Effects of neuregulin-1 on autonomic nervous system remodeling post-myocardial infarction in a rat model
Xin Lai, Liang Zhong, Hai-xia Fu, Song Dang Xin Wang Ning Zhang Gao-ke Feng Zi-qiang Liu Xi Wang,Long Wang
1 Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Cardiovascular Research Institute, Wuhan University, Wuhan, Hubei Province, China
3 Hubei Key Laboratory of Cardiology, Wuhan, Hubei Province, China
4 Wuhan Medical & Healthcare Center for Women and Children, Wuhan, Hubei Province, China
5 Department of Cardiology, Henan Province People’s Hospital, Zhengzhou, Henan Province, China
How to cite this article:Lai X, Zhong L, Fu HX, Dang S, Wang X, Zhang N, Feng GK, Liu ZQ, Wang X, Wang L (2017) Effects of neuregulin-1 on autonomic nervous system remodeling post-myocardial infarction in a rat model Neural Regen Res 12(11):1905-1910.
Funding: This work was supported by a grant from the National Key Basic Research Development Program, the “973” Program, No.2012CB518604; the National Natural Science Foundation of China, No. 81260052; the Natural Science Foundation of Hubei Province, No.2014CKB497, 2014BKB075, and 2015BKA339; and the Natural Science Foundation of Henan Province of China, No. 201602262.
Introduction
Neuregulin-1 (NRG-1), a member of the epidermal growth factor family, plays an important regulatory role in the development and repair of the nervous system (Murphy et al.,2002; Lemmens et al., 2007; Melenhorst et al., 2008; Ding et al., 2014; Yamada et al., 2014). Moreover, some scholars believe that NRG-1 and its receptors (ErbB2, ErbB3, and ErbB4) are crucial in cardiac development (Mei and Xiong,2008; Pasca et al., 2014). NRG-1 has been found to be able to induce hypertrophy and inhibit cell apoptosis in rat ventricular myocytes (Zhao et al., 1998; Baliga et al., 1999;Rohrbach et al., 1999). Recent studies also found that NRG-1 activates tyrosine kinase, thus causing various cardiovascular biological effects, including regulating the structure and function of cardiomyocytes and its apoptosis and proliferation, and promoting angiogenesis (Odiete et al., 2012;Mendes-Ferreira et al., 2013). Experimental studiesin vivohave found that NRG-1 can significantly improve acute and chronic ischemic cardiomyopathy and myocarditis, and phase II clinical trials revealed that the short-term administration of recombinant human NRG-1 can improve chronic cardiac function in patients with heart failure (Gao et al.,2010; Jabbour et al., 2011). However, phase III clinical trials of recombinant human NRG-1 for heart failure are ongoing.Notably, in patients with breast cancer accepting anti-ErbB2 monoclonal antibody trastuzumab treatment, heart failure occurred partially (Feldman et al., 2000; Schneider et al.,2001). Therefore, NRG-1 and its receptors are being considered gradually for the cardiovascular system.
A large amount of norepinephrine is released from sympathetic nerves during acute myocardial ischemia, and sympathetic sprouting and sympathetic remodeling reach a peak at 1 week after myocardial infarction (MI) (Wu et al., 2012;Holmes et al., 2016; Prabhu and Frangogiannis, 2016). At the same time, sympathetic nerve reinnervated by budding results in sympathetic hyperinnervation, and this imbalanced innervation plays an important role in the occurrence of ventricular arrhythmias (Li et al., 2004; Zhou et al., 2004;Boogers et al., 2010; Alston et al., 2011). Meanwhile, vagus nerve injury, necrosis, regeneration, and reconstruction also occur post-MI (Suo et al., 2013).
Most studies focused on the roles of NRG-1 in nerve repair and neurohumoral regulation of heart injuries (Monje et al., 2006; Calvo et al., 2010; Cahill et al., 2013), but the effects of NRG-1 on the neural remodeling post-MI remain unclear. We hypothesized that NRG-1 might play a role in neural remodeling following MI. Thus, we prepared a rat model of MI to investigate how NRG-1 impacts cardiac function and neural remodeling after MI.
