类风湿关节炎患者外周血滤泡辅助性T细胞水平检测
安乐美,李 娟,季兰岚,李光韬,张卓莉
(北京大学第一医院风湿免疫科, 北京 100034)
·论著·
类风湿关节炎患者外周血滤泡辅助性T细胞水平检测
安乐美,李 娟,季兰岚,李光韬,张卓莉△
(北京大学第一医院风湿免疫科, 北京 100034)
目的:检测外周血滤泡辅助性T细胞(follicular T helper cells , Tfh cells)的比例及其表面标志,分析与类风湿关节炎(rheumatoid arthritis , RA)患者疾病活动度的关系。方法: 收集40例类风湿关节炎患者及20例正常对照组的外周血,分离外周血单个核细胞(peripheral blood mononuclear cells, PBMC)及血清,流式细胞术检测PBMC中CD4+CXCR5+Tfh细胞(CXCR5, 趋化因子受体, C-X-C chemokine receptor type 5)的比例及其表面程序性细胞死亡1(programmed death-1, PD-1)、可诱导的协同刺激性因子(inducible co-stimulator, ICOS)、CD40配体(CD40 ligand, CD40L)及白介素21受体(interleukin-21 receptor, IL-21R)的表达情况,实时荧光定量PCR方法检测B 细胞淋巴瘤-6(B-cell lymphoma 6, Bcl-6)、白介素-21(interleukin-21, IL-21)及IL-21R的表达,酶联免疫吸附法(enzyme-linked immunosorbent assay , ELISA)检测血清中IL-21和B 细胞趋化因子CXC配体13 (B cell-attracting chemokine CXC ligand 13, CXCL13)水平。结果: (1)流式细胞术结果提示,与正常对照组比较,RA患者组外周血CXCR5+CD4+Tfh细胞表达与正常对照组相比明显升高(16.75±3.92vs. 7.49±1.84,P<0.001);低疾病活动度或缓解组RA外周血CXCR5+CD4+Tfh细胞表达与正常对照组相比明显升高(16.62±3.43vs. 7.49±1.84,P<0.001);中疾病活动度组RA外周血CXCR5+CD4+Tfh细胞表达与正常对照组相比明显升高(16.82±3.07vs. 7.49±1.84,P<0.001);高疾病活动度组RA外周血CXCR5+CD4+Tfh细胞表达与正常对照组相比明显升高(16.87±5.50vs. 7.49±1.84,P<0.001);RA患者外周血ICOS、PD-1、IL-21R在CD4+CXCR5+细胞的表达与正常对照组相比显著升高 (ICOS+CXCR5+CD4+细胞, 8.37±4.28vs. 3.72±1.81,P<0.001; PD-1+CXCR5+CD4+细胞, 1.57±1.10vs. 0.24±0.30,P=0.035; IL-21R+CXCR5+CD4+细胞, 4.60±4.05vs. 0.20±0.19,P=0.006),而CD40L在CD4+CXCR5+细胞的表达在两组间差异无统计学意义(3.38±3.71vs. 0.54±0.34,P=0.135)。(2)RA患者组较正常对照组外周血中IL-21R mRNA升高(5.00±4.94vs. 0.74±0.55,P<0.001),而转录因子Bcl-6 mRNA [4.54(3.33, 7.23)vs. 5.31(2.81, 7.44),P=0.329]以及IL-21 mRNA [0.72(0.26, 3.45)vs. 0.56(0.27, 3.71)P=0.195]表达差异无统计学意义(P>0.05);Bcl-6、IL-21和IL-21R mRNA的表达在不同组RA患者之间差异也无统计学意义(P>0.05)。(3)RA患者血清中IL-21、CXCL13水平明显高于正常对照组[IL-21, (200.49±154.56) ng/Lvs. (8.21±5.95) ng/L,P<0.001; CXCL13, (43.09±1.28)ng/Lvs. (93.72±49.72) ng/L,P<0.001];相关分析显示IL-21、CXCL13浓度与RA疾病活动度正相关(r>0.4),而血清类风湿因子(rheumatoid factor, RF)和抗环瓜氨酸多肽抗体(anti-citrullinated protein antibody, anti-CCP)与RA疾病活动度无相关性(r<0.2)。结论: 外周血Tfh细胞及其相关细胞因子在RA患者中显著升高,说明Tfh细胞可能参与RA的发病机制,阻断Tfh细胞的产生,有望成为治疗RA患者的新的作用靶点。
