Methotrexate combined with methylprednisolone for the recovery of motor function and differential gene expression in rats with spinal cord injury
Jian-tao Liu, Si Zhang, Bing Gu,, Hua-nan Li, Shuo-yu Wang, Shui-yin Zhang
1 Jiangxi Key Laboratory of Bioprocess Engineering, Jiangxi Science & Technology Normal University, Nanchang, Jiangxi Province, China
2 Department of Spine Surgery, Af filiated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi Province, China
RESEARCH ARTICLE
Methotrexate combined with methylprednisolone for the recovery of motor function and differential gene expression in rats with spinal cord injury
Jian-tao Liu1, Si Zhang1, Bing Gu1,*, Hua-nan Li2, Shuo-yu Wang2, Shui-yin Zhang1
1 Jiangxi Key Laboratory of Bioprocess Engineering, Jiangxi Science & Technology Normal University, Nanchang, Jiangxi Province, China
2 Department of Spine Surgery, Af filiated Hospital of Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi Province, China
Graphical Abstract
Molecular mechanism of methotrexate (MTX) combined with methylprednisolone (MP) in the treatment of spinal cord injury (SCI)
Abstract
Methylprednisolone is a commonly used drug for the treatment of spinal cord injury, but high doses of methylprednisolone can increase the incidence of infectious diseases. Methotrexate has anti-inflammatory activity and immunosuppressive effects, and can reduce inflammation after spinal cord injury. To analyze gene expression changes and the molecular mechanism of methotrexate combined with methylprednisolone in the treatment of spinal cord injury, a rat model of spinal cord contusion was prepared using the PinPoint™ precision cortical impactor technique. Rats were injected with methylprednisolone 30 mg/kg 30 minutes after injury, and then subcutaneously injected with 0.3 mg/kg methotrexate 1 day after injury, once a day, for 2 weeks. TreadScan gait analysis found that at 4 and 8 weeks after injury, methotrexate combined with methylprednisolone significantly improved hind limb swing time, stride time, minimum longitudinal deviation, instant speed, footprint area and regularity index. Solexa high-throughput sequencing was used to analyze differential gene expression. Compared with methylprednisolone alone, differential expression of 316 genes was detected in injured spinal cord treated with methotrexate and methylprednisolone. Te 275 up-regulated genes were mainly related to nerve recovery, anti-oxidative, anti-inflammatory and anti-apoptotic functions, while 41 down-regulated genes were mainly related to proinflammatory and pro-apoptotic functions. Tese results indicate that methotrexate combined with methylprednisolone exhibited better effects on inhibiting the activity of inflammatory cytokines and enhancing antioxidant and anti-apoptotic effects and thereby produced stronger neuroprotective effects than methotrexate alone. Te 316 differentially expressed genes play an important role in the above processes.
Key Words:nerve regeneration; spinal cord injury; methotrexate; methylprednisolone; gait; gene expression pro?le; inflammation; oxidative stress; apoptosis; nerve repair; Solexa gene sequencing; secondary lesion; neural regeneration
Introduction
The incidence of spinal cord injury (SCI) caused by many events, including traffic accidents, mining and construction accidents, seismic and natural disasters, has tended to rise year on year. Te pathology of SCI usually includes primary injury and secondary injury (Tator, 1991). Primary injury often occurs in the spine, leading to SCI (Ray et al., 2016; Yu et al., 2016).Te secondary damage includes inflammation, oxidative stress,neuronal apoptosis, intracellular and extracellular ion imbalance and a series of pathological reactions (Luo et al., 2013).Terefore, to avoid second injury and to promote the survival of axons and neurons, therapies aim to reduce or eliminate the destructive pathological response and to promote the regeneration, repair and functional reconstruction of nerve tissue in the chronic phase.
Methylprednisolone (MP) acts through a variety of mechanisms to prevent the occurrence of secondary SCI and is currently the only food and drug administration-approved drug for the treatment of acute SCI (Bracken, 2012). However, high doses of MP lead to many side effects, including glucocorticoid-induced infection, diabetic complications, and impaired wound healing, and can endanger the lives of patients. Terefore, many experts suggest that high-dose MP shock therapy should not be used as a conventional treatment, although it can be used as a selective treatment strategy (Zhang et al., 2013).
Methotrexate (MTX) is a common anti-rheumatic drug that can improve the disease state and has anti-inflammatory and immunosuppressive effects (Keith et al., 2012). MTX is generally used to compensate for the poor ef ficacy of glucocorticoid or other anti-rheumatic drugs. MTX can also be combined with hormones in early rheumatoid arthritis, so as to reduce hormone doses, thereby alleviating hormone side effects (Pincus et al., 2015). MTX can be taken long-term because of low cost,various routes of administration, steady long-term ef ficacy and high safety; namely, it can be taken as an anchor drug (Saeki et al., 2012). Low doses of MTX can be used in the treatment of SCI to ameliorate inflammation (Sanli et al., 2012), oxidative stress and apoptosis (Bakar et al., 2013; Kertmen et al., 2013),thereby preventing the occurrence and development of secondary injury.
Terefore, large-dose MP pulse therapy has been implemented in the early stage of SCI, followed by low-dose MTX maintenance treatment to compensate for the toxic effects of MP.Under this regimen, MTX can protect the remaining synaptic and neuronal functions. Most studies have explored the therapeutic effect on SCI of MTX or MP alone. Tis study, however,explored the molecular mechanisms of combined MP and MTX administration for treating SCI by high-throughput sequencing.
Materials and Methods
Animals
Fifty male Sprague-Dawley rats weighing 245 ± 20 g were provided by Hunan Slack Kingma Company, China (license No.43004700001369). All rats were housed at room temperature (25± 2°C) and 65% humidity with a 12 hour light/dark cycle. Bedding was changed every two days. The rats were allowed free access to food and water.
To allow rats to adapt to the treadmill environment, fifty rats were adaptively trained on a ZH-PT computer controlled animal treadmill (Anhui Zhenghua, Huaibei, China) for 5 days before the operation. All rats were randomly assigned to the sham group (sham operation), SCI group (model establishment), MP group (SCI + MP alone), MTX group (SCI + MTX alone) and MP + MTX group (SCI + MP combined with MTX). Ten rats from each group were used for gait analysis, six for histology and the remaining four for Solexa gene sequencing.
All procedures were approved by the Experimental Animal Ethics Committee of Jiangxi University of Traditional Chinese Medicine (approval No. A20150107). Te experiment followed the United States National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication No.85-23, revised 1986).
Surgical procedure for SCI
SCI models were prepared using a modi fied method reported by Gu et al. (2011). Rats were anesthetized with 5% chloral hydrate and fixed on a stereotaxic apparatus in the prone position. After shaving the thoracolumbar back region, the skin was disinfected with iodophor. Taking the T10spinous process as the center, a 4.0 cm-long median longitudinal incision was made to separate the muscle and fascia from both sides of the spine. Te spinous process and lamina were gently removed, and the spinal dura mater was exposed. Te PinPointTMPrecision Cortex Striker (diameter 3.0 mm, Hatteras Instrument Cary, NC, USA) was moved to the top of the T10dural surface and centered on the median vessel.When the contact head, which has a high-precision contact sensor, touched the surface of the dura mater, an audible warning was generated. Subsequently, the three-dimensional coordinates were determined. T10injury was produced with an impact depth of 1.0 mm; an impact velocity of 2.0 m/s; and a residence time of 200 ms. Immediately after rat tail spasm and retraction flutter of lower extremities, cold gauze was used to stop bleeding. Muscles and skin were sutured layer by layer.
The sham group was operated as above, but no impact was performed. The preparation and postoperative nursing of SCI models were in accordance with procedures in a previous study(Gu et al., 2011). After surgery, rats were injected with 5 mL Ringer’s lactate solution to compensate for blood loss. Te rectal temperature was maintained at 37 ± 1°C with a thermostatically controlled heating pad. Penicillin and gentamicin sulfate (2.5 mL/kg) were intramuscularly injected twice a day for 7 days. If urinary tract infection occurred, the antibiotics were administered for 10 days. At a fixed time, urination was induced 2 to 3 times every day, until spontaneous urination.
Terapeutic schedule
MP (Methylprednisolone Sodium Succinate for Injection;batch number: Z07305) was provided by P fizer Pharmaceutical Belgian Company (Belgian, Ixelles). MTX (0.05 g/1 mL, batch number: 271021-2) was purchased from the Lingnan Pharmaceutical Co., Ltd. (Guangzhou, China).
Te necks of rats in sham and SCI groups were subcutaneously injected with 0.5 mL normal saline 30 minutes after injury.The rats in the MP group were injected with MP (30 mg/kg,187 μL; P fizer, Belgian, Ixelles)viathe tail vein. Te rats in the MTX group were subcutaneously injected with MTX (0.3 mg/kg, 1.5 μL; Lingnan Pharmaceutical Co., Ltd.), once every other day, for 2 weeks. Te rats in the MP + MTX group were injected with MP (30 mg/kg, 187 μL)viathe tail vein 30 minutes beforeinjury, and were then subcutaneously injected with MTX (0.3 mg/kg, 1.5 μL) at 1 day after injury, once a day, for 2 weeks.
Gait analysis
Te hind limb gait, including hind limb swing time, stride time,minimum longitudinal deviation, instant speed, footprint area and regularity index, was recorded using the small animal gait analyzer (Clever Sys. Inc., Reston, VA, USA). The video files were analyzed using Treadscan 4.0 system software (Clever Sys.Inc.) (Zhang et al., 2012). Gaits were tested at 2, 4, and 8 weeks after injury. Before the test, each rat ran freely for 30–45 seconds and was then placed on the walking table of the gait analyzer. Te speed of the walking table increased from 7 cm/s to 15 cm/s over 20 seconds. Each rat was tested three times.
Histology
Sections containing injured spinal cord tissue were generated as previously described (Pinzon et al., 2008). Briefly, at 8 weeks, all animals were anesthetized and perfused intracardially with normal saline solution followed by 4% paraformaldehyde solution.Cord segments of about 1 cm surrounding the injured site were dissected, embedded in paraf fin, and cut vertically into serial sections. One section at the center of impact (+0 mm) and sections at around 3 and 5 mm from the impact site were selected and stained with Harris’ hematoxylin and eosin for routine pathological examination.
