Globose basal cells for spinal cord regeneration
Durai Murugan Muniswami, Indirani Kanakasabapathy, George Tharion
1 Department of Physical Medicine & Rehabilitation, Christian Medical College, Vellore, Tamil Nadu, India
2 Department of Anatomy, Christian Medical College, Vellore, Tamil Nadu, India
How to cite this article:Muniswami DM, Kanakasabapathy I, Tharion G (2017) Globose basal cells for spinal cord regeneration. Neural Regen Res 12(11):1895-1904.
Funding: This study was financially supported by Department of Biotechnology, Ministry of Science & Technology, Government of India.
Introduction
Spinal cord injury (SCI) damages the cord initially by mechanical trauma followed by secondary cascades of ischemia,edema, inflammatory processes, free radical generation, excitotoxicity and a series of catastrophic pathophysiological events, which results in paraplegia or tetraplegia. The injured axons fail to regenerate because of many factors, including the presence of inhibitory molecules, lack of permissive growth environment within lesion sites (Filbin, 2003; Silver and Miller, 2004; Cregg et al., 2014) and lack of neurotrophic support (Liu et al., 1999a, b; Tuszynski et al., 1996; Bradbury et al., 1999). Over past decades, several exogenous chemicals,growth factors, cells are experimented in animal models of SCI. Cell transplantation therapy is thought to be a promising strategy for SCI repair (Tso and McKinnon, 2015).
Intraspinal transplantation of neural stem cells (NSCs)from subventricular zone in a rat model of cervical SCI led to significant respiratory recovery (Sandhu et al., 2017).Immature neurons in the hippocampus tend to migrate and form neural network following brain injury for repair (Ibrahim et al., 2016). The hippocampus and subventricular zone are the sources of NSCs for transplantation to treat SCI, but these sources are highly invasive for clinical applications.Among the possible sources of autologous cells for use to treat SCI, olfactory mucosa is a promising source that contains olfactory ensheathing cells (OECs) and neural progenitor cells.
In olfactory mucosa, there is a continuous replacement of olfactory sensory neurons throughout adult life of mammals(Mackay-Sim, 2003). The old neurons are vulnerable to death by environmental insults and new neurons arise from the olfactory epithelium for replacement. This neurogenesis is facilitated by a specialized gial “OECs” in the olfactory mucosa.OECs are being used in the pre-clinical and clinical practice around the world with different outcomes (Li and Lepski,2013). Both OECs and neurons of olfactory mucosa significantly enhanced axonal outgrowth in cortical slices as compared to controls (Ishihara et al., 2014). The olfactory epithelium is comprised of horizontal basal cells (HBCs), globose basal cells (GBCs) and sustentacular cells. The basal cells proliferate and differentiate into fully mature neurons (Graziadei,1973). The capacity to replace the damaged or lost olfactory sensory neurons by olfactory epithelium is well confirmed in several studies (Smith, 1951; Schultz, 1960; Hurtt et al., 1988;Delaleu and Sicard, 1995; Schwob et al., 1995).
HBCs are electron-dense, flat shaped, slow dividing(quiescent) keratin positive cells, which act as a reserve multipotent stem cells and it could be isolated by NCAM-1 and ICAM-1/β1 integrin (Chen et al., 2004; Viktorov et al., 2006). These cells have high proliferative potential,when cultured in nerve growth factor (NGF), transforming growth factor-α (TGF-α), epidermal growth factor (EGF)media, which gives rise to globose stem cells, glial cells and horizontal neural cell adhesion molecule (NCAM)-1+progenitor cells (Holbrook et al., 1995; Carter et al., 2004; Leung et al., 2007). Transplantation of HBCs for treatment of SCI in rat models showed partial recovery in terms of the Basso,Beattie and Bresnahan (BBB) locomotor rating scale score and remyelination (Ohnishi et al., 2013).
GBCs are electron-lucent, spherical shaped and reside superficially in the olfactory mucosa above the HBC layer. This is the precursor of the olfactory receptor neurons located in the olfactory epithelium as a small population of transit amplifying (mitotically active) cells called GBCs (Calof and Chikaraishi, 1989; Gordon et al., 1995). It is generally agreed that GBCs are the immediate neuronal progenitor cells exhibiting the neurogenic basic helix-loop-helix transcription factors during development and regeneration of olfactory epithelium (Guillemot et al., 1993; Gordon et al., 1995; Cau et al., 1997; Manglapus et al., 2004). Transplantation and genetic fate mapping studies indicate that the keratin-negative GBC population contains multipotent stem cells and also some populations which act as reserve cells in olfactory epithelium (Goldstein et al., 1998; Chen et al., 2004; Gokoffski et al.,2011; Jang et al., 2014).In vitrostudies have shown that olfactory neurons are defined by NCAM expression (Mahanthappa and Schwarting, 1993; DeHamer et al., 1994; Satoh and Takeuchi, 1995), and are OMP-immunoreactive cells (Pixley,1992; MacDonald et al., 1996; Wayne et al., 1996). Among the basal cells, a group of GBCs express early-stage differentiation markers like GBC-1 (Goldstein and Schwob, 1996), m-musashi (Sakakibara et al., 1996), and MASHI (Guillemot et al.,1993; Gordon et al., 1995). GBCs were fluorescence-activated cell sorting (FACS) done using markers like Ascl1+(Guo et al., 2010), GBC-1 (Goldstein and Schwob, 1996), GBC-2(Chen et al., 2004), GBC-3 (Jang et al., 2007), Lgr5+(Chen et al., 2014) forin vitroandin vivostudies (Duan and Lu, 2015).
After destroying olfactory epithelium by MeBr gas in C57BL/6 mice, green fluorescence protein (GFP)-labeled GBCs were infused into nasal cavity, and they engrafted and gave rise to neurons, GBCs and sustentacular cells. Evidence suggests that GBCs of olfactory epithelium are responsible for replacing damaged cells (Chen et al., 2004; Jang et al.,2007). Several studies suggest that transplantation of olfactory mucosal progenitor cells has a promising therapeutic effect in cochlear damage (Pandit et al., 2011), SCI (Xiao et al.,2005, 2007) and Parkinson’s disease (Murrell et al., 2008).Therefore, olfactory epithelium has been considered to be an important source for adult neural stem/progenitor cells.
In this study, we isolated rat GBCs using GBC-3 antibody,in vitrocharacterized them for neuropotency, transplanted them into the injured rat spinal cord, and evaluated the outcomes of GBCs transplantion by BBB scores, motor-evoked potential, and histological observation.
Materials and Methods
Twenty-two adult Albino Wistar rats were obtained from the Laboraty Animal Center of the Christian Medical College, Vellore, India. They were used for cell culture (n= 10)and SCI experiments (n= 12). The study was approved by Institutional Review Board (IRB) and Institutional Animal Ethics Committee of Christian Medical College, Vellore(IAEC No. 1/2010), India.