关节炎, 类风湿;T淋巴细胞, 辅助诱导;细胞因子类;受体, CXCR5;白细胞介素21
类风湿关节炎( rheumatoid arthritis, RA)是以侵袭性多关节炎为主要表现的异质性、系统性、自身免疫性疾病,RA的基本病理变化为滑膜炎,滑膜成纤维细胞过度增生,大量T淋巴细胞和巨噬细胞浸润以及血管翳形成[1]。RA的病因尚不清楚,可能与遗传、感染、环境、免疫紊乱有关,其中免疫因素更重要。当抗原进入人体后,激活辅助性T淋巴细胞分泌细胞因子等炎性因子,激活B淋巴细胞分化为浆细胞,进而分泌包括类风湿因子在内的大量免疫球蛋白,同时诱发炎症反应[2]。尽管RA发病机制仍未明确,但CD4+T淋巴细胞一直是研究的重点。滤泡辅助性T细胞(follicular helper T cells, Tfh)是新近发现的一种不同于Th1、Th2、Th17细胞的CD4+T细胞亚群,主要存在于次级淋巴器官滤泡中,参与生发中心的形成、维持,辅助B细胞成熟、增殖,促使免疫球蛋白发生类别转换[3-4]。Tfh细胞特异的转录因子是Bcl-6,高表达趋化因子受体CXCR5(C-X-C chemokine receptor type 5, CXCR5)、程序性细胞死亡1(programmed death-1,PD-1)、可诱导的协同刺激性因子(inducible co-stimulator, ICOS) 等细胞表面膜分子,分泌白介素-21(interleukin-21, IL-21)等细胞因子,同时缺少Blimp-1(prdm1)[5]。外周血T细胞被认为是外周记忆性T细胞,虽然其数量较少,但具有与Tfh细胞相似的功能特性。本文用流式细胞术检测RA患者和正常人外周血中Tfh细胞表面标志在CD4+细胞的表达,实时荧光定量PCR法检测外周血IL-21、IL-21R、Bcl-6 mRNA的表达水平以及酶联免疫吸附法测定血浆中IL-21、CXCL13水平;同时分析评价Tfh细胞相关指标与RA疾病活动度之间的相关性,旨在探索外周血中Tfh细胞与RA发病之间的关系,初步探讨Tfh细胞在RA发病机制中的作用,并为可能的治疗方法提供新策略。
1 资料与方法
1.1 资料
选取2011年7月至2013年4月就诊于北京大学第一医院风湿免疫科的住院或门诊RA患者40例,所有患者均符合1987年或2010年美国风湿病学会(American Collage of Rheumatoid, ACR)分类标准[6-7]。排除标准:近一个月内有感染者,近期有手术外伤史者,处于妊娠、分娩或哺乳期的女性患者,本人或直系亲属中有自身免疫性疾病,长期使用激素或免疫抑制剂,并发其他疾病者。收集正常对照20例,来自北京大学第一医院健康职工。对照组不具备RA分类标准中的任何一条。本研究方案经北京大学第一医院医学伦理委员会审批并备案(2008127),所有入组患者和健康志愿者均签署知情同意书。
1.2 方法
1.2.1 资料搜集 留取患者全血标本9 mL及血清2 mL,同时收集患者的临床资料,包括患者姓名、性别、年龄、民族、肿胀关节数(number of swollen joints, SJC)、压痛关节数(number of tender joints, TJC)、患者对疾病的总体评分(patient global assessment of disease activity, PGA)、医生对疾病的总体评分(evaluator globalassessment of disease activity, EGA)等临床表现以及用药情况;收集患者的实验室资料,包括血尿常规、红细胞沉降率(erythrocyte sedimentation rate, ESR)、C反应蛋白(C-reactive protein, CRP)、类风湿因子(rheumatoid factor, RF)、抗环瓜氨酸多肽抗体(anti-citrullinated protein antibody, anti-CCP)等,为达到统一评判标准,临床资料的收集均由同一医生完成。