Total RNA extraction and Solexa gene sequencing
Rats were decapitated 8 weeks after surgery. Four rats were selected from each group for RNA extraction. An approximately 10 mm spinal cord section surrounding the damage center was isolated. Total RNA extraction and cDNA synthesis were performed as previously described (Liu et al., 2016). Briefly,total RNA from each sample was isolated using an RNeasy Protect Mini Kit (Qiagen, Dusseldorf, Germany). RNA purity and quantity were assessed with a SmartSpec Plus (Bio-Rad,Hercules, CA, USA). cDNA synthesis was performed using an iScript cDNA Synthesis Kit (Bio-Rad). Te amplification procedure was: pre-denaturation for 30 seconds at 98°C; 18 cycles of denaturation for 10 seconds at 98°C, annealing for 30 seconds at 65°C, extension for 15 seconds at 72°C; extension for 7 minutes at 72°C; holding at 8°C. The amplification products were sequenced after gel purification by HiSeq2000 high-throughput sequencing (Illumina, San Diego, CA, USA). Te obtained image files were converted into digital signals. Te results were analyzed and processed after removing impurities and linker sequences.
Quality evaluation and RNA-Seq bioinformatic analysis
Original HiSeq2000 Single-end sequencing images were transformed into raw data. Adapters and low quality, contaminated data were removed from the raw data and the length distribution was analyzed. Sequences were then matched against the rat genome. Te ratios of sequence to the genome and unique gene were calculated using SOAP software (BGI, Shenzhen, China).To annotate and classify gene functions for each sequence, all assembled unique sequences were searched against GenBank’s non-redundant database, the Swiss-Prot protein database(http://www.expasy.ch/sprot), the KEGG pathways database(Kanehisa et al., 2008), and the COG database (http://www.ncbi.nlm.nih. gov/COG/) using BLASTx and against nucleotide(nt) using BLASTn with a threshold of E <1.0E-5. According to protein (nr) annotation information, we used Blast2GO software (Conesa et al., 2005) to obtain GO comment information from Unigene. AGRIGO and WEGO software were used to conduct GO functional classification (Ye et al., 2006). R program was used to identify differentially expressed genes from different samples. Differential expression was analyzed between two groups (SCI and sham groups, MP and SCI groups, and MTX + MP and MP groups) using DEGseq software (Wang et al., 2010). Te abundance of gene expression was measured using per million mapped fragments (Mortazavi et al., 2008). Te differentially expressed genes in different treatment groups were screened using a digital gene expression pro file difference gene detection method (Audic and Claverie, 1997). TheP-value of each differentially expressed gene was computed. Te Benjamini–Hochberg false discovery rate (Pang et al., 2013) was applied to correctP-values. Te genes suppressed at an estimated |log2(-Fold change)|≥1 and with a false discovery rate adjustedP-value≤ 0.05 were considered to be differentially expressed (Wu et al.,2010). All differentially expressed genes were mapped to each term of the Gene Ontology database (http://www.geneontology.org/, release date: Aug 1st, 2012) and the gene numbers were calculated from each GO term. Te rigorous Bonferroni correction method was used forPvalue correction (Chen et al., 2014).When the corrected GO term had a cutoffvalue of no more than 0.05, it was de fined as a signi ficant enrichment.
Quantitative polymerase chain reaction (qPCR)
To detect the expression level of selected unigenes, quantitative real-time PCR was performed as described previously (Chen et al., 2014). A 5 μL aliquot of cDNA was blended with Super Mix(Invitrogen) and the PCR primers in a 20 μL reaction volume.Te iQ5 instrument (Bio-Rad, Hercules, CA, USA) was used to perform quantitative PCR under the following conditions: 95°C for 30 seconds, followed by 40 cycles of 95°C for 15 seconds and 60°C for 35 seconds. Relative expression was calculated by the 2–ΔΔCtmethod using β-actin as an internal control (Chen et al., 2011). Gene-speci fic primers used are listed inTable 1. Te RNA library used was generated from a separate sample from that used for RNA-seq. Three replicates were performed for each sample.
Statistical analysis
Data analysis was performed using SPSS 16.0.2 statistical soft-ware (SPSS, Chicago, IL, USA). All data are presented as the mean ± SD. Comparisons among groups were performed by one-way analysis of variance followed by Dunnett’s test. Gene matching randomness was analyzed using the Kolmogorov-Smirnov test (Lee et al., 2017). A value ofP< 0.05 was considered statistically signi ficant.
Results
Effects of MP and MTX on motor function in rats with SCI
Swing time
As shown inFigure 1A, the swing time in the MP group was significantly shorter than that of the SCI group (P< 0.05) from 2 to 8 weeks post-injury. At 8 weeks post-injury, improvement was significantly enhanced by combined MP and MTX compared with the use of MP alone (P< 0.05).
Stride time
As shown inFigure 1B, the stride time was significantly longer between SCI and sham groups after injury (P< 0.05). Te MP +MTX group achieved a significant better therapeutic effect between 4 and 8 weeks post-injury compared with the MP group (P< 0.05).
Minimum longitudinal deviation
As shown inFigure 1C, the minimum longitudinal deviation in the MP group was significantly decreased compared with the SCI group (P< 0.05). Te restorative effects in the MP + MTX group were significantly better at each time point compared with the MP group (P< 0.05).
Instant run speed
As shown inFigure 1D, the instant run speed was significantly decreased in the SCI group at each time point after injury compared with the sham group (P< 0.05). Te instant run speed in the MP group was significantly improved compared with the SCI group (P< 0.05). Te instant run speed in the MP + MTX group at 4 and 8 weeks post-injury was significantly increased compared with the MP group (P< 0.05).
Footprint area
As shown inFigure 1E, at 2–8 weeks after injury, the footprint area in the SCI group was significantly decreased compared with the sham group (P< 0.05). Te footprint area in the MP group was significantly increased compared with the SCI group at each time point (P< 0.05). Te improvements in the MP +MTX group were significantly more at 4 and 8 weeks post-injury compared with the MP group (P< 0.05).
Regularity index
As shown inFigure 1F, 2–8 weeks after injury, the regularity index in the SCI group was significantly lower than that of the sham group (P< 0.05). The regularity index was significantly improved in the MP group compared with the SCI group 2 weeks post-injury (P< 0.05). The MP + MTX group and the MP group showed similar restorative effects at 2 and 4 weeks,but the MP + MTX group was significantly more improved at 8 weeks compared with MP group (P< 0.05).
Effects of MP and MTX on histology in SCI rats
Te results of hematoxylin-eosin staining are shown inFigure 2. Eight weeks after injury, white matter and gray matter structures were complete in the sham group. Te number of neurons was decreased and the vacuole was very severe in the SCI group.In the MP + MTX and MP groups, there were various degrees of tissue loss and cyst formation, and all the damage had a tendency to spread to the adjacent area. Myelin sheath swelling and the loose structure of the white matter and the white matter bundle were improved in the MP + MTX group. Total glial cell proliferation, tissue voids, and the size of cysts at the injury center and in adjacent areas were significantly smaller in the MP +MTX group compared with those in the MP group.
Gene sequencing
Evaluation of sequencing results
Quality evaluation and RNA-Seq bioinformatic analysis showed that linker sequences were removed from the original data.Reads with a length of < 20 bp were filtered out. Sequences containing an unknown base ratio of > 4% were removed. Retention of valid data was > 70%, which was in line with requirements for subsequent analyses. Te gene matching ratio was up to > 50% (Table 2). Matching randomness analysis of all reads showed no significant differences in length distribution and uniform distribution; the randomness and uniformity were in line with the expected requirements.
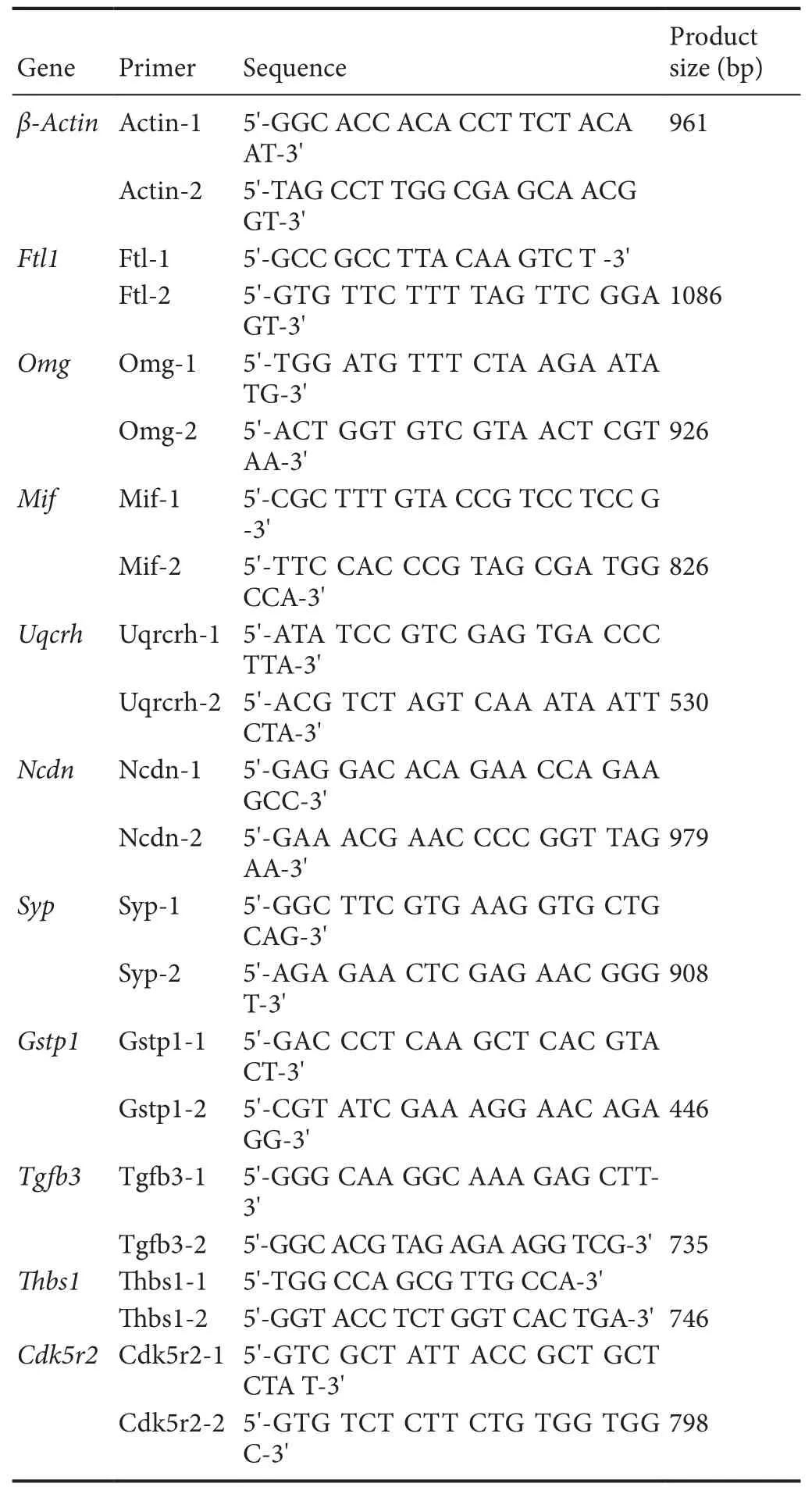
Table 1 Primer sequences used in quantitative polymerase chain reaction
Differential gene expression after SCI injury
Te difference in gene expression between two groups (SCI and sham, MP and SCI, MTX+MP and MP) showed that overall gene expression was roughly similar between groups (Figure 3).Tus, the scatters overlapped and showed a diagonal line.
