Delayed degeneration of an injured spinothalamic tract in a patient with diffuse axonal injury
Diffuse axonal injury (DAI) is one of the devastating mechanisms of traumatic brain injury (TBI) and shows extensive subcortical lesions due to shearing forces induced by rapid acceleration-deceleration and rotation of the brain (Adams et al., 1982;Meythaler et al., 2001). As a result, conventional brain imaging techniques including brain MRI have been limited in demonstrating neuronal degeneration in patients with DAI. However,diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI) has enabled three-dimensional reconstruction of the neural tracts (Jang and Seo, 2014). Since the introduction of DTI, several studies have demonstrated neuronal degeneration after TBI (Tomaiuolo et al., 2005; Hong and Jang, 2010; Kwon and Jang, 2014). However, little is known about delayed neuronal degeneration in patients with TBI.
The spinothalamic tract (STT) is the somatosensory neural tract responsible for pain and touch from the contralateral extremities and body (Hong et al., 2011). Many studies have reported on injury of the STT in patients with brain injury (Goto et al., 2008;Hong et al., 2012; Seo and Jang, 2013, 2014; Kim et al., 2015). In addition, injury of the STT has been suggested as the most plausible pathogenetic mechanism of central pain. However, to the best of our knowledge, no DTT study on delayed degeneration of an injured STT following DAI has been reported.
In this study, we reported on a patient who showed delayed degeneration of an injured STT following DAI, which was demonstrated with DTI.
A 27-year-old male who had suffered from head trauma resulting from colliding with a truck while sitting in a passenger seat in a sedan experienced loss of consciousness for 3 months.He underwent a compressive operation for a depressed skull fracture in the leftfrontal lobe. Brain MRI at onset corresponded to DAI (Figure 1A): grade 3 (hemorrhagic DAI lesions in the corpus callosum and pons) (Adams et al., 1982; Parizel et al., 1998). He had begun to feel pain in both hands and legs, and trunk since approximately 5 years after onset of head trauma.The characteristics and severity of pain were as follows: constant tingling and pricking sensation without allodynia or hyperalgesia (Visual Analogue Scale score: 3). Somatosensory function was determined using the subscales for tactile sensation and kinesthetic sensation of the Nottingham Sensory Assessment was normal (Lincoln et al., 1998). The electromyography study showed no evidence of peripheral nerve injury or radiculopathy. The study protocol was approved by the institutional review board of Yeungnam University Hospital (YUMC 2015-07-064).
DTI data were acquired twice (1 and 7 years after onset) using a 6-channel head coil on a 1.5T Philips Gyroscan Intera (Philips,Ltd., Best, the Netherlands) with 32 gradients. Imaging parameters were as follows: acquisition matrix = 96 × 96, reconstructed to matrix = 192 × 192, field of view = 240 × 240 mm2, repetition time = 10,398 ms, echo time = 72 ms,b= 1,000 s/mm2, and a slice thickness of 2.5 mm. Analysis of DTI data was performed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library (FSL:www.fmrib.ox.ac.uk/fsl) based on the probabilistic tractography method (5,000 streamline samples, 0.5 mm step lengths, curvature thresholds = 0.2) (Smith et al., 2004). For fiber tracking of the STT, the seed region of interest was placed on the isolated STT area (posterolateral to the inferior olivary nucleus and anterior to the inferior cerebellar peduncle in the medulla) (Jang and Kwon, 2013). Two target regions of interest were placed on the portion of the ventro-postero-lateral nucleus of the thalamus and primary somatosensory cortex on the axial images (Jang and Kwon, 2013). The threshold of 2 streamlines was applied for the results of fiber tracking.
Partial tearing at the subcortical white matter in both STTs observed on 1-year DTT and both partially torn STTs showed severe degeneration at the subcortical white matter on 7-year DTT (Figure 1B).