Materials and Methods
Animals
Forty-five adult male Sprague-Dawley rats aged 12 weeks and weighing 250–300 g were provided by the Animal Experiment Center of Wuhan University in China (No.42000500005679). Rats were housed in temperature-controlled and humidity-controlled large cages with sawdust bedding and given access to tap water and foodad libitumfor 7 days. All animal care and experimental procedures were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 85-23, revised 1986).This research was approved by the Administration Committee of Experimental Animals, Hubei Province, China. Rats were randomized into three groups (n= 15 per group): sham operation group, MI group, and MI plus NRG-1 group.
Establishment of the MI model
The left anterior descending coronary artery of the rats in the MI and MI plus NRG-1 groups was ligated to establish the MI model, as previously described (Wang et al., 2014). In brief, after anesthesia with 3.6% chloral hydrate (1 mL/100 g, Sinopharm Chemical Reagent Co., Ltd, Shanghai, China)was administered, the rats were connected to the electrocardiograph and then disinfected. We cut the skin along the left side of the sternum; separated fascia and muscle with hemostatic forceps; performed thoracotomy at the third and fourth ribs to expose the mediastinum and pericardium;found the left atrial appendage, pulmonary artery cones,and veins; identified the left anterior descending coronary artery and ligated it using a 5-0 (or 6-0) suture; and closed the chest and skin layer by layer. A successful MI model was confirmed when the color of the ischemic area became pale and ST-segment elevation was detected in leads I, II,and aVL by electrocardiography. In the sham group, rats received the same procedures, except leftanterior descending coronary artery ligation.
Experimental interventions
Rats in the MI plus NRG-1 group were given an intraperitoneal injection of NRG-1 (10 µg/kg) after MI, once daily for 7 consecutive days (Lemmens et al., 2011). At 4 weeks after MI, echocardiography was performed to evaluate cardiac function, and myocardial tissues from the infarct border zone were collected to detect the levels of tyrosine hydroxylase (TH), growth associated protein-43 (GAP43), nerve growth factor (NGF), choline acetyltransferase (CHAT), and vesicular acetylcholine transporter (VACHT) in the assessment of neural remodeling (Ajijola et al., 2015).
Assessment of heart function
Heart function was tested using the Philips IE33 ultrasound system (GE Healthcare, Milwaukee, WI, USA) at 4 weeks post-MI. Each rat was measured under anesthesia,and the indexes included the left ventricular end-systolic inner diameter (LVESD), leftventricular diastolic diameter(LVEDD), leftventricular end-systolic volume (LVESV), leftventricular end-diastolic volume (LVEDV), leftventricular ejection fraction (LVEF%), and left ventricular fractional shortening (LVFS%) were recorded and measured. The following equation was used: LVEF% = (LVEDD3– LVESD3)/LVEDD3× 100% and LVFS% = (LVEDD – LVESD)/LVEDD × 100%.(1:500; Abcam), anti-CHAT (1:500; Bioss, Beijing, China),anti-VACHT (1:500; Abcam), or anti-GAPDH (1:10,000;Abcam) overnight at 4°C. After the membrane was washed three times, the blots were incubated with secondary HRP conjugated goat anti-rabbit antibody (1:10,000; Pierce,Rockford, IL, USA) for 30 minutes at room temperature.Membranes were detected by enhanced chemiluminescence(Beyotime Biotechnology, Jiangsu, China) and exposed to film in the dark. The optical density intensity of each band was measured using AlphaEaseFC so ft ware (Alpha Innotech