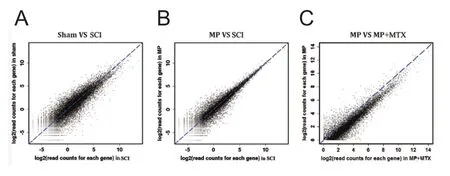
Figure 3 Scatter plots of gene expression differences between two groups after normalization.
According to GO classification, differentially expressed genes were split into three categories: biological processes, cellular components and molecular functions; each category contains a large number of subgroups. All gene GO classifications included reproduction, transcription factor activity, cell killing, immune process, catalytic activity, transport activity, metabolism, and anti-oxidation. Fifty-four regulatory gene subclasses were related to life activities.
There were 443 differentially expressed genes between the SCI and sham groups (269 genes were up-regulated and 174 genes were down-regulated). GO enrichment analysis showed that the upregulated genes in the SCI group were mainly involved in wound repair, ionic equilibrium, stress response, organ development, biosynthesis, signal transduction, inflamma-tion, oxidation and cell apoptosis, suggesting that pathological manifestations after injury might be related to the regulation and control of these genes. Among them,Dcn[Q01129, log2(-Fold change) = 2.44,P= 0.0074] can regulate and control neurons and glial cells in the central nervous system to produce decorin (Jungmann et al., 2012). Decorin has anti-fibrosis and anti-inflammation effects (Esmaeili et al., 2014), which are involved in the wound repair process. The upregulation of Dcn expression might be associated with activation of the wound repair mechanism. TeFtl1[P29391, log2(Fold change)= 2.944,P= 0.00151],Ftl2[P49945, log2(Fold change) = 2.678,P= 1.091E-81] andTcirg1genes [Q13488, log2(Fold change) =2.278,P= 0.00481] are responsible for regulating the intracellular and extracellular ion balance (Capecci and Forgac, 2013;Tao et al., 2014). Te upregulation of this kind of gene might reflect the activation of the ion balance regulatory pathway in response to ion imbalance.
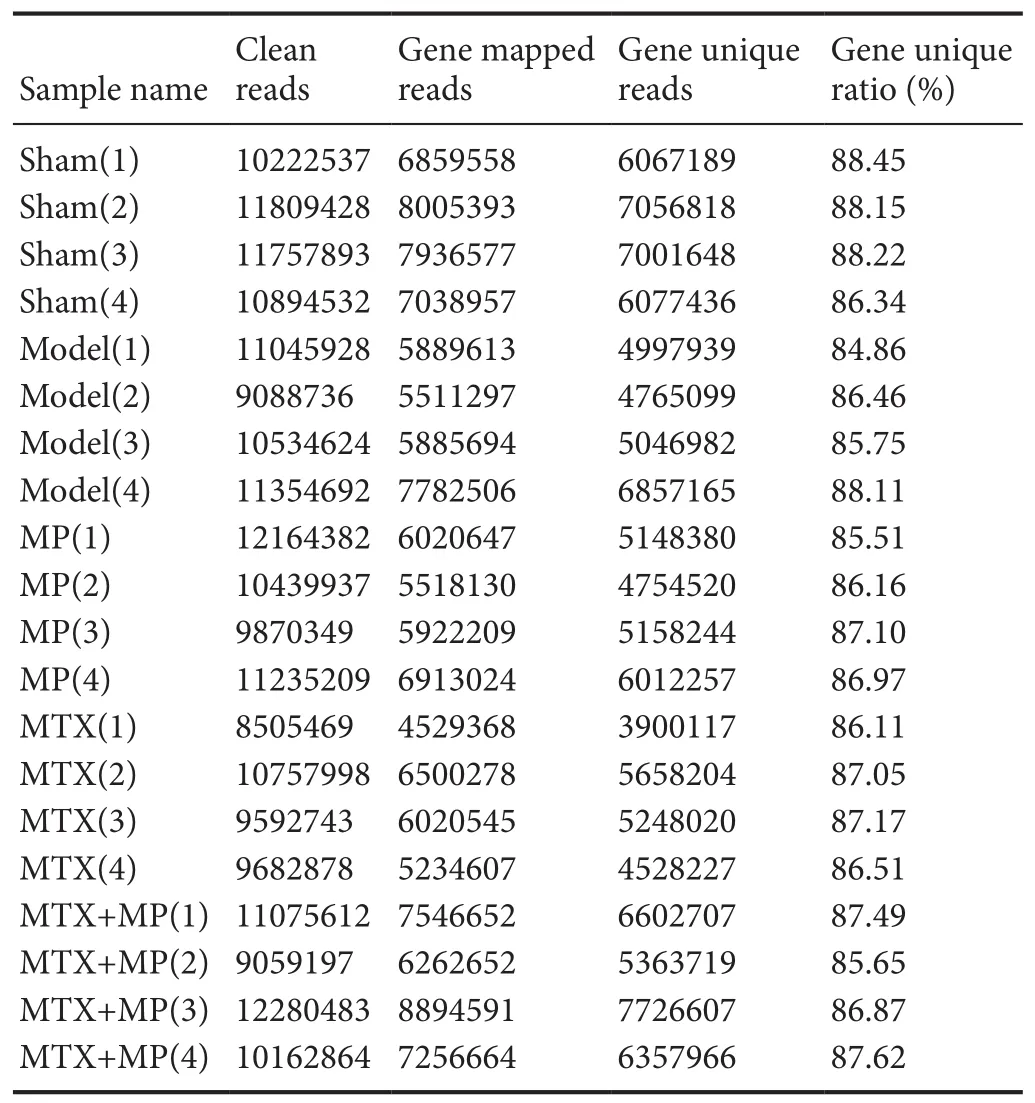
Table 2 Te matching of each sample sequence with the gene and the unique matching statistics
Compared with the sham group, genes related to regulation of neuronal regeneration were down-regulated in the SCI group (Table 3), which corresponded to neurophysiological dysfunction after SCI, and might be the most direct reason for sense and motor function-related disorders. Genes with greater differential expression includedHsp90aa1, encoding heat shock protein 90, [P82995, log2(Fold change) = −4.21,P= 2.21E-05],which corrects the misfolding of synuclein and regulates the neuroprotective effect in neurodegenerative diseases (Sundaramurthi et al., 2012). The gene encoding cyclin-dependent kinase,Cdk5r2, [Q13319, log2(Fold change) = −3.31,P= 2.55E-09] plays a crucial role in the oligodendrocyte differentiation and myelination repair process (Bankston et al., 2013). Te gene encoding contactin-2,Cntn2, [P22063, log2(Fold change) =−3.38,P= 0.0116] is a member of the neural recognition family.It participates in the formation and maintenance of the nervous system (Mohebiany et al., 2014). TeOmggene [Q63912, log2(−Fold change) = −2.85,P= 2.89E–12] can regulate neurons in the central nervous system and oligodendrocytes to express the myelin protein. Myelin is strongly associated with development and maturation of the nervous system (Vourc’h and Andres, 2004). In addition, genes down-regulated in the SCI group compared with the sham group involved biological oxidation, substance and energy metabolism, organ development, ion transportation, and signal transduction, indicating that the down-regulation of these genes might be related to the pathological process after injury.

Table 3 Examples of down-regulated GO items of differentially expressed genes associated with regeneration of neurons in spinal cord injury and sham groups
Effect of drug therapy on differential gene expression
(1) Differential gene expression between MP and SCI groups
There were 52 differentially expressed genes (33 up-regulated and 18 down-regulated) between MP and SCI groups. GO enrichment analysis showed that the upregulated genes associated with SCI injury and repair mainly reflected nervous system activities, inflammatory processes, biological oxidation, and apoptosis (Table 4).
Te up-regulated genes associated with nervous system activities were involved with positive regulation of axonal regeneration, synaptic transportation, and oligodendrocyte generation,which directly reflected the promoting role of MP in nerve regeneration after SCI. Among them,Gstp1[P04906, log2(Fold change) = 2.11,P= 0.00285], encodes glutathione-S-transferase, an isoenzyme that marks oligodendrocyte maturation in mammals (Tamura et al., 2007). TeMifgene [P34884, log2(Fold change) = 2.02,P= 0.0163)] encodes macrophage migration inhibition factor, a pluripotent cytokine that participates in inflammation and immune responses, and can promote the regeneration of peripheral nerves and inhibit Schwann cell apoptosis(Nishio et al., 2002).
