.4 Polarization photographs of aqueous mixtures of CTAB(100 mmol∙kg-1)and 3MS at 25°C (a)80 mmol∙kg-1;(b)90 mmol∙kg-1

Table 1 Zero-shear viscosity(η0)and relaxation time(tR)of mixed aqueous solutions of 100 mmol∙kg-1CTAB with varying 3MS/4MS/5MS concentrations at 25°C
To obtain more information about the microstructure of this system,we applied the cryogenic transmission electron microscopy(Cryo-TEM)method.Samples that contain CTAB(100 mmol∙kg-1)and 3MS/4MS(either 40 or 100 mmol∙kg-1)were prepared.The cryo-TEM image(Fig.5a)clearly shows that the worm-like micelles have formed in the CTAB/3MS solution (C3MS=40 mmol∙kg-1),which is consistent with the results of the rheological measurements.Fig.5b shows a mass of vesicles withan average vesicle diameter of approximately 100-150 nm. However,it is worth mentioning that many multilayer vesicles are also observed(red arrow in Fig.5b),which is detected in neither the CTAB/5MS solution in the literature3nor the CTAB/4MS solution(Fig.5c).Unilaminar vesicles mainly form in the CTAB/ 4MS and CTAB/5MS solutions and this difference also reflects on the appearance(Fig.6),the turbid state might be due to the formation of multilayer vesicles in CTAB/3MS mixed solution.An increase in the concentration of 3MS changes the viscosity and the aggregation(transform from short micelles to worm-like micelles, and finally change to vesicles)of the solutions.The reason for this is the change in the effective head-group area of the CTAB/3MS mixed systems.The morphology of the self-assembled aggregates is controlled by the spontaneous curvature of the liquid-micelle interface and depends on the packing parameter,where v represents the hydrophobic chain volume,a is the average area per polar head,and l is the surfactant alkyl chain length33.In dilute solution(C3MS<20 mmol∙kg-1),although the binding of 3MS molecules to CTAB micelles causes a screening of the headgroup charge and a reduction of effective head-group area,the effect is weaker than the effect that an increase in the effective head-group area caused by the embedded 3MS molecules in CTAB micelles. As a result,the effective head-group area still increases and the shapes of micelles remain spherical.When the 3MS concentration is greater than 30 mmol∙kg-1,most of the head-group charges are screened.As a result,a reduces and p increases and worm-like micelles form.However,when C3MSincreases beyond 80 mmol∙ kg-1,the head-group area is further reduced.This is because the resultant molecular geometry screens the electrostatic repulsion between the head groups so that they can pack more tightly together.This leads to the formation of low-curvature aggregates. If p is within 0.5 Fig.5 Cryo-TEM micrographs of aqueous CTAB(100 mmol∙kg-1)mixed with 3MS/4MS (a)3MS:40 mmol∙kg-1;(b)3MS:100 mmol∙kg-1;(c)4MS:100 mmol∙kg-1 Fig.6 Appearances of CTAB(100 mmol∙kg-1)with 3MS(left), 4MS(middle),and 5MS(right)(100 mmol∙kg-1)mixed solutions 3.2Effect of temperature on the CTAB/3MS system Ten samples containing 100 mmol∙kg-1CTAB and varying concentrations of 3MS have been investigated at room temperature.Typically,when a micellar solution is heated,the micellar contour length,L,decays exponentially with temperature16,34.The reduction in micellar length leads to an exponential decrease in rheological properties such as zero-shear viscosity η0and relaxation timetR34-36.Indeed,when C3MSwas lower than 40 mmol∙kg-1, h0decayed exponentially with temperature(Fig.7a),which is in accordance with Arrhenius′law.However,for two samples in which C3MSequal to 10 and 20 mmol∙kg-1,the influence of temperature was scarcely observed because of the low viscosity.When C3MSwas higher than 50 mmol∙kg-1,large changes were observed in the rheological properties. As shown in Fig.7b,we can observe that for samples containing 3MS in the concentration range of 50-70 mmol∙kg-1,the