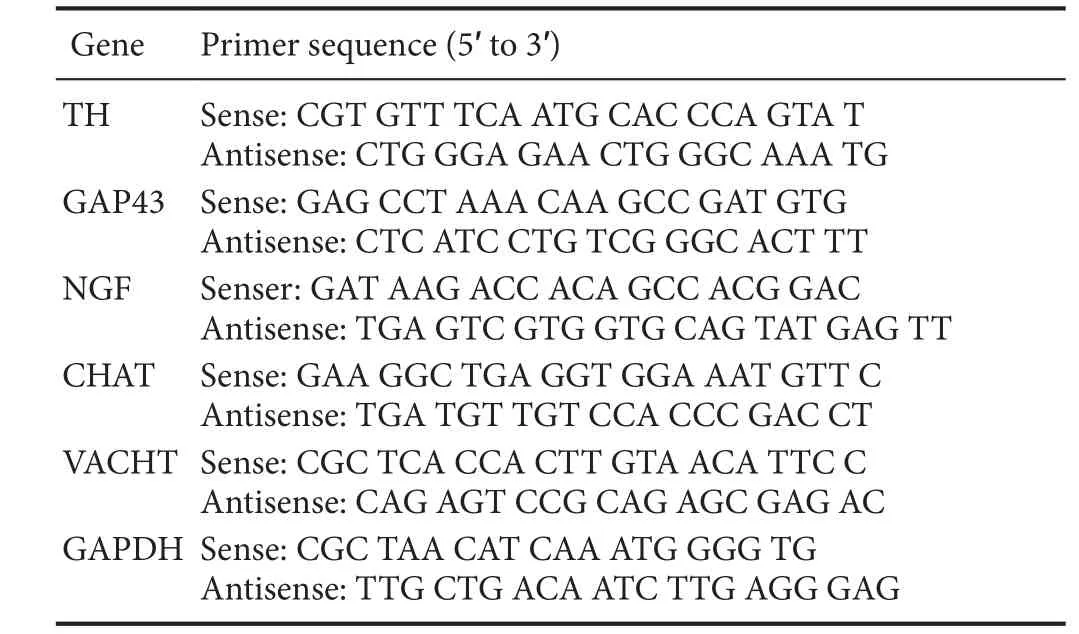
Table 1 Gene sequences
Measurements of the neural remodeling markers (TH,GAP43, NGF, CHAT, and VACHT)
Quantitative real-time polymerase chain reaction (qRT-PCR)Approximately 100 mg of myocardial tissues from the infarct border zone were collected at 4 weeks after MI. Total RNA was extracted by Trizol reagent (15596-026; Invitrogen, Carlsbad, CA, USA), cDNA was obtained with the First Strand cDNA Synthesis Kit (FSK-100; Toyobo, Kita-ku,Osaka, Japan), and then the fragments were amplified with the SYBR Green-based assays kit (Invitrogen) according to the manufacturer’s instructions. Next, qRT-PCR was conducted using the StepOne™ Real-Time PCR System(ABI, Life Technologies, Rockville, MD, USA). RT-PCR conditions were 42°C/30 minutes and 80°C/5 minutes for reverse transcription; 95°C/60 seconds for pre-denature; and 95°C/15 seconds, 58°C/20 seconds, and 72°C/20 seconds.All steps were performed for 40 cycles. The gene sequences are shown inTable 1. The mRNA expression levels of TH,GAP43, NGF, CHAT, VACHT, and GAPDH were detected,and GAPDH was used for normalization. The relative gene expression in the sample was calculated as 2-ΔΔCT. Experiments were performed in triplicate.
Western blot assay
Fifty µg of protein was extracted from the ischemic myocardial tissues at 4 weeks after MI. The tissues were lysed in radioimmunoprecipitation assay (AS1004, Aspen, Wuhan,China), and lysis buffer and protein concentrations were determined using the BCA kit (AS1086, Aspen). The proteins were used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. The membranes were blocked by incubation with 5%bovine serum albumin in a TBS-Tween buffer (10 mM Tris-HCl, 150 mM NaCl, and 0.5% Tween-20) for 1 hour at room temperature, and subsequently, incubated with different primary antibodies: rabbit anti-TH (Santa, Shanghai, China),anti-GAP43 (1:1,000; Abcam, Cambridge, UK), anti-NGF Corp., San Leandro, CA, USA). Results are shown as the optical density ratio to GAPDH.
Statistical analysis
All data were analyzed by SPSS 16.0 (SPSS Inc., Chicago, IL,USA). Values are presented as the mean ± SD. The statistical significance of differences was analyzed using one-way analysis of variance with the Student-Newman-Keulspost hoctest for comparisons among groups. Values ofP< 0.05 were considered statistically significant.
Results
Effect of NRG-1 on cardiac function
At 4 weeks after MI, echocardiography results showed significant differences in LVESD, LVEDD, LVESV, LVEDV,LVEF%, and LVFS% among groups. Compared with the sham group, the MI group showed a significant deterioration in cardiac function; that is, LVESD, LVEDD, LVESV,and LVEDV were significantly increased, and LVEF% and LVFS% were significantly decreased (P< 0.05). After NRG-1 injection, LVESD, LVEDD, LVESV, and LVEDV significantly decreased; whereas, LVEF%, and LVFS% significantly increased (P< 0.05), suggesting a significant improvement in cardiac function (Table 2).