Isolation, culture, neuronal induction, and GFP labeling of GBCs
Culture of epithelial stem cells
Ten male Albino Wistar rats, aged over 3 months old,weighing 100–250 g, were used for tissue collection following intraperitoneal anesthesia with ketamine (90 mg/kg) and xylazine (10 mg/kg). In anesthetized rats, olfactory mucosa was removed from the posterior regions of nasal septum and placed in ice cold DMEM/F12 (Gibco; Grand island, New York, USA) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 25 ng/mL amphotercin-B. The olfactory mucosa was incubated for 30 minutes at 37°C in 2.4 U/mL dispase II (Roche; Tokyo, Japan). The olfactory epithelium was carefully separated from the underlying lamina propria under the dissection microscope. The olfactory epithelium was incubated with 0.05% trypsin-EDTA (Gibco; Grand island, New York, USA) in low calcium Ringer solution (Claris Lifesciences Ltd, Ahmedabad, India) for 5–10 minutes at 37°C, followed by dissociation enzyme cocktail (collagenase/hyaluronidase/trypsin inhibitor; 1, 1.5, 0.1 mg/mL respectively; Sigma, St. Louis, MO, USA) in Ringer’s solution for 15 minutes at 37°C with trituration. The olfactory epithelium is gently triturated for about 10–20 times to separate the cells.Dissociated cells were subsequently transferred to a 15 mL conical tube and the enzymes were inactivated by adding 10 mL of DMEM/F12. The cell suspension was centrifuged at 200 ×gfor 10 minutes. The supernatant was aspirated and the cell pellet was resuspended in culture media and then plated in culture flask coated with poly-D-lysine at a density of 4–5 × 104/cm2. Cultures were incubated at 37°C in 5%CO2and expansion medium was refreshed every other day.Expansion medium was composed of DMEM/F12 (1:1; Gibco), 2% fetal bovine serum (Gibco), N2 supplement (Gibco)and epidermal growth factor (EGF; 25 ng/mL; Gibco).
Fluorescence-activated cell sorting of GBCs
GBCs were sorted using GBC-3 antibody (a giftfrom James E. Schwob, Woochan Jang; Department of Anatomy and Cellular Biology, Tufts University School of Medicine, Boston, MA, USA). 80–90% confluent cultures were trypsinized and washed with Hank’s Balanced Salt Solution (HBSS).Cell pellets were incubated with primary antibody (GBCIII mouse monoclonal IgM, 1:100) for 20 minutes on ice.Then they were washed with PBS by centrifugation and incubated with secondary antibody (Donkey anti-mouse IgMCy3 conjugated, 1:50; Jackson ImmunoResearch Inc., West Grove, PA, USA) for 20 minutes. Finally, after PBS washes,cells were sorted by ARIA-BD. Sorted cells were plated in culture media and then transplanted at a density of 5 × 105cells on the 9thday after injury in a rat model of SCI.
Characterization of GBCs by immunohistochemistry
Cells were fixed in 4% paraformaldehyde for 15 minutes at room temperature and were washed with PBS (Gibco)three times. Blocking and permeabilization were done in 2% goat serum/2% bovine serum albumin (BSA, Jackson ImmunoResearch Inc.) with 0.1% Triton X-100/PBS. Cells were incubated with primary antibody at 4°C overnight.After PBS washes, cells were incubated with secondary antibodies for 2 hours at room temperature. They were then washed and mounted with 4′,6-diamidino-2-phenylindole(DAPI)-containing vectashield (Vector Laboratories, Burlingame, CA, USA). Coverslips were immediately transferred to glass slides and examined under the fluorescent microscope. The following primary and secondary fluorescent antibodies were used in this study as mentioned below. Mouse monoclonal anti-beta III tubulin (1:50 dilution; Millipore,Temecula, CA, USA), mouse monoclonal anti-MAP2 IgG1(1:100 dilution; Millipore), mouse monoclonal anti-NeuN IgG1 (1:50 dilution; Millipore), mouse monoclonal anti-neurofilament H&M IgG1 (1:100 dilution; Millipore), with appropriate secondary antibody goat anti-mouse Rhodamine(1:50 dilution; Millipore), goat anti-mouse IgG1-FITC (1:50 dilution; Southern Biotech, Birmingham, AL, USA).
Flow cytometry of GBCs
Cultured GBCs were trypsinized and washed with PBS. Two to five lakhs of cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature, then washed with PBS following blocking and permeabilization in 2% goat serum/2% bovine serum albumin (BSA) with 0.1% Triton X-100/PBS, and incubated with 5–10 μL of primary antibody for 20 minutes on ice. Excess unbound antibodies were washed with PBS and removed. This was then incubated for 20 minutes with 5–10 μL of fluorescent tagged appropriate secondary antibody. Finally unbound secondary antibody was washed with PBS. Cell suspension was aspirated and analyzed using a fluorescence-activarted cell sorter (BD FACS Aria, CA, USA) for NSC marker (neural cell adhesion molecule, Nestin, SOX2), mesenchymal stem marker(CD54, CD90, CD73, CD29, CD105) and haematopoietic marker (CD45, CD34). The unstained cells were used as control. The following primary and secondary fluorescent antibodies were used. Mouse anti-rat CD54-FITC conjugated (1:50 dilution; BD Biosciences, Franklin Lakes, NJ, USA),monoclonal mouse anti-rat CD90-FITC conjugated (1:100 dilution; Millipore), mouse monoclonal IgG1 anti-CD34-FITC conjugated (1:100 dilution; Santa Cruz Biotechnology,Dallas, TX, USA), mouse monoclonal anti-rat CD45-PE conjugated (1:100 dilution; BD Biosciences), monoclonal mouse anti-beta 1 integrin (1:50 dilution; Millipore), monoclonal mouse anti-rat CD73 (1:50 dilution; BD Biosciences),goat polyclonal IgG anti-CD105 (1:25 dilution; Santa Cruz Biotechnology), monoclonal anti-NCAM mouse IgG1 (Sigma, St. Louis, Missouri, USA), anti-SOX2 clone 6G1.2-FITC conjugated (Millipore), anti-nestin mouse IgG (Millipore),with appropriate secondary antibodies goat anti-mouse IgG2b R-phycoerythrin (RPE) (1:50 dilution; Southern Biotechnology Associates, Inc., Birmingham, AL, USA), goat anti-mouse IgG1-RPE conjugated (1:50 dilution; Southern Biotechnology Associates, Inc.,), donkey anti-goat IgG-perCp conjugated (1:100 dilution; Jackson ImmunoResearch Inc.).
Neurosphere formation
Neuronal induction medium composed of DMEM/F12 (1:1)supplemented with 2% fetal bovine serum (FBS, Gibco), B27 supplement (Gibco), 20 mM retionic acid (Sigma) and 12.5 ng/mL bFGF (Invitrogen) was used. Cells were maintained in neuronal induction media for 12 days (Greco et al., 2008).After 12 days these cells were immunostained for neuronal markers [βIII tubulin, microtubule-associated protein 2(MAP-2), neuronal nuclei (NeuN), neurofilament (NF)] and images were captured using Leica DMI6000 fluorescent microscope.
Neuronal induction
Neuronal induction medium composed of DMEM/F12 (1:1)supplemented with 2% fetal bovine serum (FBS, Gibco), B27 supplement (Gibco), 20 mM retionic acid (Sigma) and 12.5 ng/mL bFGF (Invitrogen) was used. Cells were maintained in neuronal induction media for 12 days (Greco et al., 2008).After 12 days these cells were immunostained for neuronal markers [βIII tubulin, microtubule-associated protein 2(MAP-2), neuronal nuclei (NeuN), neurofilament (NF)]and images were captured using Leica DMI6000 fluorescent microscope.
GFP labeling of cells
0.5 × 105/mL cells were grown in complete medium overnight. 50 μL 1 × 107IFU/mL pre-made lentiviral particles for fluorescent proteins (catalog number: LVP001; GenTarget Inc., San Diego, CA, USA) was added to the culture. After 72 hours of transduction, the transduction rate was checked in fluorescent microscope. GFP labeled cells were used for transplantation.
Animal experiments Experiment design
The study was approved by the Institutional Animal Ethics Committee of Christian Medical College (IAEC No. 1/2010),Vellore, India and the experiment was performed according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines. Rats were randomly divided into two groups (n= 6 rats in each group). Rats in the GBCs group received GBCs transplantation (5 lakh cells) after injury, and those in the control group were administered DMEM without cells.