根据上述临床数据,计算28个关节的疾病活动评分[the disease activity score in 28 joints, ESR-based or CRP-based, DAS28(ESR) or DAS28(CRP)]、临床疾病活动性指数(clinical disease activity index,CDAI)、简化的疾病活动指数(simplified disease activity index, SDAI);根据DAS28(ESR)将RA患者分为3组,即低疾病活动或缓解组、中等疾病活动度组、高疾病活动度组[8]。
1.2.2 实验方法 (1)流式细胞仪检测:分离外周血单个核细胞(peripheral blood mononuclear cells, PBMC)后逐级降温冻存至液氮中,使用时复苏冻存细胞,计数后将细胞转移至流式管中,每管500 μL(细胞浓度维持在5×105个/mL左右)。向流式管中加入抗体CD4、CD19、PD-1、ICOS、IL-21R、CD40L及其同型对照,均为5 μL,CXCR5及其同型对照均为1.5 μL。流式1中加入CD4、CD19、 CXCR5,混匀;流式管2内加入CD4、CD19、CXCR5相应抗体的同型对照,混匀;流式管3中加入CD4、CXCR5、PD-1、ICOS,混匀;流式管4中加入CD4、CXCR5、PD-1、ICOS相应抗体的同型对照,混匀;流式管5中加入CD4、CXCR5、IL-21R、CD40L,混匀;流式管6中加入CD4、CXCR5、IL-21R、CD40L相应抗体的同型对照,混匀。室温孵育30 min,洗涤重悬细胞,使用BD FACSAria流式细胞仪(BD公司,美国)对处理好的标本进行检测,每份标本至少获取50 000个细胞进行分析。用Flow Jo 7.6软件(San Diego公司, 美国) 对获得的数据进行分析。(2)实时荧光定量PCR:使用Primer 3软件设计引物,Bcl-6上游引物 5′-AAGGCCAGTGAAGCAGAGA-3′,下游引物 5′-CCGATAGGCCATGATGTCT-3′ ;IL-21上游引物 5′-TTCAGAAGGCCCAACTAAAG-3′,下游引物 5′-TGAAGGGCATGTTAGTCTGT-3′;IL-21R上游引物 5′-ACGAAGGTCTGAATCCCGA-3′,下游引物 5′-TACACGGGTGACATCGCCAA-3′;β-actin上游引物5′-CACGGCTGCTTCCAGCTC-3′,下游引物 5′-CACAGGACTCCATGCCCAG-3′。各引物委托北京天辉远有限公司合成,采取两步法进行实时荧光定量PCR,首先提取PBMC中的总RNA,反转录获得cDNA;然后,分别分别加入IL-21,IL-21R和Bcl6的上、下游引物,同时以β-actin作为内参照,应用ABI 7300 RT-PCR仪,最后计算ΔCt(Ct样本-Ct内参照),半定量待测目标产物的表达。(3)ELISA:严格按照酶联免疫吸附法(enzyme-linked immunosorbent assay, ELISA)试剂盒说明书进行,其中所用主要试剂如下:淋巴细胞分离液购自美国GE公司;流式抗体CD4-PerCP、CD19-APC、CXCR5-Alexa Fluor®488、PD-1-APC、ICOS-PE、CD40L-PE、IL-21R-APC及与之对应的同型对照抗体均购自美国BD PharmingenTM公司; CXCL13、IL-21 ELISA试剂盒购自美国R&D公司。
1.3 统计学分析
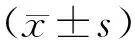
2 结果
2.1 一般临床资料比较
RA患者与正常对照组在性别、年龄上差异无统计学意义,具有可比性。40例RA患者均为汉族,其中男性10例,女性30例,男女比例为1 ∶3,平均年龄(49.6±15.0)岁,病程12~75个月(中位病程30个月),其中8例患者病程超过24个月。根据患者TJC、SJC、PGA、EGA、ESR计算DAS28(ESR),14例患者属于高疾病活动度组[high disease activity of RA,HRA, DAS28(ESR)>5.1];13例患者属于中疾病活动度组[moderate disease activity of RA,MRA, DAS28(ESR) 3.2~5.1],13例患者属于低疾病活动度或者缓解组[low disease activity or remission of RA, LRA,DAS28(ESR)<3.2]。用药情况见表1。20 例健康对照中,其中5例男性,15例女性,男女比例为1 ∶3.3,平均年龄 (47.8±8.8)岁,最小年龄39岁,最大年龄65岁。