Effect of NRG-1 on the mRNA levels of neural remodeling markers
The mRNA expression levels of TH, GAP43, NGF, CHAT,and VACHT were significantly increased in the MI and MI plus NRG-1 groups compared with the sham group (P<0.05). Compared with the MI group, there was a significant decrease in mRNA expression levels of TH, GAP43, and CHAT in the MI plus NRG-1 group (P< 0.05). Simultaneously, there was a decreasing trend in NGF and VACHT mRNA expression levels (P> 0.05;Table 3).
Effect of NRG-1 on the protein levels of neural remodeling markers
The protein expression levels of TH, GAP43, NGF, CHAT,and VACHT were significantly increased in the MI and MI plus NRG-1 groups compared with the sham group (P<0.05). The protein expression levels of TH and GAP43 in the ischemic myocardia in the MI plus NRG-1 group were significantly lower than those in the MI group (P< 0.05); the protein expression levels of NGF, CHAT, and VACHT were slightly lower in the MI plus NRG-1 group than those in the MI group (P> 0.05;Table 4andFigure 1).
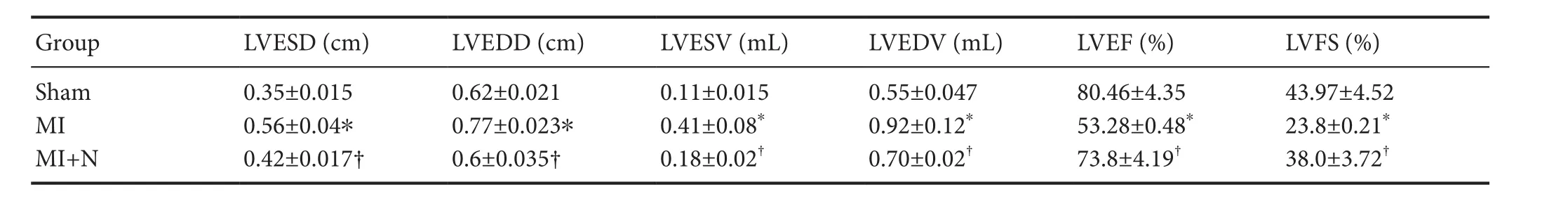
Table 2 Effect of NRG-1 on cardiac function in rats after MI
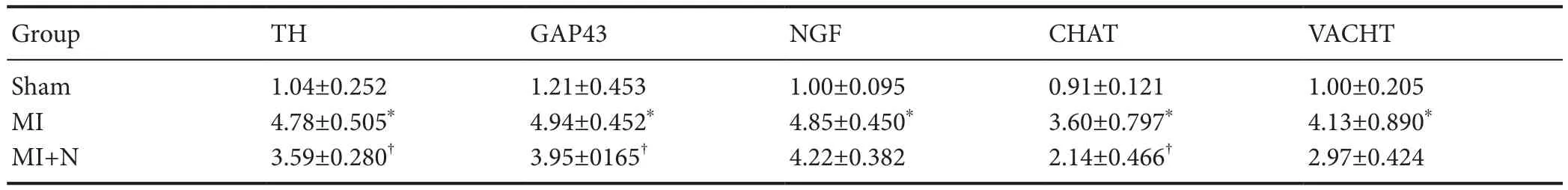
Table 3 Effect of NRG-1 on relative mRNA levels (/GAPDH) of neural remodeling markers in rat ischemic myocardia
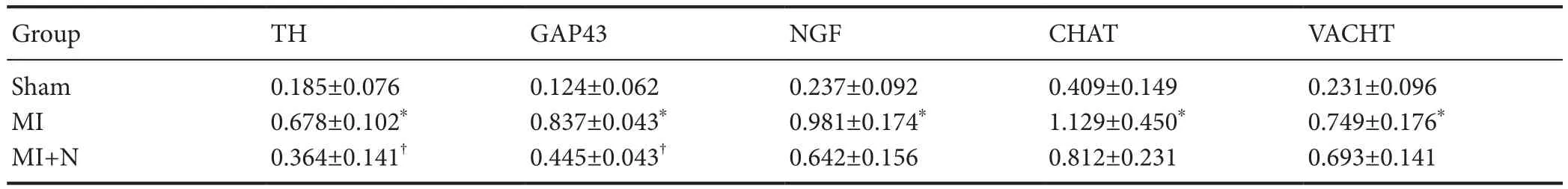
Table 4 Effect of NRG-1 on protein levels (/GAPDH) of neural remodeling markers in rat ischemic myocardia