zeroshear viscosities increase with increasing temperature until they reach a maximum,and then,on further heating,decrease.The temperature corresponding to the η0maximum increases with increasing concentration of3MS(see the plot t1vs C in Fig.7d). Similar trend can be obtained from the dynamic rheological determination for these samples with increasing temperature.Fig.8 shows the dynamic rheological spectra at various temperatures for the solutions of CTAB(100 mmol∙kg-1)and 3MS(60 mmol∙kg-1).The plots show elastic modulus G′and viscosity modulus G″as a function of frequencyω.We note that the sample exhibits the viscoelastic response expected of worm-like micelles:elastic behavior at highwor at short timescales(G′dominating over G″), and viscous behavior at lowωor long timescales(G″exceeds G′)24.The dynamic rheology measurements,thus,reveal an increase in the relaxation time,τR,up to 30°C,followed by a decrease at higher temperatures.The steady-shear rheological data under different temperatures for solutions containing CTAB(100 mmol∙kg-1)and 3MS(60 mmol∙kg-1)are shown in Fig.9.On the one hand,in all cases,a Newtonian plateau appears at low shearrates,followed by shear thinning at higher shear rates.The zeroshear viscosity,η0,obtained from the steady-shear viscosity measurements increases at 20-30°C and then decreases at higher temperatures(Fig.7b).On the other hand,the viscosity at high shear rates is much less sensitive to temperature,a feature that has also been seen in other studies on worm-like micellar solutions34,37. Fig.7 Zero-shear viscosity as a function of temperature for different CTAB/3MS solutions at a fixed 100 mmol∙kg-1CTAB content(a-c),and the temperature corresponding to theη0maximum(d) 3MS is slightly soluble in cold water,but soluble in hot water. Thus,at room temperature,dissociated anionic 3MS partitions almost to the micelles with the aromatic portion submerged in the hydrophobicinteriorofthemicelles.Thesolubilityof3MSinwater increases with increasing temperature,which means a downward trend that anionic 3MS binds to the micelles.This may cause some of the weakly binding counterions to desorb fromthe micelles and be released into solutions.Consequently,as the temperature increases,the negative charges of the micellar surface reduce and, simultaneously,theheadgroupareaalsochanges.Theoveralleffect of this is to reduce the interfacial curvature of the aggregates leading to micelle growth with increasing temperature3,38-40.In addition,there is another competing temperature effect caused by the increased solubility of 3MS at higher temperatures.The micellar surface is considerably positively charged resulting in increased electrostatic repulsion.Therefore,the head group area will increase and impede the growth of micelles.When 3MS is in the state of high concentration,the temperature should be increased to dissolve excess 3MS in order that the quantity of 3MS in micelles can reach its optimal value and the most stable structure of CTAB(100 mmol∙kg-1)micelles can form,which will also result in the maximum viscosity.Therefore,theη0maximum increases at higher temperatures with increasing 3MS concentration (Fig.7d). Fig.8 Dynamic rheological curves of the sample containing CTAB(100 mmol∙kg-1)and 3MS(60 mmol∙kg-1)as a function of temperature For the Fig.7c,the zero-shear viscosities of the samples containing 3MS in the range of 80 to 100 mmol∙kg-1also increasewith increasing temperature until they reach a maximum,and then decrease on further heating.Unlike the aforementioned samples, however,these samples are bluish dilute solutions at room temperature,which gradually become pale,transparent viscoelastic solutions on increasing the temperature.It is worth mentioning that the transform is thermo-reversible,that is,vesicles convert to worm-like micelles on heating and can be re-formed on cooling. Moreover,the