表1 类风湿关节炎患者的临床特征(n=40)Table 1 Clinical characteristics of rheumatoid arthritis patients (n=40)
Other drugs taken were anti-tumor necrosis factor α(2 patients with active RA), hydroxychloroquine (5 patients), sulfasalazine (2 patients); some patients took>1 drug. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; RF, rheumatoid factor; DAS28, disease activity score assessed in 28 joint areas (ESR-based or CRP-based); CDAI, clinical disease activity index; SDAI, simplified disease activity index; NSAIDs, nonsteroidal anti-inflammatory drugs.
2.2 流式细胞分析结果
2.2.1 RA患者外周血CD4+CXCR5+Tfh细胞频率明显升高 对RA患者及健康志愿者中的CD4+CXCR5+Tfh细胞进行流式细胞术检测,结果发现RA患者外周血CD4+CXCR5+Tfh细胞占CD4+细胞的百分比明显高于正常对照组 (P<0.05)。进一步对RA患者进行分组研究,发现外周血CD4+CXCR5+Tfh细胞在RA患者各组间差异无统计学意义(图1)。
2.2.2 CD4+CXCR5+Tfh细胞上PD-1、ICOS、CH40L和 IL-21R 的表达情况 为明确不同疾病活动度RA患者之间及与正常对照者外周血CD4+CXCR5+Tfh细胞在细胞表型上的差异,用流式细胞术检测了CD4+CXCR5+Tfh细胞上PD-1, ICOS, IL-21R及CD40L的表达情况,结果发现, RA患者中,ICOS, PD1, IL-21R在 CXCR5+CD4+细胞表达较正常对照组升高(P<0.05),而CD40L在CXCR5+CD4+Tfh细胞的表达水平则在两组间差异无统计学意义(P>0.05,图2)。
2.3 RT-PCR实验结果
Bcl6和IL-21在Tfh细胞分化发育过程中起重要作用,因此本研究检测了PBMC中相关mRNA的表达,结果显示,IL-21R在两组之间差异有统计学意义(P<0.05),Bcl6、IL-21 mRNA表达在RA患者和正常对照组间差异无统计学意义 (P>0.05,图3)。
2.4 RA患者血清CXCL13和IL-21水平升高
血清ELISA结果示:RA组和正常对照组血浆IL-21的浓度分别为(200.49±154.56) ng/L和(8.21±5.95) ng/L,两组间差异有统计学意义(P<0.001)。不同RA分组之间差异也有统计学意义(P<0.05),MRA和HRA均显著高于LRA(P<0.05)。RA组和正常对照组血浆CXCL13的浓度分别为(93.72±49.72) ng/L、(43.09±1.28) ng/L,两组间差异有统计学意义(P<0.05)。不同RA分组之间差异也有统计学意义(P<0.05,图4)。
双变量相关分析显示:血清IL-21浓度和CXCL13浓度呈正相关(r=0.643,P<0.001);血清CXCL13、IL-21与RA的疾病活动指数及RF、抗CCP的相关分析见图5和表2。
3 讨论
RA是一种以进行性滑膜炎症和关节破坏为特征的系统性自身免疫病,发病率较高,是最常见的风湿病之一。多年来,虽然对其病因及发病机制进行了大量的研究,以寻求更有效的治疗方法,但至今仍然因病因不明而使疾病无法根治。人类 Tfh 细胞是新近发现的一种CD4+T细胞亚群[9],研究表明Tfh细胞参与多种自身免疫性疾病的发病,如系统性红斑狼疮、干燥综合征、Grave’s病和桥本氏甲状腺炎[10-12]。