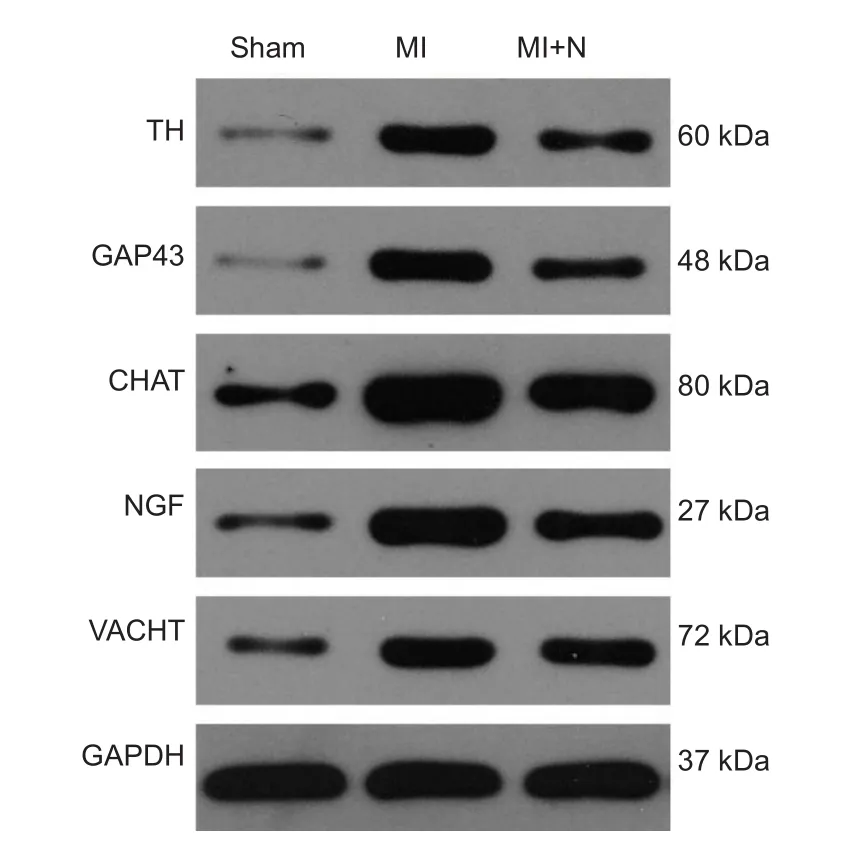
Figure 1 NRG-1 injection regulated protein levels of neural remodeling markers in rat ischemic myocardia.Target protein expression was analyzed by western blot assay with antibodies against TH, GAP43, NGF, CHAT, and VACHT relative to GAPDH. MI: Myocardial infarction; N: neuregulin-1; TH: tyrosine hydroxylase; GAP43: growth associated protein-43; NGF: nerve growth factor; CHAT: choline acetyltransferase; VACHT: vesicular acetylcholine transporter; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Discussion
Some studies have shown that in the NRG-1 knockout mouse model of MI, cardiac systolic function was significantly impaired, but it was improved significantly after NRG-1 intervention (McCormick et al., 2015; Vandekerckhove et al., 2016; Yasui et al., 2016; Zhang et al., 2016). In an MI model, mitochondrial dysfunction and apoptosis were obviously reduced after intravenous administration of NRG-1, thereby reducing leftventricular remodeling post-injury(Guo and Wang, 2012). Gu et al. (2010) found that NRG-1 significantly up-regulated cardiac myosin light chain kinase and myosin light chain phosphorylation post-MI; the values of LVEF%, LVFS%, LVEDD, and LVESD were significantly increased, and cardiac function was significantly improved.In the current study, we observed that NRG-1 improved cardiac function post-MI.