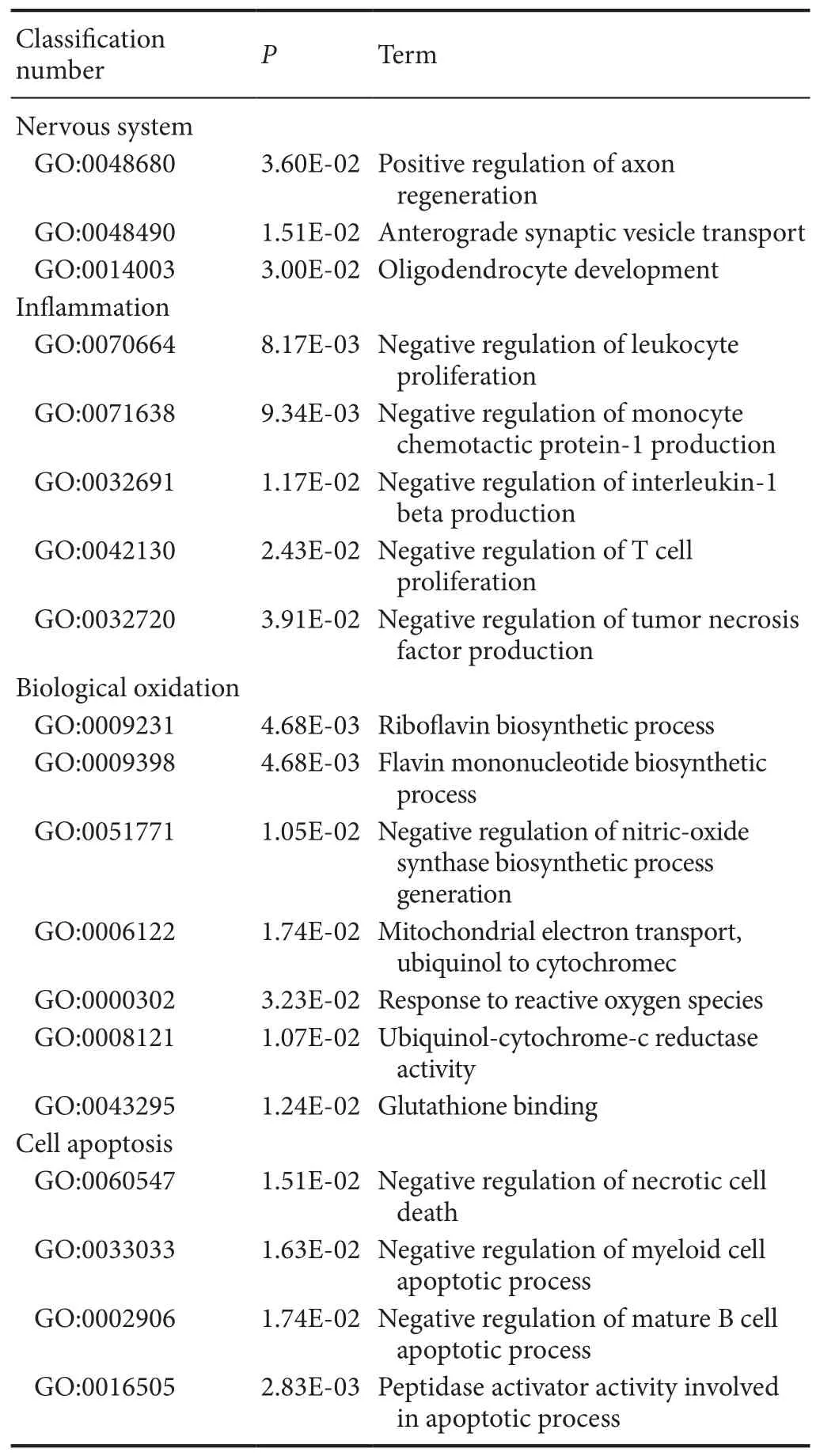
Table 4 Examples of up-regulated GO items of differentially expressed genes associated with secondary damage mechanisms in spinal cord injury and methylprednisolone groups
Among the upregulated genes were multiple GO items associated with the inflammatory reaction. Among them, the factors that directly reflect the anti-inflammatory effect of MP were negative regulation of leukocyte proliferation, generation of monocyte chemoattractant protein (MCP-1), interleukin (IL)-1β, and tumor necrosis factor (TNF), and T cell proliferation.Te anti-inflammatory effect might be an important mechanism of MP for promoting nerve function restoration after SCI. TeRT1-Bagene [P20037, log2(Fold change) = 3.24,P= 0.00186],encoding class II tissue antigen, participates in the genetic control and regulation of the immune response, and can decrease inflammation after injury by inhibiting the proliferation of T cells (Havik et al., 2007). TeMifgene, encoding macrophage migration inhibitory factor, can also inhibit the inflammatory reaction by limiting the excessive phagocytosis of macrophages that occurs in the pathological process (Muller et al., 2014).
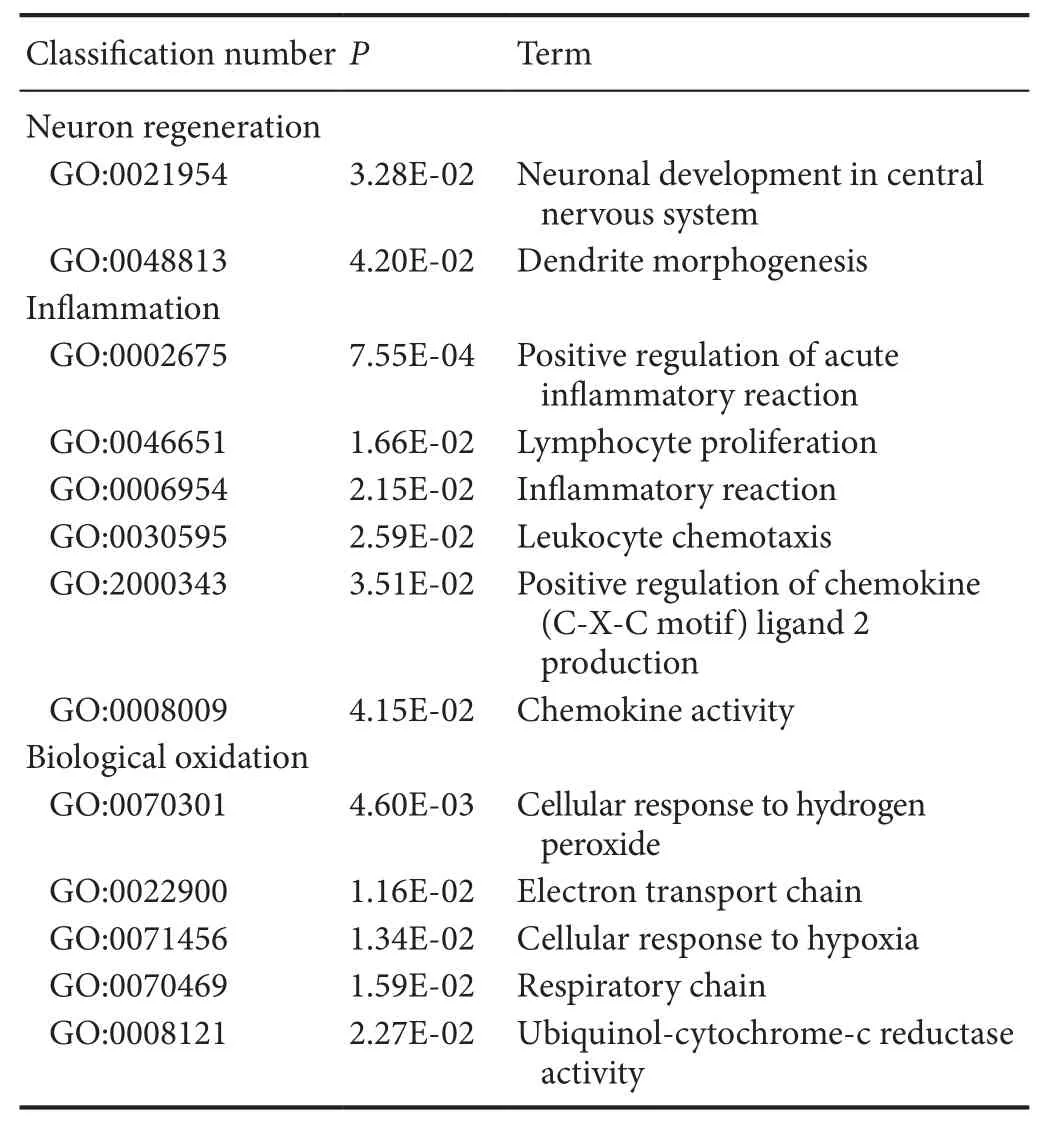
Table 5 Examples of down-regulated GO items of differentially expressed genes associated with secondary damage mechanisms in spinal cord injury and methylprednisolone groups
In addition, genes associated with oxidation process and cell apoptosis were up-regulated. GO enrichment analysis showed that genes re flecting inhibition of oxidative stress by MP were mainly involved in positively regulating the biosynthesis of inducible nitric oxide synthase (iNOS) and negatively regulating the binding activities of ribo flavin and glutathione. We speculated that MP could regulate oxidation in damaged tissue and play a role in the inhibition of oxidative stress. TheRfkgene[8CFV9, log2(Fold change) = 2.54,P= 0.00695], encoding riboflavin kinase that catalyzes ribo flavin to produce a coenzyme of antioxidase, regulates the biological oxidation process to ensure normal metabolism (Schramm et al., 2014).Uqcrh[P99028,log2(Fold change) = 3.34,P= 0.00742] is a target gene of peroxisome proliferator activated receptor-1α (PGC-1α) in normal mitochondrial metabolism. Te expression ofUqcrhwas signi ficantly down-regulated by oxidative stress and other pathological states (Zaza et al., 2013). Te regulation of apoptosis by MP was re flected in the negative regulation of myeloid cell apoptosis and necrosis.