Laminectomy and SCI
Female adult Albino wistar rat, weighing 100–250 g, were anaesthetised by intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). Ophthalmic ointment was applied to the eyes to prevent drying during the operation.The fur on the mid dorsal region was shaved and the skin was cleaned with povidone-iodine solution (7.5%, w/v).Tega-derm was applied over the shaved skin to prevent fur contamination during the surgery. A 2.0 cm long incision was made over the lower thoracic area. The muscle and connective tissue were bluntly dissected to expose the T8–11vertebrae. T10laminectomy was completed using a microsurgery bone rongeur. Care must be taken to avoid damage to the spinal cord. SCI was induced by dropping a 10 g rod, a custom-fabricated impactor device provided by the Department of Bioengineering, Christian Medical College, Vellore, India,from a 25 cm height to the expose spinal cord (Tharion et al., 2011). Absorbable suture (vicryl, Johnson-Johnson Ltd)was used to ligate the incised muscle and skin. Meloxicam (1 mg/kg; Intas Pharmaceuticals Ltd, Gujarat, India) as analgesic, Enrofloxacin (2.5 mg/kg; Intas Pharmaceuticals Ltd.) as antibiotic, and Ringer lactate 5 mL/100 g were administered subcutaneously as post-operative care. Animals had free access to food and water throughout the study. Bladder and bowel were expressed manually as post-operative care.
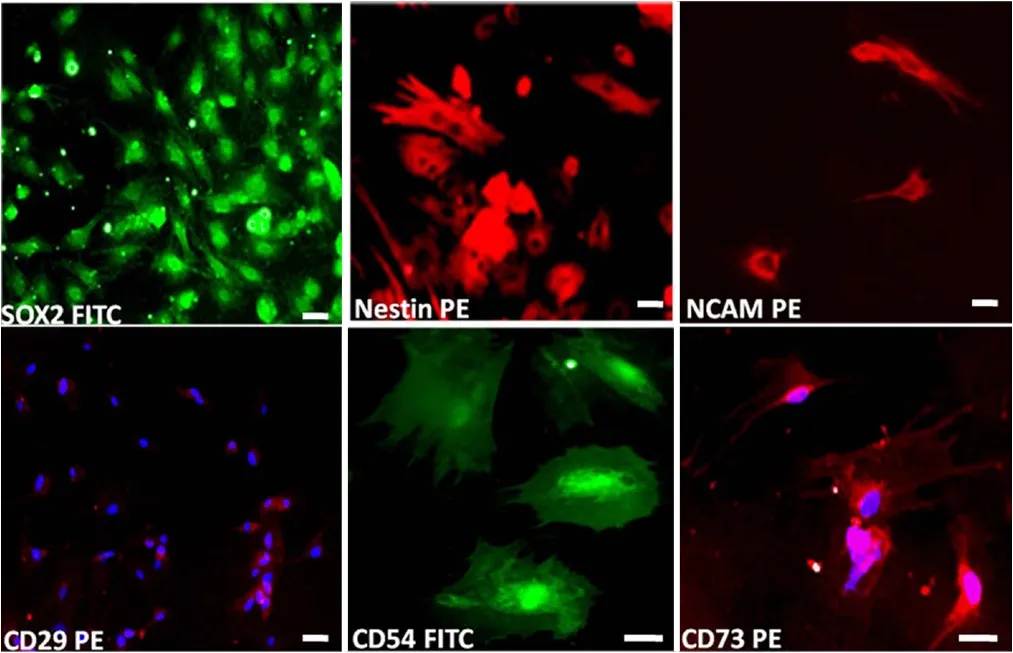
Figure 1 Immunohistochemical characterization of passage 2 globose basal cells (GBCs).GBCs expressed NSC markers SOX2, nestin, and neural cell adhesion molecule and were also immunostained for CD29, CD54 and CD73 (scale bars: 20 μm). Green indicates fluorescein isothiocyanate (FITC) conjugated, red indicates R-phycoerythrin (PE) conjugated, and blue indicates nuclear stain 4′,6-diamidino-2-phenylindole (DAPI) conjugated.
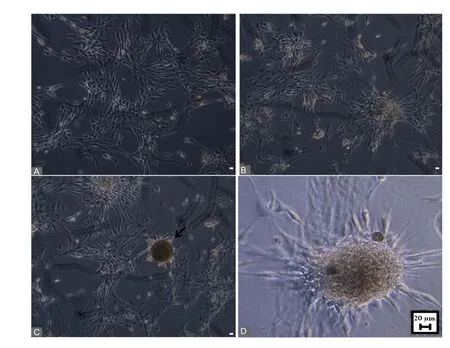
Figure 3 Globose basal cells cultured in different media (DMEM/F12,N2 supplement, epidermal growth factor, and basic fibroblast growth factor).(A) Globose basal cell culture on the first day. (B) Aggregation of cells on the second day. (C) Neurosphere formation on the third day (black arrow). (D) Phase contrast magni fied view of neurosphere. Scale bars:20 μm. Phase contrast image captured using Leica DMI6000 microscope.
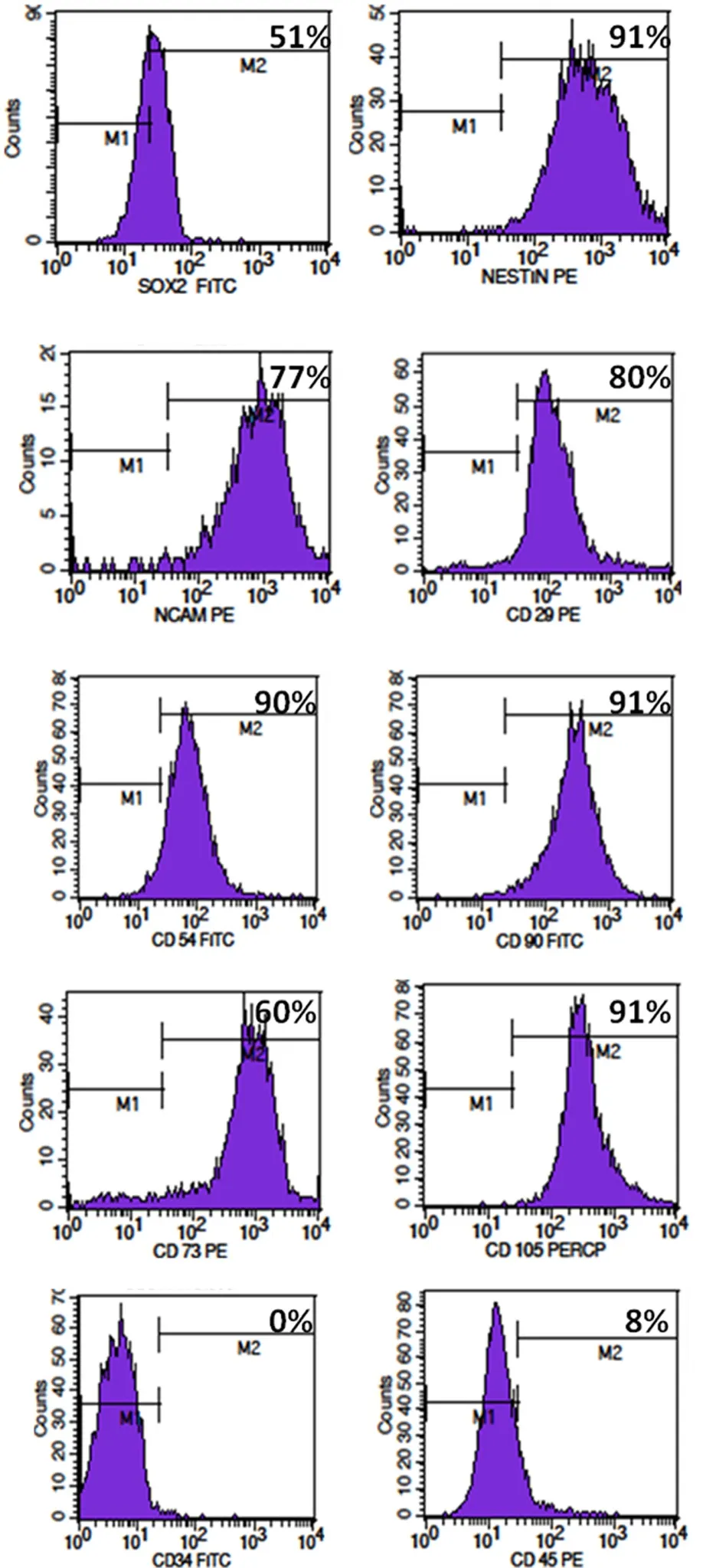
Figure 2 Flow cytometry analysis of passage 2 globose basal cells(GBCs) for neural stem cell markers and cell surface markers.Flow cytometry analyses revealed that in passage 2 GBCs, the expression of neural stem cell markers SOX2, nestin, and NCAM was 51%,91%, and 77%, respectively, and the expression of mesenchymal stem cell markers CD29, CD54, CD90, CD73, and CD105 was 80%, 90%,91%, 60%, and 91%, respectively. GBCs were negative for hematopoeitic markers CD34 and CD45, and their expression was 0% and 8%,respectively.