NRG-1 can promote the proliferation of Schwann cells and increase the density and area of dendritic spines of neurons (Limpert and Carter, 2010). For peripheral nerves,NRG-1 promotes myelination and plays a significant role in axonal degeneration, axonal regeneration, remyelination,and innervation (Gambarotta et al., 2013; Shin et al., 2014).It has been reported that sympathetic nerve hyperinnervation and denervation result in neural remodeling, but generally, sympathetic nerve remodeling is also accompanied with vagus nerve remodeling (Yu et al., 2010). Additionally, our study found sympathetic and vagus nerve remodeling after MI, which was expressed as a significantly increased GAP43 level, as well as increased CHAT and VACHT levels.
TH was generated by norepinephrine and located in the cytoplasm of adrenergic nerve fibers. GAP43, a kind of neuronal specific protein, exists in axons, which marks neuronal growth by neuronal synthesis. NGF can promote the growth of neuritis and induce cardiac sympathetic hyperinnervation (Lu et al., 2012). Chen et al. (2014) reported that TH,GAP43, and NGF significantly increased post-MI as confirmed by western blot analysis and immunohistochemistry,and aerobic exercise inhibited the cardiac sympathetic nerve sprouting and restored B1-AR/B3-AR balance. Our study’s results also showed that the expression levels of TH, GAP43,and NGF were significantly increased post-MI, but after the NRG-1 intervention, their expression levels decreased,suggesting that NRG-1 can inhibit sympathetic remodeling
post-MI. CHAT is a vagus nerve marker. Suo et al. (2013)found that CHAT-positive nerve fiber density and its mRNA expression level were higher post-MI. Besides, VACHT is a cardiac vagal nerve fiber marker. Xi et al. (2004) reported that vagal innervation density appeared to significantly increase after MI. In the current study, after the NRG-1 intervention, the expression levels of CHAT and VACHT were not significantly different when compared with those in the MI group post-MI, except the mRNA level of CHAT, implying that NRG-1 may not inhibit vagus nerve regeneration and repair following MI.
In summary, NRG-1 intervention effectively down-regulates sympathetic nerve mRNA and protein expression levels, thus inhibiting the sympathetic nerve remodeling post-MI, which reaches a new equilibrium of the autonomic nervous system to protect cardiac function by reducing sympathetic nerve tension. But the further mechanism of nerve remodeling is not involved in the current study, and we will explore the possible mechanisms in the further research.
Author contributions:XW, LW and XL participated in study conception and design. GKF, LZ and XL collected and analyzed data, and ensured the integrity of the data. HXF, SD and XL wrote the paper. XW,NZ, ZQL and XL served as principle investigators, and were in charge of paper authorization. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:The study protocol was approved by Administration Committee of Experimental Animals of Wuhan University of China. All animal care and animal surgeries were in accordance with the Guide for Care and Use of Laboratory Animals (Public Health Service, 1996, NIH Publication No. 85-23).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Ajijola OA, Yagishita D, Reddy NK, Yamakawa K, Vaseghi M, Downs AM, Hoover DB, Ardell JL, Shivkumar K (2015) Remodeling of stellate ganglion neurons following spatially targeted myocardial infarction: neuropeptide and morphologic changes. Heart Rhythm 12:1027-1035.
Alston EN, Parrish DC, Hasan W, Tharp K, Pahlmeyer L, Habecker BA (2011) Cardiac ischemia-reperfusion regulates sympathetic neuropeptide expression through gp130-dependent and independent mechanisms. Neuropeptides 45:33-42.
Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA,Sawyer DB, and Kelly RA (1999) NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70 (S6K), and MEK-MAPKRSK. Am J Physiol Heart Circ Physiol 277:2026-2037.
Billman GE (2009) Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol Heart Circ Physiol 297:H1171-1193.
Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, Dibbets- Schneider P, Stokkel MP, van der Wall EE, Schalij MJ, Bax JJ (2010) Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzyl guanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol 55:2769-2777.
Cahill ME, Remmers C, Jones KA, Xie Z, Sweet RA, Penzes P (2013)Neuregulin1 signaling promotes dendritic spine growth through kalirin. J Neurochem 126:625-635.
Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist J, Loeb JA, Bennett DL(2010) Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci 30:5437-5450.
Chen T, Cai MX, Li YY, He ZX, Shi XC, Song W, Wang YH, Xi Y,Kang YM, Tian ZJ (2014) Aerobic exercise inhibits sympathetic nerve sprouting and restores b-adrenergic receptor balance in rats with myocardial infarction. PLoS One 9:e97810.