本研究发现CXCR5+、ICOS+、PD-1+、IL-21R+细胞在CD4+细胞上表达明显升高,与既往在系统性红斑狼疮中的研究结果一致[11]。Tfh细胞在B细胞特异性抗原发育和体液免疫反应中起重要作用,因此增高的Tfh细胞可能反映活跃的免疫反应[13]。本研究发现外周血PD-1+、ICOS+、IL-21R+在CXCR5+CD4+细胞上表达增高,说明Tfh细胞活化后从淋巴滤泡迁入至外周血发挥其功能,而外周血中CD40-CD40L依赖B细胞的活化,所以RA患者CD40L在CXCR5+CD4+细胞上表达与正常对照组间差异无统计学意义[14]。增多的Tfh细胞相关表面标志与RA的疾病活动度[ESR, CRP, DAS28(ESR), DAS28(CRP), CDAI或SDAI]没有相关性,说明外周血Tfh细胞可能是RA的固定表型,不随病情的变化而变化。
A, expression of CXCR5 on CD4 lymphocytes in health controls; B, expression of CXCR5 on CD4 lymphocytes in RA patients; C, expression of CXCR5 on CD4 lymphocytes between RA patients and healthy controls; D, relative prevalence of CXCR5CD4 cells among different RA groups. HC, healthy controls; RA, rheumatoid arthritis; Guided by DAS28(ESR), LRA, low disease activity or remission of RA; MRA, moderate disease activity of RA; HRA, high disease activity of RA. *P<0.001.
图1 CXCR5CD4 Tfh细胞在RA患者与正常对照组之间的表达
Figure1 CXCR5CD4 Tfh cells expressed in RA patients and healthy controls
A, PD-1 expressed onCD4CXCR5 Tfh cells in RA patients and HC; B, ICOS expressed on CD4CXCR5 Tfh cells in RA patients and HC; C, IL-21R expressed on CD4CXCR5 Tfh cells in RA patients and HC; D, CD40L expressed on CD4CXCR5 Tfh cells in RA patients and HC. HC, healthy controls; RA, rheumatoid arthritis; PD-1, programmed death 1 positive; ICOS, inducible T cell costimulator; IL-21R, interleukin-21 receptor; CD40L, CD40 ligand. *P<0.001.
图2 PD-1,ICOS, IL-21R和CD40L在CD4CXCR5 Tfh细胞表达
Figure2 PD-1, ICOS, IL-21R and CD40L expressed on CD4CXCR5 Tfh cells in RA patients and HC
A, Bcl-6 mRNA expressed in RA and HC; B, IL-21 mRNA expressed in RA and HC; C, IL-21R mRNA expressed in RA and HC; Bcl-6, B-cell lymphoma 6; IL-21, Interleukin-21; IL-21R, Interleukin-21 receptor; HC, healthy controls; RA, rheumatoid arthritis. * P<0.001.