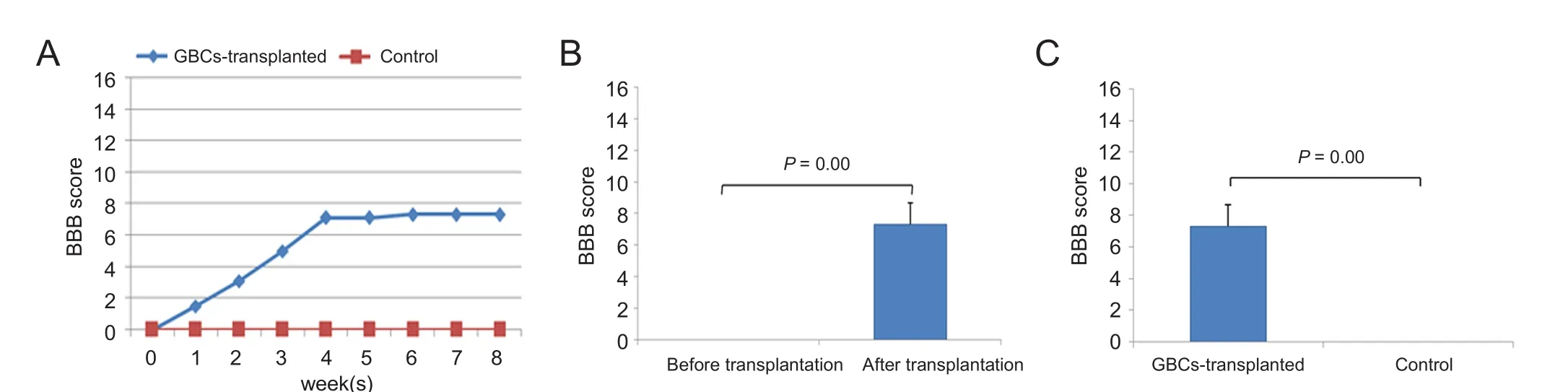
Figure 6 Hindlimb motor recovery in spinal cord injury rats following globose basal cell (GBC) transplantation.(A) The Basso, Beattie and Bresnahan (BBB) locomotor scale score increased from 0 to 7.3 over time in the GBCs group (n = 6), and no hindlimb movement was observed in the control group (n = 6). (B) In the GBCs group, the BBB score was 0 prior to cell transplantation (i.e., on the 9th day after SCI), but significant functional recovery was observed at the end of the 8th week (P < 0.001). (C) At the end of the 8th week, there was significant difference in hindlimb motor function recovery between GBCs and control groups (P < 0.001). Error bars indicate the standard deviations.

Figure 7 Hindlimb motor evoked potential in spinal cord injury rats following globose basal cells (GBCs) transplantation.Motor cortex stimulation and their responses were recorded in hindlimb muscle in the 8th week after spinal cord injury. There is no EMG amplitude seen in the control group (A). In the GBCs group, a representative EMG amplitude (arrow) was shown (B). There was significant difference in the mean amplitude between GBCs and control groups (P < 0.05) (C). Amplitude varies based on regeneration of injured spinal cord. Error bars indicate the standard deviations.

Figure 4 Immunohistochemical characterization of neurospheres after 3 days.Neurosphere expresses neural stem cell markers SOX2 and nestin.FITC-positive SOX2 expression is in green, PE-positive nestin expression is in red, and nuclei are visualized with DAPI (blue). Scale bars: 20 μm.

Figure 5 Induced neuronal differentiation of globose basal cells after 12 days.Differentiated globose basal cell express neuron markers βIII tubulin,microtubule-associated protein 2 (MAP2), neuronal nuclei (NeuN) and neurofilament (scale bars: 20 μm). Red indicates rhodamine conjugated,green indicates fluorescein isothiocyanate (FITC) conjugated, and blue indicates nuclear 4′,6-diamidino-2-phenylindole (DAPI) staining.
Cell transplantation
Cell transplantation was done on the 9thday after SCI. Behavioral assessment (BBB) was conducted prior to cell transplantation as described below. Rats were re-anesthetized(intraperitoneal injection of 90 mg/kg ketamine/10 mg/kg xylazine), and the original incision was re-opened and the dorsal laminectomy was extended to the T8–11vertebrae. Under a surgical microscope, the wound was explored and the injured spinal cord segment as well as the normal spinal cord a few millimer above and below the injured segment were exposed. On the day of transplantation, GBCs were harvested, pelleted, and transferred into a 25 µL Hamilton syringe(approximately 100,000 cells/µL). Total 5 × 105cells were injected at multiple sites, in and around the injured spinal cord with the aid of a sterile Hamilton syringe. Control rats received DMEM media alone without cells. Following cell transplantation, the surgical wound was closed and routine post-operative care was given.
Behavioral assessment
The Basso, Beattie, Bresnahan (BBB) Locomotor Rating Scale (Basso et al., 1995) is an operationally defined 21 point scale, designed to assess hindlimb locomotor recovery after impact injury to the spinal cord in rats. No complete hindlimb movement indicates score “zero” and the normal rat hindlimb gait score is “21”. This locomotor scale categorizes combinations of rat hindlimb joint movements, trunk position and stability, stepping, coordination, paw placement,toe clearance and tail position, representing sequential recovery stages that rats attain after SCI. All rats received bladder expression before the open field testing to eliminate behaviors due to bladder fullness. Rats were allowed to walk in the open field (45 cm × 60 cm rectangular tray) and videographed by a Sony HD video recording handycam.Assessment was done by an investigator, who was blinded to the experiments. All rats were assessed for BBB score before transplantation (i.e.) on the 9thday after SCI and then once a week after cell transplantation till the 8thweek.
Motor evoked potential studies
The EMG stimulator device and recording software (CMC-daq) used in this study were designed by Department of Bioengineering, Christian Medical College, Vellore, India.Transcranial electrical stimulation of motor cortex by placing superficial bipolar electrode on the scalp was done in the anesthetized rat (intraperitoneal injection of 90 mg/kg ketamine and 10 mg/kg xylazine). The EMG signals were recorded from the lower limb gastrocnemius muscles. 1.5 V alkaline batteries were used in pulse stimulator. The signals were amplified, filtered (10 to 5,000 Hz), and sampled at 2 kHz: 0.050 s/sweep of EMG channel. The output of the data acquisition were 0.05 s/screen, 1.5 mV/div. The time of the data for each segment is displayed in the centre, bottom of the window and the amplitude of the data is shown on the leftside of each channel.