onset of the transition and,correspondingly,the location of the viscosity peak shift to higher temperatures as increasing the concentration of 3MS.Corresponding phenomena are also observed in the temperature ramp scan and conductivity test. As shown in Fig.10a,at low temperatures the samples maintain low viscosity,and the viscosity increases rapidly to a peak when the temperature reaches a certain number.The turning temperature for the three samples containing a fixed[CTAB]and varying [3MS](80,90,and 100 mmol∙kg-1)is 25,40,and 50°C,respectively.The resulting transition temperature point basically squares with that of Fig.7c,and becomes larger with increasing [3MS].The transition for each sample was also determined with the aid of electrical conductivity measurements by plotting the specific conductivities(κ)of solutions as a function of temperature.As shown in Fig.10b,the specific conductivities are gradually enhanced.This is because the solubility of 3MS in water increases with increasing temperature resulting in the fact that the anionic weakly bound 3MS with micelles desorbs to water and,thereby, makes contribution for the conductive ability enhancement of solution.For each sample,reproducible breaks were observed in the conductivity curves indicating the onset of transitions that vesicles convert to worm-like micelles,which is corresponding to the result above.Among the three samples,the steady-shear viscosity of the mixture of CTAB(100 mmol∙kg-1)and 3MS(100 mmol∙kg-1)is shown in Fig.11.The viscosity of this solution is independent of shear rate at low temperature,and its value is only 0.01 Pa∙s at 30°C.This rheological behavior is due to the presence of vesicles(Fig.5b).With increasing temperature,the sample switches to a shear-thinning response and,at 50°C,the zero-shear viscosity is several orders of magnitude higher.Therefore,these samples should undergo a transition from vesicles to worm-like micelles. Fig.9 Steady-shear viscosity of a mixed solution of CTAB(100 mmol∙kg-1)and 3MS(60 mmol∙kg-1)as a function of shear rate(γ)at different temperatures Fig.10 Viscosity(a)and conductivity(b)as a function of temperature for three CTAB/3MS solutions containing 100 mmol∙kg-1CTAB and varying concentrations of 3MS Fig.11 Steady-shear rheology(viscosity as a function of shear rate)for a sample containing CTAB(100 mmol∙kg-1)with 3MS (100 mmol∙kg-1)at various temperatures The reason for the change in structure from vesicles to wormlike micelles is,again,the temperature dependent change in 3MS solubility.At low temperatures,almost an equimolar amount of 3MS is adsorbed at the aggregate surface because of the low solubility of 3MS.This means that most of 3MS molecules are associated with CTAB and the formation of vesicles is on account of the hydrophobic and electrostatic interactions.Heating increases the solubility of 3MS and some of the weakly bound 3MS molecules will disassociate from the vesicles.This desorption changes the molecular geometry and,hence,reduces the inter-facial curvature of the aggregates.Accordingly,the behavior induces a transition from vesicles to worm-like micelles3. Asimilar trend in the changes to aggregate morphology can be obtained by varying the concentration of CTAB in samples.This method can provide a new way to use small molecules to control aggregates in surfactant solutions such as worm-like micelles and vesicles.The method can be applied by adding 3MS to a fixed CTAB solution or changing the temperature of the solutions and then may have utilization in many different fields.Among all the factors,the C3MS/CCTABratio is probably the most important element.Indeed,repeated experiments at different CTAB concentrations have confirmed that the C3MS/CCTABratio largely controls the onset of the vesicle-to-micelle transition. This work has demonstrated that aqueous mixed solutions of 