Compared with the SCI group, the genes down-regulated in the MP group were associated with neuronal differentiation and regeneration, inflammatory reaction and biological oxidation(Table 5). Te genes participating in the regulation of neuronal differentiation and regeneration were involved in neural genesis and dendritic morphogenesis. Te down-regulation of this kind of gene might re flect MP therapy having inhibitory effects on nerve regeneration; however, the ultimate effect on the restoration of neural function is the sum of positive and negative effects. Te down-regulated genes associated with in flammatoryreaction mainly re flected the activation of chemotactic factors.Pf4[P06765, log2(Fold change) = −2.31,P= 2.93E-05] is an important marker gene of the inflammatory reaction (Liu et al., 2014). It encodes platelet factor-4, which can increase the chemotaxis of immune cells, and promote their migration to inflammatory sites, so as to aggravate the in flammatory reaction.Te down-regulation of this kind of gene further illustrated that MP had an anti-inflammatory effect. Compared with the SCI group, there were associated oxidation processes highlighted in the MP group, but they did not directly affect oxidative stress.
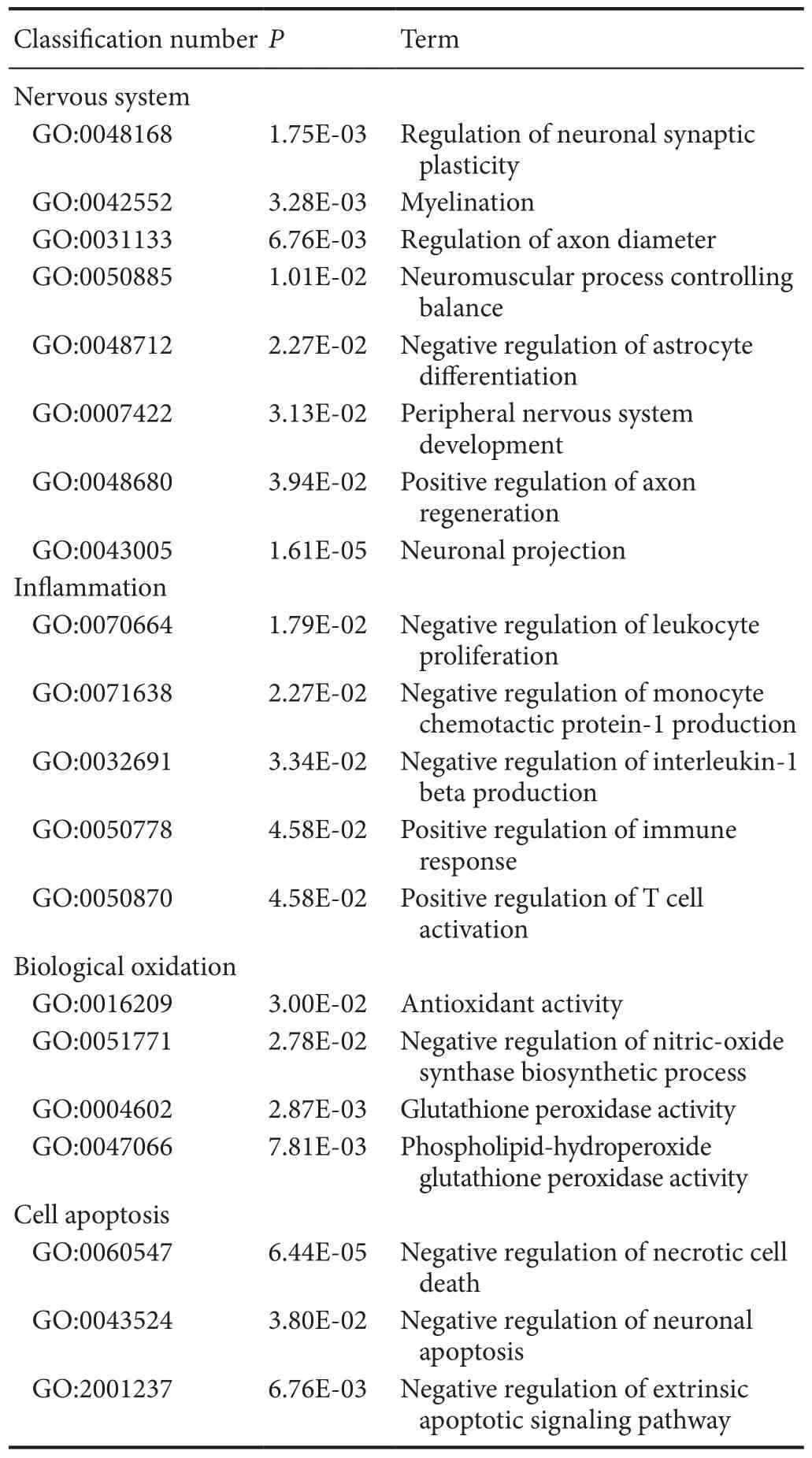
Table 6 Examples of up-regulated GO items of differentially expressed genes associated with secondary damage mechanisms in methylprednisolone and methylprednisolone + methotrexate groups
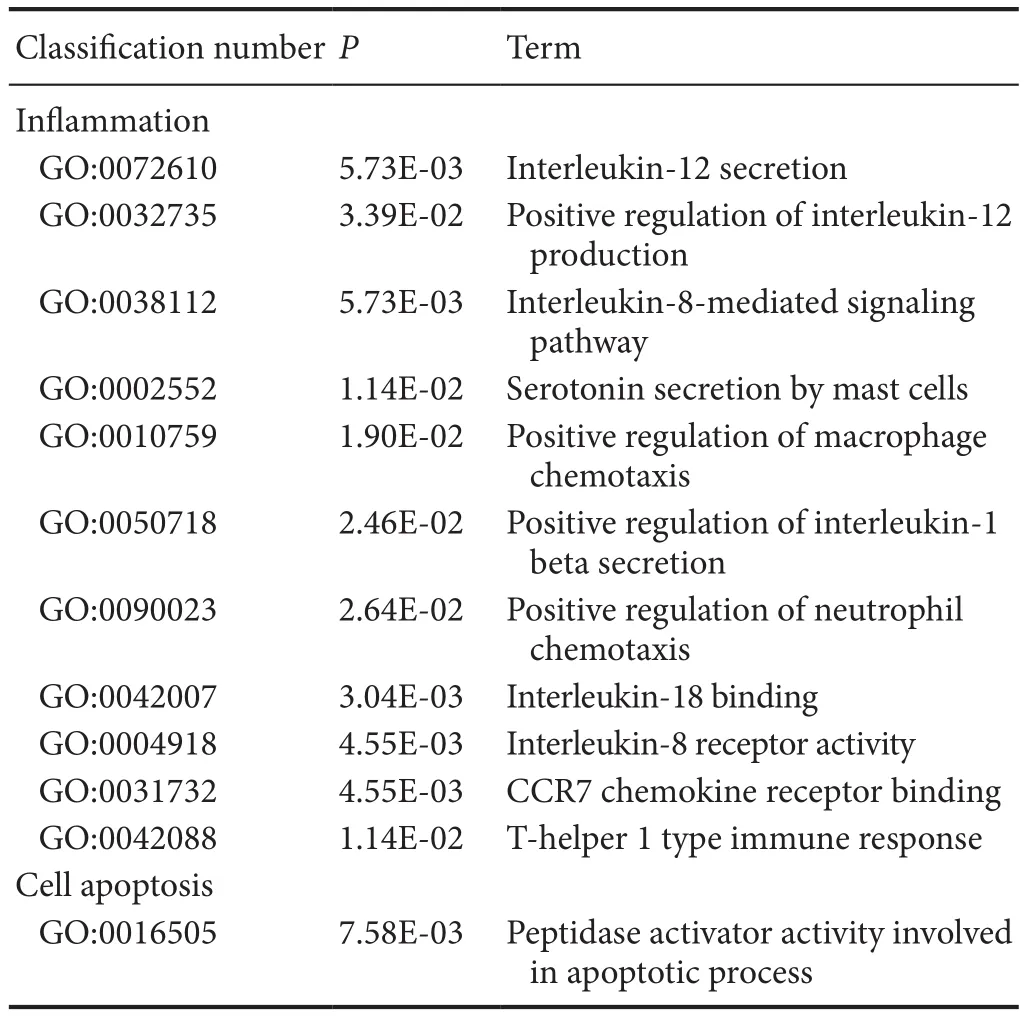
Table 7 Examples of down-regulated GO items of differentially expressed genes associated with secondary damage mechanisms in methylprednisolone and methylprednisolone + methotrexate groups

Figure 4 qPCR analysis of 10 genes shown to be differentially expressed by Solexa sequencing.
(2) Analysis of differential gene expression between MP +MTX and MP groups
There were 316 genes that were differentially expressed between MP + MTX and MP groups (275 genes were up-regulated and 41 were down-regulated). GO enrichment analysis showed that there were a large number of associated items that promote nerve restoration among the up-regulated genes that also involve anti-inflammation, anti-oxidation and anti-apoptosis(Table 6). The down-regulated genes were mainly involved in pro-inflammatory and pro-apoptotic processes (Table 7).
Compared with the MP group, the up-regulation of genes that promote neural restoration in the MP + MTX group was related to the regulation of neuronal synaptic plasticity, myelinformation, control of neuromuscular balance, and regeneration of neurons and axons. TeNdcngene [O35095, log2(Fold change) = 2.47,P= 0.0161], encoding neural cartilage protein,and theSypgene [P07825, log2(Fold change) = 2.72,P= 0.0143],encoding synaptic vesicle protein, play neurotrophic effects on promoting axonal growth and modulating synaptic plasticity in the central nervous system (Oku et al., 2013; Park et al., 2015).TeMalgene [Q64349, log2(Fold change) = 2.39,P= 0.00377],encoding myelin and lymphocyte protein, is mainly produced by oligodendrocytes and Schwann cells. It regulates the peripheral nervous system to produce myelin. It might promote myelin wrapping by regulating the formation of membrane components (Buser et al., 2009).
Among the up-regulated genes, the anti-inflammatory GO genes mainly negatively regulate the proliferation of leukocytes,and the generation of IL-1β and chemokines. Among them, theMifgene [P34884, log2(Fold change) = 2.84,P= 0.014] is involved in inhibiting excessive phagocytosis of macrophages. TeGstp1gene [P04906, log2(Fold change) = 2.70,P= 0.000151],encoding glutathione-S-transferase, is also an important marker of the inflammatory reaction (Iorio et al., 2015).