Recording was done for control (n= 6) and cell transplanted rats (n= 6) at the end of 8 weeks post SCI/cell transplantation. Single channel recorded EMG signals were analyzed for amplitude in mV. This parameter was carried out to evaluate the functional integrity of the spinal cord.
Histology
Two weeks after GFP labeled GBCs transplantation, transplanted rats (n= 2) were anesthetized by intraperitoneal injection of 90 mg/kg ketamine/10 mg/kg xylazine and transcardially perfused with 4% paraformaldehyde solution. 1 cm length of spinal cord centered on the injury epicenter was removed and post-fixed in 30% sucrose/phosphate-buffered saline at 4°C overnight. Twenty micrometer (µm) thick longitudinal serial cryo-sections were cut and mounted on poly-L-lysine coated slides. Representative tissue sections were incubated with 0.3% Triton X-100/phosphate-buffered saline at 4°C overnight. After PBS washes, they were incubated with primary antibody (mouse monoclonal anti-beta III tubulin,1:50 dilution; Millipore) at 4°C overnight. After PBS washes, they were incubated with secondary antibody (goat anti-mouse Rhodamine, 1:50 dilution; Millipore) for 2 hours at room temperature. Finally tissue sections were washed with PBS and visualized under the confocal microscope.
Statistical analysis
SPSS16.0 software (SPSS, Chicago, IL, USA) was used to statistically analyze hindlimb motor recogery using BBB scores and the amplitude of motor evoked potential. Wilcoxon test and Mann-WhitneyUtest were used to determine the significant differences between different groups/within group.Continuous variables for each group were represented as the mean ± SD. A level ofP< 0.05 was considered statistically significant.
Results
Immunophenotype characterization of GBCs
GBC-3 cells were muunohistochemically positive for SOX2,nestin, NCAM, CD29, CD54, and CD73 (Figure 1). Flow cytometry analyses of GBC-3 revealed that the expression of NSC markers SOX2, nestin, neural cell adhesion molecule was 51%, 91%, and 77%, respectively, and the expression of typical mesenchymal stem cell markers CD29, CD54, CD90,CD73, CD105 was 80%, 90%, 91%, 60%, 91%, and GBC-3 was negative for hematopoietic markers CD34 and CD45(Figure 2). GBCs, upon exposure to neurosphere medium,aggregated and formed neurospheres on the 3rdday (Figure 3). These neurospheres exhibited the characteristics of neural stem/progenitor cell markers SOX2 and nestin (Figure 4).The presence of SOX2 and nestin represents NSC like properties.In vitroneuronal induction was performed using neuronal induction media for 12 days. The differentiated cells expressed mature neuronal markers βIII-tubulin, MAP-2,NeuN and NF (Figure 5). GBCs possessed the culture characteristics including formation of neurospheres, expression of NSC markers, and neuronal differentiation properties.These findings suggest that GBCs are multipotent, neural stem/progenitor cells in the olfactory neuroepithelium.
Hindlimb motor function
Following spinal cord injury, BBB score was zero on the 9thday. 5 × 105cells were transplanted on the 9thday post-SCI.In each week post-transplantation, BBB scores gradually progressed and reached 7.3 ± 1.4 at the end of the 8thweek,whereas in the control group, locomotor recovery did not progress and stayed in the baseline level.
In the GBCs group, there was significant difference in BBB score between before and after transplantation (0vs.7.3 ±1.4,P< 0.001). Significant difference in BBB score was also found between control and GBCs groups (0vs.7.3 ± 1.4,P<0.001). The mean BBB score was 1.5 ± 0.8, 3.1 ± 0.9, 5.0 ± 0.8,7.1 ± 1.4, 7.1 ± 1.4, 7.3 ± 1.4, 7.3 ± 1.4 and 7.3 ± 1.4 in the first, second, third, fourth, fifth, sixth, seventh, and eighth weeks after cell transplantation, respectively. This suggests significant functional recovery as compared to control group(P< 0.001) (Figure 6).
Motor evoked potentials of hindlimbs
Transcranial electrical stimulation and their EMG response from the hindlimb gastrocnemius muscle were done on anesthesized rats. A representative electromyograph of control (Figure 7A) and transplanted rats (Figure 7B) shows difference in wave pattern. Transplanted rats (1.2 ± 0.5 mV)showed increased amplitude changes, but control group(0.2 ± 0.1 mV) exhibited less amplitude. Statistical analysis showed significant improvement (P< 0.05) in amplitude among the groups (Figure 7C), which demonstrate regeneration of injured spinal cord after GBCs transplantation.
Histology of rat spinal cord
GFP-labelled GBCs were seen around the injury epicenter of the rat spinal cord (Figure 8C). GFP-labeled cells were immunostained for βIII-tubulin, a marker of mature neuron(Figure 8F). The results demonstrate that the transplanted GBCs survived and differentiated in the injured spinal cord,which could be responsible for the transplantation-mediated repair of injured spinal cord.
Discussion
Stem cell exhibits the properties of self-renewal and differentiation. Multipotent cells have the ability to give rise to different types of cells within a tissue. Harvesting of neural stem cells (NSC) from sub-ventricular zone, hippocampus requires highly invasive surgery, which is a major obstacle. In contrast, olfactory epithelial stem/progenitor cells are accessible from nasal cavity without lasting damage to the donor.
Mesenchymal stem cells (MSCs) are easily harvested,quickly expanded and have immunomodulatory, neurotrophic and neuroprotective properties, which can be clinically used for autologous transplantation. This could be a potential stem cell for transplantation in SCI with minimal risk of tumorigenicity (Uccelli et al., 2008; Torres-Espín et al., 2013; Doulames and Plant, 2016). But, multipotent nature of MSC is restricted to mesodermal lineages with limited ability to differentiate into neuronal and glial lineages(Hofstetter et al., 2002; Hodgetts et al., 2013; Vawda and Fehlings, 2013). Evidence suggests that MSCs do not integrate with host tissue following transplantation and yield moderate functional recovery (Prockop, 2003; Prockop et al., 2010;Ankrum et al., 2014; Sandner et al., 2016).
Transplantation of human embryonic stem cells (ESCs)-derived oligodendrocyte progenitor cells in SCI rat model decreased lesion size, preserved host neurons, and improve forelimb function (Sharp et al., 2010; Sun et al., 2013). Because ESCs are harvested from the blastocysts, so they are subjected to ethical constraints and pose the risk of teratoma formation following transplantation (Nisbet et al., 2003).
Induced pluripotent stem cells (iPSCs) created by reprogramming of somatic cells using viral vectors are an attractive option in the treatment of SCI. iPSCs will be advantageous over ESCs and MSCs in terms of pluripotency and clinically autologous transplantation. However, there is a high risk of insertional mutagenesis caused by vectors and epigenetic memory of the source tissue. iPSCs-transplanted groups showed gradual improvement in motor function(Doulames and Plant, 2016). Neural stem/progenitor cell transplantation has been found to promote axonal growth across the injury, facilitating synaptogenesis and remyelinating host axons, thereby resulting in significant functional improvement in SCI models (Ogawa et al., 2002; Lu et al.,2003; Iwanami et al., 2005). NSCs from the subventricular zone were transplanted in T10SCI rat models and obvious improvement in motor functin was found (Ye et al., 2016).