图3 RA患者与正常对照组之间Bcl-6、IL-21和IL-21R mRNA的表达
Figure3 Expression of Bcl-6, IL-21 and IL-21R mRNA
A, level of CXCL13 in sera of RA patients and HC;B, level of CXCL13 in different RA subgroups; C, level of IL-21 in sera of RA patients and HC; D, level of IL-21 in different RA subgroups; HC, healthy controls; RA, rheumatoid arthritis. IL-21, Interleukin-21; CXCL13,B cell-attracting chemokine CXC ligand 13; LRA, low disease activity or remission of RA; MRA, moderate disease activity of RA; HRA, high disease activity of RA. *P<0.001.
图4 CXCL13、IL-21在RA患者和正常对照组血清中的表达
Figure4 CXCL13 and IL-21 levels in sera of RA patients and HC
A, relationship between serum CXCL13 and ESR; B, relationship between serum CXCL13 and DAS28(ESR); C, relationship between serum CXCL13 and DAS28(CRP); D, relationship between serum CXCL13 and CDAI; E, relationship between serum CXCL13 and SDAI; F, relationship between serum CXCL13 and IL-21. ESR, erythrocyte sedimentation rate; CRP, C reactive protein; DAS28(ESR) or DAS28(CRP), the disease activity score in 28 joints, ESR-based or CRP-based; CDAI, clinical disease activity index; SDAI, simplified disease activity index; IL-21, Interleukin-21; CXCL13,B cell-attracting chemokine CXC ligand 13.图5 血清CXCL13浓度与RA活动度指标[ESR、DAS 28(ESR)、DAS 28(CRP)、CDAI、SDAI]及血清IL-21浓度
Figure5 Relationship between serum CXCL13 and RA disease activity indexes and serum IL-21 in RA patients

表2 细胞因子浓度与疾病活动度指标、自身抗体定量资料的相关性分析
ESR, erythrocyte sedimentation rate; CRP, C reactive protein; DAS28(ESR) or DAS28(CRP), the disease activity score in 28 joints, ESR-based or CRP-based;CDAI, clinical disease activityindex; SDAI, simplified disease activity index; IL-21, Interleukin-21; CXCL13,B cell-attracting chemokine CXC ligand 13; RF, rheumatoid factor; anti-CCP, anti-citrullinated protein antibody.
RA患者和正常对照组之间转录因子Bcl-6的表达差异无统计学意义,分析原因可能是 Bcl-6的表达为转录后调控,检测Bcl-6 蛋白比Bcl-6 mRAN更能反映问题的本质[15]。此外,IL-21 mRNA的表达在RA患者和正常对照组之间差异无统计学意义,说明IL-21也可在其他组织中表达,如滑膜。IL-21主要由活化的CD4+T细胞分泌,所以IL-21可能在活动度非常高的RA患者中表达升高[16]。
本研究发现RA患者中血浆CXCL13升高,且与RA患者的病情活动度正相关,这促使我们检测CXCL13的受体CXCR5的表达。外周血中的CXCR5+细胞群增多,是否与 CXCL13的趋化作用有关,需要进一步验证,而在SLE中,CXCL13与系统性红斑狼疮疾病活动指数相关[17]。血浆CXCL13与CD4+CXCR5+之间的相关性说明CXCL13可作为代替CD4+CXCR5+的评价指标。
本研究发现RA患者中循环Tfh细胞表达增高,然而改善病情抗风湿药治疗后是否降低Tfh表达的相关表达尚不能确定。如果Tfh细胞异常,可能在不同程度上影响T细胞B细胞的相互作用,导致体液免疫和细胞免疫的异常,从而导致RA发病。
[1]Mitchell DM, Spitz PW, Young DY, et al. Survival, prognosis, and causes of death in rheumatoid arthritis[J]. Arthritis Rheum, 1986, 29(6): 706-714.