Among the up-regulated genes, the antioxidant genes were mainly related to the activation of antioxidase activity and inhibition of peroxidase activity.Prdx1[P0CB50, log2(Fold change)= 2.60,P= 0.0484], encoding peroxide reductase-1, is an important antioxidase that scavenges for free radicals and protects against oxidative damage.Gpx4[O70325, log2(Fold change) =2.40,P= 0.00914], encoding glutathione peroxidase, is also a major endogenous antioxidant enzyme in animals. It catalyzes the disproportionation reaction of superoxide anions (Storey,1996).Gstp1[P04906, log2(Fold change) = 2.79,P= 0.00468]was an up-regulated gene. Its expression is inversely proportional to that of iNOS in the pathological process (Raposo et al.,2013).
Among the up-regulated genes, the anti-apoptosis GO genes were associated with the negative regulation of apoptosis and apoptosis signaling pathways. Among them,Tgfb3[Q07258,log2(Fold change) = 2.57,P= 0.0114] is important for cell morphology genesis, differentiation and tissue reconstruction. It can enhance the protective effects of various neurotrophic factors and is an important cytokine for neuronal survival (Krieglstein et al., 2002).
Compared with the MP group, proinflammatory genes were down-regulated in the MTX + MP group. These were mainly related to interleukin secretion, mast cells, macrophages and neutrophils, and inflammatory factor chemotaxis. Among them,Cxcr1[P70612, log2(Fold change) = −2.99,P= 0.0447],Il18bp[Q9Z0M9, log2(Fold change) = −2.01,P= 5.49E-05]andCcl19[O70460, log2(Fold change) = −2.63,P= 0.00230]can regulate secretion of IL-8, IL-18 and IL-19 and binding with their respective receptors. Tese interleukins can promote the chemotaxis of macrophages in pathological processes and induce inflammation (Galindo et al., 2009; Bishayi et al., 2015;Xuan et al., 2015).Tbs1[P35441, log2(Fold change) = −2.99,P= 0.00771] can promote macrophage chemotaxis and its expression was confirmed to be increased in inflammation-associated pathological processes (De Luna et al., 2010). In addition,pro-oxidant and pro-apoptotic genes also appeared among the down-regulated genes.Gapdh[P16858, log2(Fold change) =−2.57,P= 0.0146] encodes glyceraldehyde-3-phosphate dehydrogenase, an important enzyme in glycolysis and aerobic metabolism. However, a recent study found its expression was strongly associated with the apoptosis of neurons in neurodegenerative diseases (El Kadmiri et al., 2014).
Quantitative PCR results
Ten genes shown to be significantly differentially expressed by Solexa sequencing were randomly selected and their relative mRNA expression determined. Te results were consistent with the results of Solexa sequencing (Figure 4). Tese results indicated that the Solexa sequencing data reflected the real situation of gene expression in each experimental group;i.e., the information obtained by gene expression profile sequencing was of high reliability.
Discussion
The present study showed that the combination of MP with MTX significantly improved damaged motor function caused by SCI in rats. Both compounds and MP combined with other drugs have been widely studied; however, to our knowledge,this is the first study to show motor function recovery in rats after traumatic SCI by a combination of MP and MTX at the level of the transcriptome. Our study illustrates that the restorative effects of this combination therapy may be through the anti-inflammatory reaction, inhibition of oxidative stress and apoptosis and other common gene regulation pathways.
Gait analysis
Treadscan gait analysis can provide an all-around gait analysis for the assessment of motor function in SCI rats (Beare et al., 2009; Myers et al., 2012; Schira et al., 2012). In our study,the effects of MP and MTX, alone and in combination, were compared by Treadscan gait analysis. Measures included hind limb swing time, stride time, minimum longitudinal deviation,instant run speed, hindlimb footprint area and regularity index.Forgione showed that footprint area was significantly decreased in incomplete spinal cord contusion model rats (Forgione et al.,2014). Te results of this study also showed that SCI significantly reduced the area of the posterior footprint, and the treatment of MP combined with MTX significantly improved the posterior surface area after SCI; the regularity index was decreased in the SCI group compared with sham group, which was consistent with a previous study (Chiang et al., 2014).
Gene sequencing
The possible mechanism of different combinations of drugs that promote recovery of motor function in rats was explored through expression profile sequencing analysis. Here, differential gene expression analysis in SCI and sham groups showed that neural development- and function-associated genes were down-regulated after SCI, directly reflecting the damage to nerve structure and function. Moreover, genes associated with energy metabolism, transcription, catalysis, ion exchange, and signal transduction were differentially expressed. Tese results,at the genetic level, are consistent with a previous study (Tang et al., 2014).
MP treatment
In this study, gene expression profile analysis after MP treat-ment showed that the expression of genes associated with axon regeneration, synaptic transportation, and oligodendrocyte genesis were up-regulated, confirming that MP had an effect on nerve function recovery after injury. In addition, the expression of genes regulating neurogenesis and dendritic morphogenesis in the central nervous system were down-regulated, indicating that MP treatment had certain side effects on neurological recovery. Tis may contribute to the reason why severe SCI fails to be completely cured by MP. However, the specific mechanism of action remains to be further examined.
The inflammatory reaction is an important mechanism of secondary SCI. A large number of experimental results show that high-dose MP treatment in the acute stage can decrease the infiltration of inflammatory cells, and effectively control the inflammatory reaction (Alibai et al., 2010; Zhang et al., 2014).Our gene expression profile analysis showed that MP treatment can up-regulate the expression of anti-inflammatory genes and down-regulate the expression of genes promoting the inflammatory reaction, which demonstrated the anti-inflammatory effect of MP. These results explained the anti-inflammatory effects of MP in three ways: (1) negative regulation of immune cell activation and immune response: genes that negatively regulate leukocyte and T cell proliferation were up-regulated. Genes positively regulating the inflammatory and immune responses were down-regulated. (2) Negative regulation of the pro-inflammatory effect of chemokines: The negatively regulating Mcp-1 was up-regulated and genes activating chemokine activity were down-regulated (Hassanshahi et al., 2013; White et al., 2009).(3) Negative regulation of inflammatory factor expression: The genes negatively controlling IL-1β and TNF generation were up-regulated. IL-1β and TNF were mainly produced by activated monocytes/macrophages, and could promote neutrophil phagocytosis, cause an inflammatory reaction, participate in fever,blood coagulation and other pathological processes (Linton and Toman, 2014). Gene expression analysis, therefore, indicated a trend against the inflammatory reaction after MP treatment.
Oxidative stress plays an important role in secondary spinal cord injury. Boyaci et al. (2015) reported that MP treatment could effectively reduce oxidative stress products after SCI and improve the level of antioxidants. In our study, many oxidation-related genes were differentially expressed after MP treatment. Te genes directly reflecting oxidative stress inhibition by MP, such as iNOS (negative regulation of NO biosynthesis), and positive regulation of glutathione binding activity were up-regulated. iNOS overexpression can catalyze the generation of peroxynitrite after injury (Maggio et al., 2012). Glutathione itself is an important antioxidant (Horiyama et al., 2014) and endogenous glutathione can be consumed in a few hours or even a few minutes after injury (Lucas et al., 2002).
Apoptosis of neurons is an important factor in irreversible damage and secondary injury. Neuronal apoptosis can not only lead to local nerve degeneration, but can also cause stop nutrition of distal nerve fibers, leading to necrosis. Wang et al. (2013) reported that intrathecal injection and intravenous injection of MP could effectively inhibit the apoptosis of nerve cells and promote the recovery of motion function in the hind limbs of SCI rabbit models. Te results of this study showed that the anti-apoptotic effect of MP was the up-regulation of genes that negatively regulate necrosis and myeloid cell apoptosis-associated genes.
MP + MTX combined therapy
MTX is applied in clinical practice as an anti-inflammatory and antitumor drug. Previous studies have shown that lowdose MTX has a protective effect on nerves after SCI (Lee et al.,2008; Sanli et al., 2012; Bakar et al., 2012). The present study also found that MTX might play a neuroprotective role in SCI repair.
The results showed that MTX could reduce the levels of myeloperoxidase and malondialdehyde in injured tissue, suggesting that MTX has a dual function of anti-in flammation and inhibition of oxidative stress. We also demonstrated that MTX plays an important role in protecting axon and myelin integrity,and inhibiting the ultrastructural changes of injured spinal cord.Long-term application of MTX could increase uric acid levels in blood and urine (Chen et al., 2012; Aoyama et al., 2011). Uric acid has a neuroprotective effect, suggesting that MTX inhibited the development of secondary injury and protected nerve tissue from SCI.
Combined therapy of two or more drugs has become a hot topic in clinical research. Many studies have adopted other drugs in combination with MP (Luo et al., 2013; Yin et al., 2013; Cavus et al., 2014; Li et al., 2016), which achieved better effects. From our results, MP combined with MTX improved treatment of SCI in several aspects compared with MP alone. First, the expression of many nerve restoration promoting genes was up-regulated,such as genes regulating neuronal synaptic plasticity, myelinogenesis, control of neuromuscular balance, and regeneration of neurons and axons, indicating that combined therapy with two drugs showed signi ficant advantages in the treatment of SCI in protecting the integrity of neuronal structures and in promoting neurons to rebuild functional connectivity compared with MP treatment alone. Second, there were two anti-in flammatory advantages: (1) negative regulation of the proliferation and binding activity of immune cells. Genes negatively regulating leukocyte proliferation were up-regulated, while genes positively regulating mast cell secretion, and macrophage and neutrophil chemotaxis were down-regulated. (2) Negative regulation of the production and activity of pro-inflammatory factors. The genes negatively regulating IL-1β and chemotactic factor production were up-regulated. However, genes that positively regulate positively regulating interleukins (IL-8, IL-18, and IL-19), their receptors, and chemotactic factor activities were down-regulated. Tese in flammatory factors can promote neutrophil migration to the site of injury and release a series of active products, resulting in local inflammation (Kwon et al., 2011; Khalatbary and Zarrinjoei, 2012).Tird, the inhibition of oxidative stress reactions has advantages.Differential gene expression analysis showed that the combined administration could positively regulate the activity of antioxidant enzymes (glutathione peroxidase and peroxiredoxin 1) and negatively regulate the activity of iNOS. Endogenous antioxidase is rapidly depleted after SCI. Therefore, the up-regulation of genes regulating antioxidase activity could replenish the antioxidase and inhibit peroxidation. In addition, genes regulating iNOS activity were down-regulated, also suggesting that the combined therapy had a better inhibitory effect on oxidative stress. Nitric oxide is produced from L-arginine by iNOS, and can react with hydroxyl free radicals to produce highly toxic free radicals (Yadav et al., 2014). Studies have shown that high-levels of nitric oxide can also promote cell apoptosis. Finally, the inhibition of nervecell apoptosis reflected the negative regulation of genes promoting neuronal apoptosis enzymes.