The regenerative potential of the olfactory system has attracted scientific interest. In the last decades, much attention has been taken by scientific community on olfactory ensheathing cells, because of unique glial characteristics of astrocytes(CNS) as well as Schwann cells (PNS) and their regenerative potential (Raisman, 2004; Mackay-Sim, 2005). OEC transplantation demonstrated moderate therapeutic effect in SCI,whereas transplantation of heterogenous glial and stem cells(OECs, olfactory nerve fibroblasts, GBCs, HBCs) or whole olfactory mucosa tissue in SCI yields maximum efficacy. The results indicate that both glial and stem cells of olfactory mucosa plays a role in regeneration (Lima et al., 2010, 2006).
Among the different types of stem cells like MSCs, ESCs,iPSCs, NSCs are the foremost choice for neural regeneration.So, we evaluated the stem/progenitor cells (GBCs, HBCs) of olfactory neuro-epithelium in SCI, but in this study we focused only on GBCs. GBCs contain two populations of cells.One population of cells are immediate neuronal progenitors that differentiate into olfactory sensory neuron (Goldstein et al., 1998; Huard et al., 1998; Newman et al., 2000). The other population of GBCs are multipotent that can differentiate into non-neuronal cells (supporting cells, Bowman’s gland) of the olfactory epithelium (Chen et al., 2004). HBCs have been isolated, cultured and differentiated into neurons and glia, suggesting multipotency (Carter et al., 2004). Both GBCs and HBCs demonstrate multipotent nature, but only GBCs are able to produce HBCs (Huard et al., 1998).
Methyl bromide gas injury leads to HBC activation by down-regulation of p63. The activated HBCs give rise to GBCs, thus tissue maintenance takes place, suggesting p63 regulates the reserve state of HBCs, but not the stem cell status. GBCs are active stem cells that give rise to all the mature cells of the tissue. HBCs are reserve stem cells and remain dormant until tissue injury. Under normal condition of olfactory epithelium, HBCs are rarely involved in turnover of olfactory sensory neurons (Schnittke et al., 2015).
GBC-3 is a monoclonal antibody that recognizes the cell surface antigen-40 kDa laminin receptor and is specific for GBC population of olfactory epithelium (Jang et al., 2007).In our study, FACS sorted GBC-3 positive cells express the NSC marker of SOX2, nestin, neural cell adhesion molecule and MSC markers. On plating these GBCs in EGF, N2 supplement and bFGF medium, it forms “neurosphere” a cultural characteristic of NSCs. NSCs from the brain were reported to grow as “neurosphere” in culture (Reynolds and Weiss, 1992). Neurosphere-forming cells expressed 91-98% of βIII-tubulin, and 36–78% of nestin under different culture conditions (Zhang et al., 2004). In an another study,cells from human olfactory mucosa behave like neural stem/progenitors and form “neurosphere” which gives rise to neurons and glia (Roisen et al., 2001; Winstead et al., 2005).
In olfactory epithelium, NEUROD1-labeled cells also coexpress cell cycle markers such as PCNA, Ki67 and Edu and these GBCs exhibit a greater progenitor cell capacity (Lee et al., 2000; Gao et al., 2009; Kuwabara et al., 2009; Boutin et al., 2010; Guo et al., 2010; Puligilla et al., 2010). SOX2 tightly regulates NeuroD1 by repressing it in the dentate gyrus.NEUROD1-labeled cells coexpress SOX2 (Gao et al., 2009;Kuwabara et al., 2009). Neuronal differentiation is repressed by SOX2, which is co-expressed with NeuroD1, a factor that regulates the neuronal differention of the same progenitors,suggesting pleiotropic function of SOX2 in the olfactory epithelium (Graham et al., 2003; Ellis et al., 2004; Bani-Yaghoub et al., 2006; Boutin et al., 2010; Puligilla et al., 2010). In our study, we further investigated the expression of SOX2 and nestin in the neurospheres, demonstrating neural stem/progenitor cell characteristics of GBCs.
Although peripheral olfactory stem cells originate from neural crest-derived cells, it expresses neural cell-related genes and also exhibits the properties of bone marrow mesenchymal stem cell (BM-MSC), thus named “olfactory ectomesenchymal stem cells” (OE-MSC) (Delorme et al., 2010;Girard et al., 2011; Féron et al., 2013). In our study, GBCs also express the panel of MSC markers CD29, CD54, CD90,CD73, CD105 like bone marrow MSCs.
Earlier studies have shown that transplantation of neural stem/progenitor cells. BMSCs on the 9thday post-SCI yielded better survival and differentiation (Okano, 2002; Parr et al., 2008). So, we chose the 9thday after SCI for transplantation as the therapeutic window period. As a preliminary study, a single dose of 5 lakhs of cell transplantation was carried out rather than use of different doses. Direct injection of cells into the injured cord was employed to ensure the maximum effects, though various routes of cell administration like lumbar puncture and intravenous route are employed in other studies.
In this study, hindlimb locomotor recovery was evaluated weekly by the BBB scale. The GBCs transplanted groups showed promising score of 7.3 at the end of the 8thweek after transplantation. The control group had no improvement in functional recovery, suggesting that there was no spontaneous regeneration after SCI. Moreover, in the GBCs group, EMG amplitude was markedly increased, indicating axonal regeneration. In addition, histological studies showed that GFP-labelled cells survived around the injury epicentre of the injured spinal cord. These cells stained positive for βIII tubulin, suggesting differentiation to neural cells which could be responsible for the hindlimb motor recovery. Thus we conclude that GBCs exhibit therapeutic effects in experimental models of SCI.
Taken together, GBCs from the olfactory epithelim could be an alterative source of NSCs for autologous neurotransplantation, which can be collected less invasively for therapeutic transplantation following SCI.
Author contributions:DMM was responsible for literature retrieval, experiment conduction, data acquisition and analysis, statistical analysis, and prepared the paper. IK conceived and designed the study,particilated in literature retrieval, edited and reviewed the paper. GT participated in study conception and design, experiment conduction,data analysis, and edited and reviewed the paper. All authors approved the final version of this paper.
Conflicts of interest:None declared.
Research ethics:The study was approved by Institutional Review Board(IRB) and Institutional Animal Ethics Committee of Christian Medical College (IAEC No. 1/2010), Vellore, India.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.

Figure 8 Histology of injured rat spinal cord following globose basal cells (GBCs) transplantation.Two weeks after green fluorescent protein (GFP) labeling cell transplantation, longitudinal 20 μm thick cryosections of GBCs-transplanted spinal cord were prepared. Phase contrast (A), GFP-labelled GBCs in the spinal cord around injury epicenter (B), merged image (C). GFP-labeled GBCs (green) in spinal cord (D). βIII-tubulin-PerCp positive cells(red) in the spinal cord (E). Merged image of D and E, yellow color (white arrow) shows GBCs differentiated into neurons in the spinal cord (F).Scale bars: 20 μm.
Ankrum JA, Ong JF, Karp JM (2014) Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol 32:252-260.
Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B,Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR,Sikorska M (2006) Role of Sox2 in the development of the mouse neocortex. Dev Biol 295:52-66.
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1-21.
Boutin C, Hardt O, de Chevigny A, Coré N, Goebbels S, Seidenfaden R, Bosio A, Cremer H (2010) NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc Natl Acad Sci U S A 107:1201-1206.
Bradbury EJ, Khemani S, Von R, King, Priestley JV, McMahon SB(1999) NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci 11:3873-3883.
Calof AL, Chikaraishi DM (1989) Analysis of neurogenesis in a mammalian neuroepithelium: Proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron 3:115-127.
Carter LA, MacDonald JL, Roskams AJ (2004) Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci 24:5670-5683.
Cau E, Gradwohl G, Fode C, Guillemot F (1997) Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Dev Camb Engl 124:1611-1621.