[2]Mcinnes IB, Schett G. The pathogenesis of rheumatoid arthritis[J]. N Engl J Med, 2011, 365(23): 2205-2219.
[3]Szabo SJ, Kim ST, Costa GL, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment[J]. Cell, 2000, 100(6): 655-669.
[4]Toyama H, Okada S, Hatano M, et al. Memory B cells without somatic hypermutation are generated from Bcl6-deficient B cells[J]. Immunity, 2002, 17(3): 329-339.
[5]Crotty S. Follicular helper CD4 T cells (TFH)[J]. Annu Rev Immunol, 2011, 29 (1): 621-663.
[6]Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis[J]. Arthritis Rheum, 1988, 31(3): 315-324.
[7]Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative[J]. Arthritis Rheum, 2010, 62(9): 2569-2581.
[8]Anderson JK, Zimmerman L, Caplan L, et al. Measures of rheumatoid arthritis disease activity: patient (PtGA) and provider (PrGA) global assessment of disease activity, disease activity score (DAS) and disease activity score with 28-joint counts (DAS28), simplified disease activity index (SDAI), clinical disease activity index (CDAI), patient activity score (PAS) and patient activity score-Ⅱ (PASIⅡI), routine assessment of patient index data (RAPID), rheumatoid arthritis disease activity index (RADAI) and rheumatoid arthritis disease activity index-5 (RADAI-5), chronic arthritis systemic index (CASI), patient-based disease activity score with ESR (PDAS1) and patient-based disease activity score without ESR (PDAS2), and mean overall index for rheumatoid arthritis (MOI-RA)[J]. Arthritis Care Res (Hoboken), 2011, 63 (Suppl 11): S14-S36.
[9]Shahrara S, Huang Q, Mandelin AN, et al. TH-17 cells in rheumatoid arthritis[J]. Arthritis Res Ther, 2008, 10(4): R93.
[10]Zhu C, Ma J, Liu Y, et al. Increased frequency of follicular hel-per T cells in patients with autoimmune thyroid disease[J]. J Clin Endocrinol Metab, 2012, 97(3): 943-950.
[11]Simpson N, Gatenby PA, Wilson A, et al. Expansion of circula-ting T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus[J]. Arthritis Rheum, 2010, 62(1): 234-244.
[12]Linterman MA, Rigby RJ, Wong RK, et al. Follicular helper T cells are required for systemic autoimmunity[J]. J Exp Med, 2009, 206(3): 561-576.
[13]Patakas A, Platt AM, Butcher JP, et al. Putative existence of reciprocal dialogue between Tfh and B cells and its impact on infectious and autoimmune disease[J]. Immunol Lett, 2011, 138(1): 38-46.
[14]King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses[J]. Annu Rev Immunol, 2008, 26 (1): 741-766.
[15]Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis[J]. Adv Immunol, 2010, 105 (1): 193-210.
[16]Yuan FL, Hu W, Lu WG, et al. Targeting interleukin-21 in rheumatoid arthritis[J]. Mol Biol Rep, 2011, 38(3): 1717-1721.
[17]Rosengren S, Wei N, Kalunian KC, et al. CXCL13: a novel biomarker of B-cell return following rituximab treatment and synovitis in patients with rheumatoid arthritis[J]. Rheumatology (Oxford), 2011, 50(3): 603-610.