In summary, the effect of MP and MTX combined therapy on the recovery of SCI was verified by gait analysis. Te possible inherent mechanism of this treatment was inferred from gene expression profiling. Tis study selected the anti-inflammatory drug, MTX, with a flexible administration time, in combination with the traditional SCI treatment drug, MP. Te results showed that MP and MTX combination therapy could improve the deficiency of MP treatment alone, enhance the neuroprotective effect, inhibit the activities of inflammatory cytokines, strengthen the anti-oxidative and anti-apoptotic effects and increase the clinical therapeutic effect, which could provide new strategies for the control of SCI. However, indepth research into the mechanism of these therapeutic effects is required owing to the complexity and variety of the differentially expressed genes.
Author contributions:BG designed this study. JTL and SZ performed experiments. HNL, SYZ and SYW analyzed data. JTL and BG wrote the paper. All authors approved the ?nal version of the paper.
Conflicts of interest:None declared.
Research ethics:Te study protocol was approved by the Animal Ethics Committee of Jiangxi University (approval number A20150107). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Data sharing statement:Te datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iTenticate.
Peer review:Externally peer reviewed.
Open access statement:Tis is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Alibai E, Zand F, Rahimi A (2010) Erythropoietin plus methylprednisolone or methylprednisolone in the treatment of acute spinal cord injury: a preliminary report. Crit Care Med 38:U228-228.
Aoyama K, Matsumura N, Watabe M, Wang F, Kikuchi-Utsumi K, Nakaki T (2011) Caffeine and uric acid mediate glutathione synthesis for neuroprotection. Neuroscience 181:206-215.
Audic, S., Claverie, J.M (1997) Te significance of digital gene expression profiles. Genome research 10:986-995.
Bakar B, Kose EA, Kupana Ayva S, Sarkarati B, Kasimcan MO, Kilinc K(2013) Effects of low-dose methotrexate in spinal cord injury in rats.Ulus Travma Acil Cerrahi Derg 19:285-293.
Bankston AN, Li WQ, Zhang H, Ku L, Liu GL, Papa F, Zhao LX, Bibb JA, Cambi F, Tiwari-WoodruffSK, Feng Y (2013) p39, the primary activator for cyclin-dependent kinase 5 (Cdk5) in oligodendroglia, is essential for oligodendroglia differentiation and myelin repair. J Biol Chem 288:18047-18057.
Bishayi B, Bandyopadhyay D, Majhi A, Adhikary R (2015) Expression of CXCR1 (interleukin-8 receptor) in murine macrophages after staphylococcus aureus infection and its possible implication on intracellular survival correlating with cytokines and bacterial anti-oxidant enzymes.Inflammation 38:812-827.
Boyaci MG, Eser O, Kocogullari CU, Karavelioglu E, Tokyol C, Can Y(2015) Neuroprotective effect of alpha-lipoic acid and methylprednisolone on the spinal cord ischemia/reperfusion injury in rabbits. Br J Neurosurg 29:46-51.
Bracken MB (2012) Steroids for acute spinal cord injury. Cochrane Database Syst Rev 1:CD001046.
Buser AM, Schmid D, Kern F, Erne B, Lazzati T, Schaeren-Wiemers N(2009) Te myelin protein MAL affects peripheral nerve myelination:a new player influencing p75 neurotrophin receptor expression. Eur J Neurosci 29:2276-2290.
Capecci J, Forgac M (2013) Te function of vacuolar ATPase (V-ATPase)a subunit isoforms in invasiveness of MCF10a and MCF10CA1a Human breast cancer cells. J Biol Chem 288:32731-32741.
Cavus G, Altas M, Aras M, Ozgur T, Serarslan Y, Yilmaz N, Sefil F, Ulutas KT (2014) Effects of montelukast and methylprednisolone on experimental spinal cord injury in rats. Eur Rev Med Pharmacol Sci 18:1770-1777.
Chen JH, Xia XL, Yin WL (2011) A poplar DRE-binding protein gene,PeDREB2L, is involved in regulation of defense response against abiotic stress. Gene 483:36-42.
Chen JH, Tian QQ, Pang T, Jiang LB, Wu RL, Xia XL, Yin WL (2014)Deep-sequencing transcriptome analysis of low temperature perception in a desert tree, Populus euphratica. BMC Genomics 15:326.
Chen Z, Tu S, Hu Y, Wang Y, Xia Y, Jiang Y (2012) Prediction of response of collagen-induced arthritis rats to methotrexate: an (1)H-NMR-based urine metabolomic analysis. J Huazhong Univ Sci Technolog Med Sci 32:438-443.
Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M: Blast-2GO (2005) A universal tool for annotation, visualization and analysis in functional genomics research. Bioinfo 18:3674-3676.
De Luna N, Gallardo E, Sonnet C, Chazaud B, Dominguez-Perles R, Suarez-Calvet X, Gherardi RK, Illa I (2010) Role of thrombospondin 1 in macrophage inflammation in dysferlin myopathy. J Neuropath Exp Neurol 69:643-653.
El Kadmiri N, Slassi I, El Moutawakil B, NadifiS, Tadevosyan A, Hachem A, Soukri A (2014) Glyceraldehyde-3-phosphate dehydrogenase(GAPDH) and Alzheimer’s disease. Pathol Biol 62:333-336.
Esmaeili M, Berry M, Logan A, Ahmed Z (2014) Decorin treatment of spinal cord injury. Neural Regen Res 9:1653-1656.
Galindo RC, Munoz PM, de Miguel MJ, Marin CM, Blasco JM, Gortazar C, Kocan KM, de la Fuente J (2009) Differential expression of inflammatory and immune response genes in rams experimentally infected with a rough virulent strain of Brucella ovis. Vet Immunol Immunop 127:295-303.
Gu Bing, JIN Jian-bo, Hua-nan L (2011) Establishment of traumatic spinal cord injury model in rats. Chin J Clin Pharmacol Ter 16:7.
Hassanshahi G, Amin M, Shunmugavel A, Vazirinejad R, Vakilian A, Sanji M, Shamsizadeh A, Rafat Panah H, Poor NM, Moosavi SR, Taheri S(2013) Temporal expression profile of CXC chemokines in serum of patients with spinal cord injury. Neurochem Int 63:363-367.
Havik B, Rokke H, Dagyte G, Stavrum AK, Bramham CR, Steen VM(2007) Synaptic activity-induced global gene expression patterns in the dentate gyrus of adult behaving rats: Induction of immunity-linked genes. Neuroscience 148:925-936.
Horiyama S, Takahashi Y, Hatai M, Honda C, Suwa K, Ichikawa A, Yoshikawa N, Nakamura K, Kunitomo M, Date S, Masujima T, Takayama M (2014) Methyl vinyl ketone, a toxic ingredient in cigarette smoke extract, modifies glutathione in mouse melanoma cells. Chem Pharm Bull 62:772-778.
Iorio A, Polimanti R, Piacentini S, Liumbruno GM, Manfellotto D, Fuciarelli M (2015) Deletion polymorphism of GSTT1 gene as protective marker for allergic rhinitis. Clinrespir J 9:481-486.
Jungmann O, Nikolovska K, Stock C, Schulz JN, Eckes B, Riethmuller C, Owens RT, Iozzo RV, Seidler DG (2012) Te dermatan sulfate proteoglycan decorin modulates alpha2beta1 integrin and the vimentin intermediate filament system during collagen synthesis. PLoS One 7:e50809.
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y (2008)KEGG for linking genomes to life and the environment. Nucleic Acids Res 36(Database issue):D480–D484.Keith MP, Edison JD, Gilliland WR (2012) Progress toward personalized treatment of rheumatoid arthritis. Clin Pharmaco Ter 92:440-442.
Kertmen H, Gurer B, Yilmaz ER, Sanli AM, Sorar M, Arikok AT, Sargon MF, Kanat MA, Erguder BI, Sekerci Z (2013) Te protective effect of low-dose methotrexate on ischemia-reperfusion injury of the rabbit spinal cord. Eur J Pharmacol 714:148-156.
Khalatbary AR, Zarrinjoei GR (2012) Anti-inflammatory effect of oleuropein in experimental rat spinal cord trauma. Iran Red Crescent Med J 14:229-234.
Krieglstein K, Strelau J, Schober A, Sullivan A, Unsicker K (2002)TGF-beta and the regulation of neuron survival and death. J Physiol Paris 96:25-30.
Kwon BK, Casha S, Hurlbert RJ, Yong VW (2011) Inflammatory and structural biomarkers in acute traumatic spinal cord injury. Clin Chem Lab Med 49:425-433.
Lee G, Bang L, Kim SY, Kim D, Sohn, KA (2017) Identifying subtype-speci fic associations between gene expression and DNA methylation profiles in breast cancer. BMC medical genomics 10: 28.
Lee JM, Yan P, Xiao Q, Chen S, Lee KY, Hsu CY, Xu J (2008) Methylprednisolone protects oligodendrocytes but not neurons after spinal cord injury. J Neurosci 28:3141-3149.
Li XG, Lin XJ, Du JH, Xu SZ, Lou XF, Chen Z (2016) Combination of methylprednisolone and rosiglitazone promotes recovery of neurological function after spinal cord injury. Neural Regen Res 11:1678-1684.
Linton PJ, Toman ML (2014) Immunosenescence in monocytes, macrophages, and dendritic cells: Lessons learned from the lung and heart.Immunol Lett 162:290-297.