Chen M, Tian S, Yang X, Lane AP, Reed RR, Liu H (2014) Wnt-responsive Lgr5+ globose basal cells function as multipotent olfactory epithelium progenitor cells. J Neurosci 34:8268-8276.
Chen X, Fang H, Schwob JE (2004) Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol 469:457-474.
Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J (2014) Functional regeneration beyond the glial scar. Exp Neurol 253:197-207.
DeHamer MK, Guevara JL, Hannon K, Olwin BB, Calof AL (1994)Genesis of olfactory receptor neurons in vitro: Regulation of progenitor cell divisions by fibroblast growth factors. Neuron 13:1083-1097.
Delaleu JC, Sicard G (1995) Physiological and histological recovery of the olfactory mucosa of the frog after a dichlobenil injection. Chem Senses 20:433-440.
Delorme B, Nivet E, Gaillard J, Häupl T, Ringe J, Devèze A, Magnan J,Sohier J, Khrestchatisky M, Roman FS, Charbord P, Sensebé L, Layrolle P, Féron F (2010) The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev 19:853-866.
Doulames VM, Plant GW (2016) Induced pluripotent stem cell therapies for cervical spinal cord injury. Int J Mol Sci 17:530.
Duan D, Lu M (2015) Olfactory mucosa: a rich source of cell therapy for central nervous system repair. Rev Neurosci 26:281-293.
Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S,McMahon A, Rao M, Pevny L (2004) SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells,the embryo or the adult. Dev Neurosci 26:148-165.
Féron F, Perry C, Girard SD, Mackay-Sim A (2013) Isolation of adult stem cells from the human olfactory mucosa. Methods Mol Biol 1059:107-114.
Filbin MT (2003) Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci 4:703-713.
Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ,Hsieh J (2009) Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci 12:1090-1092.
Girard SD, Devéze A, Nivet E, Gepner B, Roman FS, Féron F (2011)Isolating nasal olfactory stem cells from rodents or humans. J Vis Exp doi:10.3791/2762
Gokoffski KK, Wu HH, Beites CL, Kim J, Kim EJ, Matzuk MM, Johnson JE, Lander AD, Calof AL (2011) Activin and GDF11 collaborate in feedback control of neuroepithelial stem cell proliferation and fate. Dev Camb Engl 138:4131-4142.
Goldstein BJ, Fang H, Youngentob SL, Schwob JE (1998) Transplantation of multipotent progenitors from the adult olfactory epithelium.Neuroreport 9:1611-1617.
Goldstein BJ, Schwob JE (1996) Analysis of the globose basal cell compartment in rat olfactory epithelium using GBC-1, a new monoclonal antibody against globose basal cells. J Neurosci 16:4005-4016.
Gordon MK, Mumm JS, Davis RA, Holcomb JD, Calof AL (1995)Dynamics of MASH1 expression in vitro and in vivo suggest a nonstem cell site of MASH1 action in the olfactory receptor neuron lineage. Mol Cell Neurosci 6:363-379.
Graham V, Khudyakov J, Ellis P, Pevny L (2003) SOX2 functions to maintain neural progenitor identity. Neuron 39:749-765.
Graziadei PP (1973) Cell dynamics in the olfactory mucosa. Tissue Cell 5:113-131.
Greco SJ, Zhou C, Ye JH, Rameshwar P (2008) A method to generate human mesenchymal stem cell-derived neurons which express and are excited by multiple neurotransmitters. Biol Proced Online 10:90-101.
Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL(1993) Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75:463-476.
Guo Z, Packard A, Krolewski RC, Harris MT, Manglapus GL, Schwob JE (2010) Expression of pax6 and sox2 in adult olfactory epithelium.J Comp Neurol 518:4395-4418.
Hodgetts SI, Simmons PJ, Plant GW (2013) Human mesenchymal precursor cells (Stro-1+) from spinal cord injury patients improve functional recovery and tissue sparing in an acute spinal cord injury rat model. Cell Transplant 22:393-412.
Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L (2002) Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A 99:2199-2204.
Holbrook EH, Szumowski KE, Schwob JE (1995) An immunochemical,ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol 363:129-146.
Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE (1998)Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol 400:469-486.
Hurtt ME, Thomas DA, Working PK, Monticello TM, Morgan KT(1988) Degeneration and regeneration of the olfactory epithelium following inhalation exposure to methyl bromide: Pathology, cell kinetics, and olfactory function. Toxicol Appl Pharmacol 94:311-328.
Ibrahim S, Hu W, Wang X, Gao X, He C, Chen J (2016) Traumatic brain injury causes aberrant migration of adult-born neurons in the hippocampus. Sci Rep 6:21793.
Ishihara M, Mochizuki-Oda N, Iwatsuki K, Kishima H, Ohnishi Y,Moriwaki T, Umegaki M, Yoshimine T (2014) Primary olfactory mucosal cells promote axonal outgrowth in a three-dimensional assay. J Neurosci Res 92:847-855.
Iwanami A, Kaneko S, Nakamura M, Kanemura Y, Mori H, Kobayashi S, Yamasaki M, Momoshima S, Ishii H, Ando K, Tanioka Y, Tamaoki N, Nomura T, Toyama Y, Okano H (2005) Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res 80:182-190.
Jang W, Chen X, Flis D, Harris M, Schwob JE (2014) Label-retaining,quiescent globose basal cells are found in the olfactory epithelium. J Comp Neurol 522:731-749.
Jang W, Kim KP, Schwob JE (2007) Nonintegrin laminin receptor precursor protein is expressed on olfactory stem and progenitor cells. J Comp Neurol 502:367-381.
Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L,Nakashima K, Asashima M, Gage FH (2009) Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci 12:1097-1105.
Lee JK, Cho JH, Hwang WS, Lee YD, Reu DS, Suh-Kim H (2000) Expression of neuroD/BETA2 in mitotic and postmitotic neuronal cells during the development of nervous system. Dev Dyn 217:361-367.
Leung CT, Coulombe PA, Reed RR (2007) Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci 10:720-726.
Li J, Lepski G (2013) Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int 2013:786475.
Lima C, Escada P, Pratas-Vital J, Branco C, Arcangeli CA, Lazzeri G,Maia CA, Capucho C, Hasse-Ferreira A, Peduzzi JD (2010) Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair 24:10-22.
Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD (2006) Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med 29:191-203.
Liu Y, Himes BT, Solowska J, Moul J, Chow SY, Park KI, Tessler A,Murray M, Snyder EY, Fischer I (1999a) Intraspinal delivery of neurotrophin-3 using neural stem cells genetically modified by recombinant retrovirus. Exp Neurol 158:9-26.
Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, Tessler A,Fischer I (1999b) Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci 19:4370-4387.
Lu P, Jones LL, Snyder EY, Tuszynski MH (2003) Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol 181:115-129.
MacDonald KP, Murrell WG, Bartlett PF, Bushell GR, Mackay-Sim A(1996) FGF2 promotes neuronal differentiation in explant cultures of adult and embryonic mouse olfactory epithelium. J Neurosci Res 44:27-39.
Mackay-Sim A (2005) Olfactory ensheathing cells and spinal cord repair. Keio J Med 54:8-14.
Mackay-Sim A (2003) Neurogenesis in the adult olfactory neuroepithelium. In: Handbook of Olfaction and Gustation (Doty RL, ed), pp93-113. CRC Press.
Mahanthappa NK, Schwarting GA (1993) Peptide growth factor control of olfactory neurogenesis and neuron survival in vitro: Roles of EGF and TGF-βs. Neuron 10:293-305.
Manglapus GL, Youngentob SL, Schwob JE (2004) Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. J Comp Neurol 479:216-233.