(2015-02-05收稿)
(本文编辑:刘淑萍)
Detection of peripheral follicular helper T cells in rheumatoid arthritis
AN Le-mei, LI Juan, JI Lan-lan, LI Guang-tao, ZHANG Zhuo-li△
(Department of Rheumatology and Clinical Immunology, Peking University First Hospital, Beijing 100034, China)
Objective: To detect cell frequency and surface markers of peripheral CD4+CXCR5+follicular helper T (Tfh) cells and analyze the correlation between CD4+CXCR5+Tfh cells and rheumatoid arthritis (RA) disease activity. Methods: Forty RA patients meeting the American College of Rheumatology classification criteria for RA and twenty healthy controls (HC) were included. The peripheral blood mononuclear cells and sera were collected. The expressions of CD4+CXCR5+Tfh cells (CXCR5, C-X-C chemokine receptor type 5) and inducible T cell costimulator (ICOS), programmed death 1 positive (PD-1), interleukin-21 receptor (IL-21R) and CD40 ligand (CD40L) positive on CD4+CXCR5+Tfh cells were analyzed by flow cytometry. The transcript levels of B-cell lymphoma 6 (Bcl-6), as well as IL-21 and IL-21R, were measured by real-time polymerase chain reaction. Besides, serum IL-21 and CXCL13 concentrations were determined by enzyme-linked immunosorbent assay. The potential association between Tfh cells and RA disease activity was detected. Results: The cell surface marker of CXCR5+on CD4+cells was significantly increasingly higher across the following groups versus HC: total RA patients (16.75±3.92vs.7.49±1.84,P<0.001); RA patients with low disease activity or remission (16.62±3.43vs. 7.49±1.84,P<0.001); RA patients with moderate disease activity (16.82±3.07vs. 7.49±1.84,P<0.001) and RA patients with high disease activity (16.87±5.50vs. 7.49±1.84,P<0.001). Besides, the percentages of ICOS+, PD-1+, IL-21R+on CD4+CXCR5+Tfh cells in the RA patients were significantly higher than that of HC (ICOS+CD4+CXCR5+cells, 8.37±4.28vs. 3.72±1.81,P<0.001; PD-1+CD4+CXCR5+cells, 1.57±1.10vs. 0.24±0.30,P=0.035; IL-21R+CD4+CXCR5+cells, 4.60±4.05vs. 0.20±0.19,P=0.006). But the percentage of CD40L+on CXCR5+CD4+Tfh cells in the RA patients was not significantly higher than that of HC (3.38±3.71vs. 0.54±0.34,P=0.135). The IL-21R mRNA expression was elevated significantly (5.00±4.94vs. 0.74±0.55,P<0.001) in the RA patients but not in Bcl-6 mRNA[4.54(3.33, 7.23)vs. 5.31(2.81, 7.44),P=0.329]or IL-21 mRAN[0.72(0.26, 3.45)vs. 0.56(0.27, 3.71),P=0.195]. Additionally, the serum interleukin-21 (IL-21)and CXCL13 levels in the RA patients were higher than in the healthy controls [IL-21, (200.49±154.56) ng/Lvs. (8.21±5.95) ng/L,P<0.001; CXCL13, (93.72±49.72) ng/Lvs. (43.09±1.28) ng/L,P<0.001] and were both positively correlated with RA disease activity indexes, including erythrocyte sedimentation rate, the disease activity score in 28 joints (ESR-based or CRP-based), clinical disease activity index, and simplified disease activity index. However, none of the Tfh cells, anti-citrullinated protein antibody or rheumatoid factor had any relationship with RA disease activity. Conclusion: Peripheral Tfh cells and their relevant cytokines are higher in RA patients than healthy controls, indicating Tfh cells may participate in the pathogenesis of RA, therefore, blocking the pathway of Tfh cells may be reasonable cellular targets for therapeutic intervention.
Arthritis, rheumatoid; T-lymphocytes, helper-inducer; Cytokines; Receptors, CXCR5; Interleukin-21
国家重点基础研究发展计划(973计划,2010CB529103)资助Supported by the Major State Basic Research Development Program of People’s Republic of China (973 Program, 2010CB529103)
时间:2016-10-31 16:28:45
http://www.cnki.net/kcms/detail/11.4691.R.20161031.1628.012.html
R593.22
A
1671-167X(2016)06-0951-07
10.3969/j.issn.1671-167X.2016.06.006
△ Corresponding author’s e-mail, zhuoli.zhang@126.com