Liu JT, Zhu L, Zhang S, Deng Z, Huang Z, Yuan M, Wu W, Yang K (2016)Te Autographa californica multiple nucleopolyhedrovirus ac110 gene encodes a new per os infectivity factor. Virus Res 221: 30-37.
Liu XQ, Yan Y, Bao L, Chen BD, Zhao YY, Qi RM (2014) Ginkgolide B inhibits platelet release by blocking Syk and p38 MAPK phosphorylation in thrombin-stimulated platelets. Tromb Res 134:1066-1073.
Lucas JH, Wheeler DG, Guan Z, Suntres Z, Stokes BT (2002) Effect of glutathione augmentation on lipid peroxidation after spinal cord injury. J Neurotraum 19:763-775.
Luo CK, Li WW, Wang XY, Wu P, Pang XY, Xu ZQ, Zeng H, Zhang PH,Peng W (2013) Effect of infliximab combined with methylprednisolone on expressions of NF-kappa B, TRADD, and FADD in rat acute spinal cord injury. Spine 38:E861-869.
Maggio DM, Chatzipanteli K, Masters N, Patel SP, Dietrich WD, Pearse DD (2012) Acute molecular perturbation of inducible nitric oxide synthase with an antisense approach enhances neuronal preservation and functional recovery after contusive spinal cord injury. J Neurotraum 29:2244-2249.
Mohebiany AN, Harroch S, Bouyain S (2014) New insights into the roles of the contactin cell adhesion molecules in neural development. Adv Neurobiol 8:165-194.
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 7:621-628.
Muller II, Chatterjee M, Schneider M, Borst O, Seizer P, Schonberger T,Vogel S, Muller KAL, Geisler T, Lang F, Langer H, Gawaz M (2014)Gremlin-1 inhibits macrophage migration inhibitory factor-dependent monocyte function and survival. Int J Cardiol 176:923-929.
Myers SA, DeVries WH, Gruenthal MJ, Andres KR, Hagg T, Whittemore SR (2012) Sildenafil improves epicenter vascular perfusion but not hindlimb functional recovery after contusive spinal cord injury in mice. J Neurotraum 29:528-538.
Nishio Y, Nishihira J, Ishibashi T, Kato H, Minami A (2002) Role of macrophage migration inhibitory factor (MIF) in peripheral nerve regeneration: anti-MIF antibody induces delay of nerve regeneration and the apoptosis of Schwann cells. Mol Med 8:509-520.
Oku S, Takahashi N, Fukata Y, Fukata M (2013) In silico screening for palmitoyl substrates reveals a role for DHHC1/3/10 (zDHHC1/3/11)-mediated neurochondrin palmitoylation in its targeting to rab5-positive endosomes. J Biol Chem 288:19816-19829.
Pang T, Ye C-Y, Xia X, Yin W (2013) De novo sequencing and transcriptome analysis of the desert shrub, Ammopiptanthus mongolicus,during cold acclimation using Illumina/Solexa. BMC Genomics 1:488.
Park SW, Lee JG, Seo MK, Cho HY, Lee CH, Lee JH, Lee BJ, Baek JH,Seol W, Kim YH (2015) Effects of mood-stabilizing drugs on dendritic outgrowth and synaptic protein levels in primary hippocampal neurons. Bipolar Disorders 17:278-290.
Pincus T, Sokka T, Cutolo M (2015) Te past versus the present, 1980-2004: reduction of mean initial low-dose, long-term glucocorticoid therapy in rheumatoid arthritis from 10.3 to 3.6 mg/day, concomitant with early methotrexate, with long-term effectiveness and safety of less than 5 mg/day. Neuroimmunomodulat 22:89-103.
Pinzon A, Marcillo A, Quintana A, Stamler S, Bunge MB, Bramlett HM,Dietrich WD (2008) A re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model. Brain Res 1243:146-151.
Raposo C, Nunes AKD, Luna RLD, Araujo SMD, da Cruz-Ho fling MA,Peixoto CA (2013) Sildenafil (viagra) protective effects on neuroinflammation: the role of inos/no system in an in flammatory demyelination model. Mediat In flamm 19:321460.
Ray SK, Samntaray S, Banik NL (2016) Future directions for using estrogen receptor agonists in the treatment of acute and chronic spinal cord injury. Neural Regen Res 11:1418-1419.
Saeki Y, Matsui T, Saisho K, Tohma S (2012) Current treatments of rheumatoid arthritis: from the ‘NinJa’ registry. Exp Rev Clin Immunol 8:455-465.
Sanli AM, Serbes G, Sargon MF, Caliskan M, Kilinc K, Bulut H, Sekerci Z(2012) Methothrexate attenuates early neutrophil infiltration and the associated lipid peroxidation in the injured spinal cord but does not induce neurotoxicity in the uninjured spinal cord in rats. Acta Neurochir 154:1045-1054.
Schramm M, Wiegmann K, Schramm S, Gluschko A, Herb M, Utermohlen O, Kronke M (2014) Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur J Immunol 44:728-741.
Storey KB (1996) Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 29:1715-1733.
Sundaramurthi H, Manavalan A, Ramachandran U, Hu JM, Sze SK,Heese K (2012) Phenotyping of tianma-stimulated differentiated rat neuronal b104 cells by quantitative proteomics. Neurosignals 20:48-60.
Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H (2007)Intracellular translocation of glutathione S-transferase pi during oligodendrocyte differentiation in adult rat cerebral cortex in vivo. Neuroscience 148:535-540.
Tang Y, Ling ZM, Fu R, Li YQ, Cheng X, Song FH, Luo HX, Zhou LH(2014) Time-specific microRNA changes during spinal motoneuron degeneration in adult rats following unilateral brachial plexus root avulsion: ipsilateral vs. contralateral changes. BMC Neurosci 15:92.
Tao YL, Wu Q, Guo X, Zhang ZZ, Shen YY, Wang FD (2014) MBD5 regulates iron metabolism via methylation-independent genomic targeting of Fth1 through KAT2A in mice. Brit J Hamatol 166:279-291.
Tator CH FM (1991) Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 32:107-115.
Vourc’h P, Andres C (2004) Oligodendrocyte myelin glycoprotein(OMgp): evolution, structure and function. Brain Res Rev 45:115-124.
Wang KF, Liu HY, B W (2013) Effects of intrathecal injection of methylprednisolone sodium succinate in acute spinal cord injury rabbits.Zhonghua Wai Ke Za Zhi 51:5.
Wang L, Feng Z, Wang X(2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics, 26:136-138.
White FA, Feldman P, Miller RJ (2009) Chemokine Signaling and the Management of Neuropathic pain. Mol Interv 9:188-195.
Wu J, Zhang Y, Zhang H, Huang H, Folta KM, Lu J (2010) Whole genome wide expression profiles of Vitis amurensis grape responding to downy mildew by using Solexa sequencing technology. BMC Plant Biol 10:234.
Xuan WJ, Qu Q, Zheng BA, Xiong SD, Fan GH (2015) Te chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukocyte Biol 97:61-69.
Yadav R, Goldstein S, Nasef MO, Lee W, Samuni U (2014) Synergistic activity of acetohydroxamic acid on prokaryotes under oxidative stress:the role of reactive nitrogen species. Free Radical Bio Med 77:291-297.
Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L (2006) WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34:W293-297.
Yin Y, Sun WF, Li ZW, Zhang B, Cui H, Deng LX, Xie P, Xiang J, Zou J (2013) Effects of combining methylprednisolone with rolipram on functional recovery in adult rats following spinal cord injury. Neurochem Int 62:903-912.
Yu YQ, Hu NC, Duan JA, Li DP, Liu C (2016) Neuroprotective effects of sufentanil preconditioning on spinal cord injury in mouse models.Zhongguo Zuzhi Gongcheng Yanjiu 20:5966-5972.
Zaza G, Granata S, Masola V, Rugiu C, Fantin F, Gesualdo L, Schena FP,Lupo A (2013) Downregulation of nuclear-encoded genes of oxidative metabolism in dialyzed chronic kidney disease patients. PLoS One 8:e77847.
Zhang Si, Gu B, Wang SY (2012) Application of treadscan gait analysis system in the evaluation of rat spinal cord contusion model. Acta Neuropharmacologica 2:10.
Zhang YL, Zhang LH, Shen J, Chen C, Mao Z, Li W, Gan WB, Tang PF(2014) Two-photon-excited fluorescence microscopy as a tool to investigate the ef ficacy of methylprednisolone in a mouse spinal cord injury model. Spine 39:E493-499.
Zhang ZC, Li F, Sun TS (2013) An expert consensus on the evaluation and treatment of acute thoracolumbar spine and spinal cord injury in China. Neural Regen Res 8:3077-3086.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
How to cite this article: Liu JT, Zhang S, Gu B, Li HN, Wang SY, Zhang SY (2017) Methotrexate combined with methylprednisolone for the recovery of motor function and differential gene expression in rats with spinal cord injury. Neural Regen Res 12(9):1507-1518.
Funding: Tis research was supported by the National Natural Science Foundation of China, No. 30960448; the Natural Science Foundation of Jiangxi Province, No. 20142BAB205023; the Ph.D. Start-up Fund of Natural Science Foundation of Jiangxi Science & Technology Normal University in China, No. 3000990122.
*Correspondence to:Bing Gu, Ph.D.,bguemory@hotmail.com.
orcid:0000-0001-7900-6605(Bing Gu)
10.4103/1673-5374.215263
Accepted: 2017-08-12
- 中国神经再生研究(英文版)的其它文章
- Tp53 gene mediates distinct dopaminergic neuronal damage in different dopaminergic neurotoxicant models
- Unusual neural tract between injured fornix and pedunculopontine nucleus in a patient with traumatic brain injury
- Promises and pitfalls of immune-based strategies for Huntington’s disease
- Post electrical or lightning injury syndrome: a proposal for an American Psychiatric Association’s Diagnostic and Statistical Manual formulation with implications for treatment
- Odorants could elicit repair processes in melanized neuronal and skin cells
- Neuroprotective effects of erythropoietin on neurodegenerative and ischemic brain diseases: the role of erythropoietin receptor