Murrell W, Wetzig A, Donnellan M, Féron F, Burne T, Meedeniya A,Kesby J, Bianco J, Perry C, Silburn P, Mackay-Sim A (2008) Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson’s disease. Stem Cells 26:2183-2192.
Newman MP, Féron F, Mackay-Sim A (2000) Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience 99:343-350.
Nisbet MC, Brossard D, Kroepsch A (2003) Framing Science -- the Stem Cell Controversy in an Age of Press/politics [WWW Document]. eweb:258154. URL https://repository.library.georgetown.edu/handle/10822/1001823 (accessed 12.16.16).
Ogawa Y, Sawamoto K, Miyata T, Miyao S, Watanabe M, Nakamura M, Bregman BS, Koike M, Uchiyama Y, Toyama Y, Okano H (2002)Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res 69:925-933.
Ohnishi Y, Iwatsuki K, Shinzawa K, Ishihara M, Moriwaki T, Umegaki M, Kishima H, Yoshimine T (2013) Adult olfactory sphere cells are a source of oligodendrocyte and Schwann cell progenitors. Stem Cell Res 11:1178-1190.
Okano H (2002) Stem cell biology of the central nervous system. J Neurosci Res 69:698-707.
Pandit SR, Sullivan JM, Egger V, Borecki AA, Oleskevich S (2011)Functional effects of adult human olfactory stem cells on early-onset sensorineural hearing loss. Stem Cells 29:670-677.
Parr AM, Kulbatski I, Wang XH, Keating A, Tator CH (2008) Fate of transplanted adult neural stem/progenitor cells and bone marrow-derived mesenchymal stromal cells in the injured adult rat spinal cord and impact on functional recovery. Surg Neurol 70:600-607.
Pixley SK (1992) CNS glial cells support in vitro survivial, division, and differentiation of dissociated olfactory neuronal progenitor cells.Neuron 8:1191-1204.
Prockop DJ (2003) Further proof of the plasticity of adult stem cells and their role in tissue repair. J Cell Biol 160:807-809.
Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L, Keating A (2010) Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 12:576-578.
Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW (2010) Sox2 induces neuronal formation in the developing mammalian cochlea. J Neurosci 30:714-722.
Raisman G (2004) Olfactory ensheathing cells and repair of brain and spinal cord injuries. Cloning Stem Cells 6:364-368.
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system.Science 255:1707-1710.
Roisen FJ, Klueber KM, Lu CL, Hatcher LM, Dozier A, Shields CB, Maguire S (2001) Adult human olfactory stem cells. Brain Res 890:11-22.
Sakakibara S, Imai T, Hamaguchi K, Okabe M, Aruga J, Nakajima K,Yasutomi D, Nagata T, Kurihara Y, Uesugi S, Miyata T, Ogawa M,Mikoshiba K, Okano H (1996) Mouse-Musashi-1, a neural rna-binding protein highly enriched in the mammalian CNS stem cell. Dev Biol 176:230-242.
Sandhu MS, Ross HH, Lee KZ, Ormerod BK, Reier PJ, Fuller DD (2017)Intraspinal transplantation of subventricular zone-derived neural progenitor cells improves phrenic motor output after high cervical spinal cord injury. Exp Neurol 287:205-215.
Sandner B, Ciatipis M, Motsch M, Soljanik I, Weidner N, Blesch A(2016) Limited functional effects of subacute syngeneic bone marrow stromal cell transplantation after rat spinal cord contusion injury. Cell Transplant 25:125-139.
Satoh M, Takeuchi M (1995) Induction of NCAM expression in mouse olfactory keratin-positive basal cells in vitro. Dev Brain Res 87:111-119.
Schnittke N, Herrick DB, Lin B, Peterson J, Coleman JH, Packard AI,Jang W, Schwob JE (2015) Transcription factor p63 controls the reserve status but not the stemness of horizontal basal cells in the olfactory epithelium. Proc Natl Acad Sci U S A 112:E5068-5077.
Schultz EW (1960) Repair of the olfactory mucosa with special reference to regeneration of olfactory cells (sensory neurons). Am J Pathol 37:1-19.
Schwob JE, Youngentob SL, Mezza RC (1995) Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol 359:15-37.
Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS (2010) Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells 28:152-163.
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146-156.
Smith CG (1951) Regeneration of sensory olfactory epithelium and nerves in adult frogs. Anat Rec 109:661-671.
Sun Y, Xu CC, Li J, Guan XY, Gao L, Ma LX, Li RX, Peng YW, Zhu GP(2013) Transplantation of oligodendrocyte precursor cells improves locomotion deficits in rats with spinal cord irradiation injury. PLoS One 8:e57534.
Tharion G, Indirani K, Durai M, Meenakshi M, Devasahayam SR,Prabhav NR, Solomons C, Bhattacharji S (2011) Motor recovery following olfactory ensheathing cell transplantation in rats with spinal cord injury. Neurol India 59:566-572.
Torres-Espín A, Corona-Quintanilla DL, Forés J, Allodi I, González F, Udina E, Navarro X (2013) Neuroprotection and axonal regeneration after lumbar ventral root avulsion by re-implantation and mesenchymal stem cells transplant combined therapy. Neurotherapeutics 10:354-368.
Tso D, McKinnon RD (2015) Cell replacement therapy for central nervous system diseases. Neural Regen Res 10:1356-1358.
Tuszynski MH, Gabriel K, Gage FH, Suhr S, Meyer S, Rosetti A (1996)Nerve growth factor delivery by gene transfer induces differential outgrowth of sensory, motor, and noradrenergic neurites after adult spinal cord injury. Exp Neurol 137:157-173.
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8:726-736.
Vawda R, Fehlings MG (2013) Mesenchymal cells in the treatment of spinal cord injury: current & future perspectives. Curr Stem Cell Res Ther 8:25-38.
Viktorov IV, Savchenko EA, Ukhova OV, Alekseyeva NY, Chekhonin VP (2006) Multipotent stem and progenitor cells of the olfactory epithelium. Bull Exp Biol Med 142:495-502.
Wayne M, Gillian RB, Jonathon L, John M, Kelli PA, Paul RB, Alan MS(1996) Neurogenesis in adult human. Neuroreport 7:138.
Winstead W, Marshall CT, Lu CL, Klueber KM, Roisen FJ (2005) Endoscopic biopsy of human olfactory epithelium as a source of progenitor cells. Am J Rhinol 19:83-90.
Xiao M, Klueber KM, Lu C, Guo Z, Marshall CT, Wang H, Roisen FJ(2005) Human adult olfactory neural progenitors rescue axotomized rodent rubrospinal neurons and promote functional recovery. Exp Neurol 194:12-30.
Xiao M, Klueber KM, Zhou J, Guo Z, Lu C, Wang H, Roisen FJ (2007)Human adult olfactory neural progenitors promote axotomized rubrospinal tract axonal reinnervation and locomotor recovery. Neurobiol Dis 26:363-374.
Ye J, Qin Y, Wu Y, Wang P, Tang Y, Huang L, Ma M, Zeng Y, Shen H(2016) Using primate neural stem cells cultured in self-assembling peptide nanofiber scaffolds to repair injured spinal cords in rats. Spinal Cord 54:933-941.
Zhang X, Klueber KM, Guo Z, Lu C, Roisen FJ (2004) Adult human olfactory neural progenitors cultured in defined medium. Exp Neurol 186:112-123.
- 中国神经再生研究(英文版)的其它文章
- The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction
- Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogenactivated protein kinase and nuclear factor kappa B signaling pathways
- Taking out the garbage: cathepsin D and calcineurin in neurodegeneration
- MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury
- Interferon regulatory factor 2 binding protein 2: a new player of the innate immune response for stroke recovery
- Endogenous retinal neural stem cell reprogramming for neuronal regeneration