3-methylsalicylic acid(3MS),which contains an unsaturated hydrocarbon ring,and a cationic surfactant CTAB can form spherical micelles,worm-like micelles or multilaminate vesicles.It is all up to the solution composition.More specifically,the transition of spherical micelles to worm-like micelles and even micelles to vesicles on increasing the concentration of 3MS depends on the change in the effective head-group area of surfactant molecules. Additionally,short micelles can transform into long micelles upon heating because of the increase in 3MS solubility,which causes being some 3MS molecules to dissociate from the micelle surface. When heated,it is interesting to note that the vesicles can evolve into micelles and that the thermal response causes the solutions to switch from low-viscosity Newtonian fluid to viscoelastic,shearthinning fluid.The vesicle-to-micelle transition also originates because of the desorption of bound 3MS molecules from the vesicles at high temperatures.In summary,both the onset and magnitude of the viscosity increase are tunable via sample composition and temperature.We hope that these results can shed light on a better understanding of structure-property relationships in surfactant-hydrotrope systems. References (1)Trickett,K.;Eastoe,J.Adv.Colloid Interface 2008,144,66. doi:10.1016/j.cis.2008.08.009 (2)Chu,Z.L.;Dreiss,C.A.;Feng,Y.J.Chem.Soc.Rev.2013,42, 7174.doi:10.1039/c3cs35490c (3)Davies,T.S.;Ketner,A.M.;Raghavan,S.R.J.Am.Chem. Soc.2006,128,6669.doi:10.1021/ja060021e (4)Lee,H.Y.;Diehn,K.K.;Sun,K.S.;Chen,T.H.;Raghavan,S. R.J.Am.Chem.Soc.2011,133,8461.doi:10.1021/ja202412z (5)Zhao,L.;Wang,K.;Xu,L.M.;Liu,Y.;Zhang,S.;Li,Z.B.; Yan,Y.;Huang,J.B.Soft Matter 2012,8,9079.doi:10.1039/ C2SM25334H (6)Zhang,Y.M.;Feng,Y.J.;Wang,J.Y.;He,S.;Guo,Z.R.;Chu, Z.L.;Dreiss,C.A.Chem.Commun.2013,49,4902.doi: 10.1039/c3cc41059e (7)Tsuchiya,K.;Orihara,Y.;Kondo,Y.;Yoshino,N.;Ohkubo,T.; Sakai,H.;Abe,M.J.Am.Chem.Soc.2004,126,12282. (8)Liu,C.C.;Hao,J.C.J.Phys.Chem.B.2011,115,980.doi: 10.1021/jp107946n (9)Jiang,L.X.;Wang,K.;Ke,F.Y.;Liang,D.H.;Huang,J.B. Soft Matter 2009,5,599.doi:10.1039/B813498G (10)Singh,M.;Ford,C.;Agarwal,V.;Fritz,G.;Bose,A.;John,V. T.;McPherson,G.L.Langmuir 2004,20,9931.doi:10.1021/ la048967u (11)Zhai,L.M.;Herzog,B.;Drechsler,M.;Hoffmann,H.J.Phys. Chem.B 2006,110,17697.doi:10.1021/jp0680591 (12)Buwalda,R.T.;Stuart,M.C.A.;Engberts,J.B.F.N. Langmuir 2000,16,6780.doi:10.1021/la000164t (13)Grabner,D.;Zhai,L.;Talmon,Y.;Schmidt,J.;Freiberger,N.; Glatter,O.;Herzog,B.;Hoffmann,H.J.Phys.Chem.B 2008, 112,2901.doi:10.1021/jp0749423 (14)Horbaschek,K.;Hoffmann,H.;Thunig,C.J.Colloid Interface Sci.1998,206,439.doi:10.1006/jcis.1998.5690 (15)Ghosh,R.;Dey,J.Langmuir 2014,30,13516.doi:10.1021/ la5022214 (16)Cates,M.E.;Candau,S.J.J.Phys.Condens.Matter 1990,2, 6869.doi:10.1088/0953-8984/2/33/001 (17)Magid,L.J.J.Phys.Chem.B 1998,102,4064.doi:10.1021/ jp9730961 (18)Olsson,U.;Soderman,O.;Guering,P.J.Phys.Chem.1986, 90,5223.doi:10.1021/j100412a066 (19)Rao,U.R.K.;Manohar,C.;Valaulikar,B.S.;Iyer,R.M. J.Phys.Chem.1987,91,3286.doi:10.1021/j100296a036 (20)Lin,Z.;Cai,J.J.;Scriven,L.E.;Davis,H.T.J.Phys.Chem. 1994,98,5984.doi:10.1021/j100074a027 (21)Zheng,Y.;Lin,Z.;Zakin,J.L.;Talmon,Y.;Davis,H.T.; Scriven,L.E.J.Phys.Chem.B 2000,104,5263.doi:10.1021/ jp0002998 (22)Acharya,D.P.;Kunieda,H.J.Phys.Chem.B 2003,107, 10168.doi:10.1021/jp0353237 (23)Shrestha,R.G.;Shrestha,L.K.;Aramaki,K.J.Colloid Interface Sci.2007,311,276.doi:10.1016/j.jcis.2007.02.050 (24)Wei,X.L.;Ping,A.L.;Du,P.P.;Liu,J.;Sun,D.Z.;Zhang,Q. F.;Hao,H.G.;Yu,H.J.Soft Matter 2013,9,8454.doi: 10.1039/c3sm51017d (25)Thurn,H.;Lobl,M.;Hoffmann,H.J.Phys.Chem.1985,89, 517.doi:10.1021/j100249a030 (26)Lin,Y.Y.;Qiao,Y.;Tang,P.F.;Li,Z.B.;Huang,J.B.Soft Matter 2011,7,2762.doi:10.1039/c0sm01050b (27)Shikata,T.;Hirata,H.;Kotaka,T.Langmuir 1989,5,398.doi: 10.1021/la00086a020 (28)Hoffmann,H.Structure