Ding D, Liang J, Zhang JF (2014) Schwann cells induce the differentiation of neuregulin-1-transfected bone marrow stromal stem cells into neuron-like cells. Zhongguo Zuzhi Gongcheng Yanjiu 18:8061-8065.
Feldman AM, Lorell BH, Reis SE (2000) Trastuzumab in the treatment of metastatic breast cancer: anticancer therapy versus cardiotoxicity. Circulation 102:272-274.
Gambarotta G, Fregnan F, Gnavi S, Perroteau I (2013) Neuregulin 1 role in Schwann cell regulation and potential applications to promote peripheral nerve regeneration. Int Rev Neurobiol 108:223-256.
Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, Li T, Liu X, Xu Y,Li X, Zou M (2010) A Phase II, randomized, double blind, muhicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol 55:1907-1914.
Gu XH, Liu XF, Xu DL, Li XY, Yan M, Qi Y, Yan WH,Wang WQ,Pan J, Xu Y, Xi B, Cheng LL, Jia JG, Wang KQ, Ge JB, Zhou MD(2010) Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc Res 88:334-343.
Guo YF, Wang X (2012) NRG-1 attenuates mitochondrial dysfunction in a rat model of heart failure. Chin Med J 125:807-814.
Holmes JW, Laksman Z, Gepsteinc L (2016) Making better scar:emerging approaches for modifying mechanical and electrical properties following infarction and ablation. Prog Biophys Mol Biol 120:134-148.
Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, Amor R, Liu X, Li XY, Zhou MD, Graham RM (2011)Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail 13:83-92.
Lemmens K, Doggen K, De Keulenaer GW (2007) Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease:implications for therapy of heart failure. Circulation 116:954-960.
Lemmens K, Doggen K, de Keulenaer DW (2011) Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol 300:H931-942.
Li W, Knowlton D, van Winkle DM, Habecker BA (2004) Infarction alters both the distribution and nonadreneigic properties of cardiac sympathetic neurons. Am J Physiol Heart Circ Physiol 286:H2229-2236.
Limpert AS, Carter BD (2010) Axonal neuregulin 1 type III activates NF-κB in Schwann cells during myelin formation. J Biol Chem 285:16614-16622.
Lu J, Gao X, Gu J, Zhou L, Guo S, Hao W, Zhou Z, Cao JM (2012).Nerve sprouting contributes to increased severity of ventricular tachyarrhythmias by upregulating iGluRs in rats with healed myocardial necrotic injury. J Mol Neurosci 48:448-455.
McCormick ME, Collins C, Makarewich CA, Chen ZM, Rojas M,Willis MS, Houser SR, Tzima E (2015). Platelet endothelial cell adhesion molecule-1 mediates endothelial-cardiomyocyte communication and regulates cardiac function. J Am Heart Assoc 4:e001210.
Mei L, Xiong WC (2008) Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci 9:437-452.
Melenhorst WB, Mulder GM, Xi Q, Hoenderop JG, Kimura K,Eguchi S, van Goor H (2008) Epidermal growth factor receptor signaling in the kidney: key roles in physiology and disease. Hypertension 52:987-993.
Mendes-Ferreira P, de Keulenaer GW, Leite-Moreira AF, Brás-Silva C (2013) Therapeutic potential of neuregulin-1 in cardiovascular disease. Drug Discov Today 18:836-842.
Monje PV, Bartlett Bunge M, Wood PM (2006) Cyclic AMP synergistically enhances neuregulin-dependent ERK and Akt activation and cell cycle progression in Schwann cells. Glia 53:649-659.
Murphy S, Krainock R, Tham M (2002) Neuregulin signaling via erbB receptor assemblies in the nervous system. Mol Neurobiol 25:67-77.
Odiete O, Hill MF, Sawyer DB (2012) Neuregulin in cardiovascular development and disease. Circ Res 111:1376-1385.
Pasca D, Giovannelli A, Gnavi S, Hoyng SA, de Winter F, Morano M, Fregnan F, Dell’Albani P, Zaccheo D, Perroteau I, Pellitteri R,Gambarotta R (2014) Characterization of glial cell models and in vitro manipulation of the neuregulin1/ErbB system. Biomed Res Int 2014:310215.
Prabhu SD, Frangogiannis NG (2016) The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis.Circ Res 119:91-112.
Ren CY, Wang FZ, Li G, Jiao Q, Bai J, Yu DJ, Hao W, Wang R, Cao JM (2008) Nerve sprouting suppresses myocardial I-to and I-K1 channels and increases severity to ventricular fibrillation in rat.Auton Neurosci 144:22–29.
Rohrbach S, Yan X, Weinberg EO, Hasan F, Bartunek J, Marchionni MA, Lorell BH (1999) Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation 100:407-412.
Schneider JW, Chang AY, Rocco TP (2001) Cardiotoxicity in signal transduction therapeutics: erbB2 antibodies and the heart. Semin Oncol 28:18-26.
Shin YK, Jang SY, Park SY, Park JY, Kim JK, Kim JP, Suh DJ, Lee HJ,Park HT (2014) Grb2-associated binder-1 is required for neuregulin-1-induced peripheral nerve myelination. J Neurosci 34:7657-7662.
Snider WD (1994) Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77:627-638.
Suo F, Hu HS, Xue M, Wang Y, Xuan YL Yan SH (2013) Relationship between neurturin dynamic expression after myocardial infarction and cardiac vagal neural remodeling. Shandong Daxue Xuebao 51:1-5.
Vandekerckhove L, Vermeulen Z, Liu ZZ, Boimvaser S, Patzak A,Segers V, Keulenaer G (2016) Neuregulin-1 attenuates development of nephropathy in a type 1 diabetes mouse model with high cardiovascular risk. Am J Physiol Endocrinol Metab 310:E495–504.
Wang X, Lv H, Gu YW, Wang X, Cao H, Tang YH, Chen H, Huang CH (2014) Protective effect of lycopene on cardiac function myocardial fibrosis after acute myocardial infarction in rats via the modulation of p38 and MMP-9. J Mol Hist 45:113-120.
Wu XL, Jiang H, Yu LL, Hu XR, Liu WW (2012) Desipramine pretreatment improves sympathetic remodeling and ventricular fibrillation threshold after myocardial ischemia. J Biomed Biotechnol 2012:732909.
Xi Y, Yu YW, Qu XF, Li JJ, Huang YL (2004) Study on vagus-remodeling and propranolol effects on it after myocardial infarction.Chin J Cardiol 32:257-259.
Yamada S, Marutsuka M, Inoue M, Zhang J, Abe SI, Ishibashi KI,Yamaguchi N, Eto I (2014) The interaction of the ErbB4 intracellular domain p80 with α-enolase in the nuclei is associated with the inhibition of the neuregulin1-dependent cell proliferation. Int J Biochem Mol Biol 5:21-29.
Yasui T, MasakiM T, Arita Y, Ishibashi T, Inagaki T, Okazawa M,Oka T, Shioyama W, Keiko Yamauchi-Takihara K, Komuro I,Sakata Y, Nakaoka Y (2016) Molecular characterization of striated muscle-specific Gab1 isoform as a critical signal transducer for neuregulin-1/ErbB signaling in cardiomyocytes. PLoS One 11:e0166710.
Yu HS, Zhang Y, Feng Y, Zhang L, Ma YH, Song W, Hou YM (2010)Nerve remodeling in a canine model of artial fibrillation induced by 48 hours right artial pacing. Chin J Cardiol 38:644-647.
Zhang YT, Hodgson N, Trivedi M, Deth R (2016) Neuregulin 1 promotes glutathione-dependent neuronal cobalamin metabolism by stimulating cysteine uptake. Oxid Med Cell Longev 2016:3849087.
Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA,and Kelly RA (1998) Neuregulins promote survival and growth of cardiac myocytes, persistence of erbB2 and erbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem 273:10261-10269.
Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, SharifiB, Chen PS (2004) Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 95:76-83.
- 中国神经再生研究(英文版)的其它文章
- The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction
- Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogenactivated protein kinase and nuclear factor kappa B signaling pathways
- Taking out the garbage: cathepsin D and calcineurin in neurodegeneration
- MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury
- Interferon regulatory factor 2 binding protein 2: a new player of the innate immune response for stroke recovery
- Endogenous retinal neural stem cell reprogramming for neuronal regeneration