and Flow in Surfactant Solutions; Herb,C.A.,Prud′homme.,R.K.Eds.;American Chemical Society:Washington,DC,1994;pp 2-31. (29)Lin,Z.Langmuir 1996,12,1729.doi:10.1021/la950570q (30)Regev,O.;Guillemet,F.Langmuir 1999,15,4357.doi:10.1021/la980935h (31)Li,X.;Dong,S.L.;Hao,J.C.Soft Matter 2009,5,990.doi: 10.1039/b815640a (32)Jiang,L.X.;Deng,M.L.;Wang,Y.L.;Liang,D.H.;Yan,Y.; Huang,J.B.J.Phys.Chem.B 2009,113,7498. (33)Nagarajan,R.Langmuir 2002,18,31.doi:10.1021/la010831y (34)Raghavan,S.R.;Kaler,E.W.Langmuir 2001,17,300.doi: 10.1021/la0007933 (35)Makhloufi,R.;Cressely,R.Colloid Polym.Sci.1992,270, 1035.doi:10.1007/BF00655973 (36)Ponton,A.;Schott,C.;Quemada,D.Colloids Surf.A 1998, 145,37.doi:10.1016/S0927-7757(98)00681-5 (37)Kalur,G.C.;Frounfelker,B.D.;Cipriano,B.H.;Norman,A. I.;Raghavan,S.R.Langmuir 2005,21,10998.doi:10.1021/ la052069w (38)Hassan,P.A.;Valaulikar,B.S.;Manohar,C.;Kern,F.; Bourdieu,L.;Candau,S.J.Langmuir 1996,12,4350.doi: 10.1021/la960269p (39)Menon,S.V.G.;Manohar,C.;Lequeux,F.Chem.Phys.Lett. 1996,263,727.doi:10.1016/S0009-2614(96)01279-1 (40)Narayanan,J.;Mendes,E.;Manohar,C.Int.J.Mod.Phys.B 2002,16,375.doi:10.1142/S0217979202009895 Thermoresponsive Properties of a Mixed Aqueous Solution of Cationic Surfactant and Organic Acid HAN Chuan-HongGENG Pei-PeiGUO YanCHEN Xiao-XiaoGUO Xiao-Dong ZHANG Jun-HongLIU JieWEI Xi-Lian* Rheologicalpropertiesofaqueousmixturesofthetraditionalcationicsurfactant cetyltrimethylammonium bromide(CTAB)and organic acid 3-methylsalicylic acid(3MS)were studied as a function of concentration and temperature using steady-state and frequency sweep-rheological measurements. Upon being heated,the solutions exhibited three different types of response.Among them,the most interesting response was that light blue dilute solutions formed over the 3MS concentration range of 80 to 100 mmol∙kg-1. These samples changed from dilute pale blue solutions to transparent viscoelastic ones as their aggregation state transitioned from vesicles to long worm-like micelles with increasing temperature.Moreover,the threshold temperature of the transition increased with 3MS concentration.The results of rheological temperature scanning and conductivity measurements verified this trend.Aqualitative explanation for this transformation is that bound 3MS molecules dissociate from the vesicles and join the bulk aqueous phase at high temperature. :Surfactant;Micelle;Vesicle;Thermoresponsive;Rheological property The aggregate morphology of surfactants in solution can significantly affect their physicochemical properties.Typically,dilute solutions of many surfactants exhibit low viscosity because of theformation of spherical micelles or vesicles.However,in some cases,such as with particular additives,the surfactants can assemble into other types of aggregates displaying exceptional viscoelasticities1.In recent years,the self-assembly of amphiphiles and different additives in solutions has attracted considerable attention2.These self-assembled morphologies in solutions can be manipulated by tuning the environmental conditions,such as temperature,additives,illumination,pH,gases,electric field,force field,and etc3-9. October 21,2015;Revised:January 4,2016;Published on Web:January 5,2016.*Corresponding author.Email:weixilian123@126.com;Tel:+86-635-8230613. The project was supported by the National Natural Science Foundation of China(21473084,21073081,21373106,ZR2012BQ013),Scientific Research(LDSY2014008)and Experimental Technology Science Research Project(314011402)of Liaocheng University,China. O648 10.3866/PKU.WHXB201601051 国家自然科学基金(21473084,21073081,21373106,ZR2012BQ013),聊城大学科研课题(LDSY2014008)和实验技术(314011402)资助项目





4 Conclusions
(Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology,College of Chemistry and Chemical Engineering,Liaocheng University,Liaocheng 252059,Shandong Province,P.R.China)1 Introduction