In this study, the STT was followed up on DTT in a patient who showed delayed onset of central pain which had started 5 years after head trauma. On this patient’s 1-year DTT, both STTs had already sustained mild injury because partial tearing was observed at the subcortical white matter. Because the conventional brain MRI did not show abnormality in the subcortical white matter and was compatible with DAI, we believe that the injuries of both STTs were caused by traumatic axonal injury (Adams et al., 1982;Alexander, 1995; Parizel et al., 1998). Although both STTs were partially injured on 1-year DTT, he did not complain central pain.However, after approximately 5 years after onset, the patient suffered from central pain in both hands and legs, and trunk. Consequently, delayed degeneration and central pain occurred 5 years after onset in both mildly injured STTs, and might be associated with delayed degeneration of both mildly injured STTs.
A few studies have reported on injury of the STT in patients with central pain following TBI (Seo and Jang, 2013, 2014; Kim et al., 2015). In 2014, Seo and Jang reported on damage of both STTs in a patient with central pain after mild TBI. The next year, Kim et al. (2015) demonstrated that injury of the STT was associated with the occurrence of central pain in 32 patients with mild TBI. On the other hand, regarding the central pain, Ofek and Defrin (2007)reported that a large portion of central pain in TBI started late following TBI: central pain in TBI first appeared at a mean onset time of 6.6 months after TBI (range: 6–66 months) and in 10 %of patients, onset of pain occurred after 12 months. Therefore, we think a significant portion of central pain in TBI might be associated with delayed degeneration of the STT. In-depth studies on this topic should be encouraged.
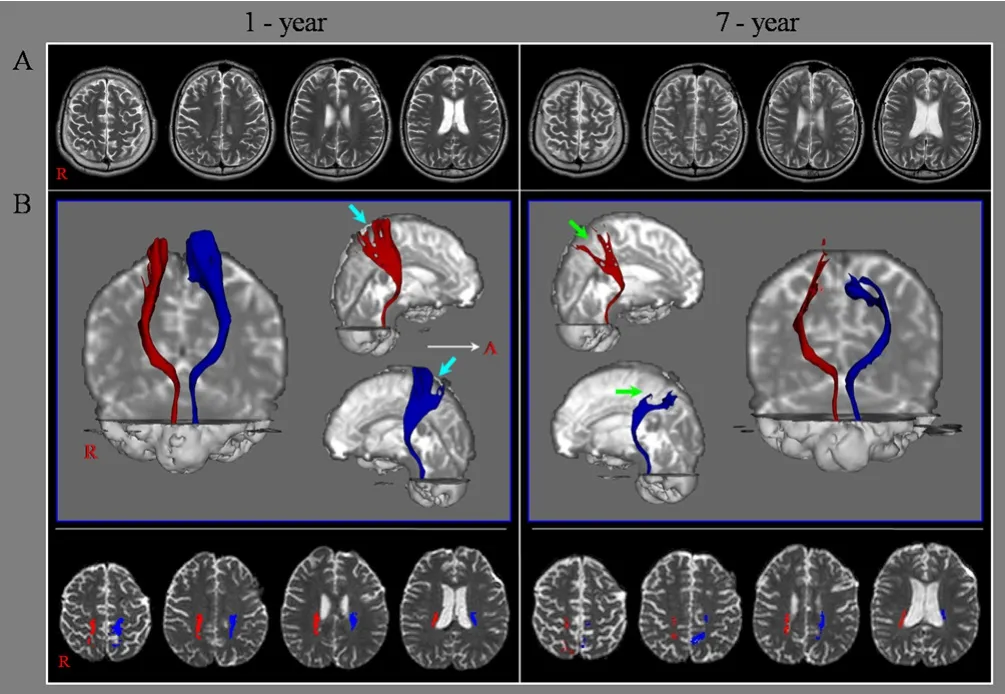
Figure 1 Brain MRI imaging and diffusion tensor tractography for a 27-year-old male patient with diffuse axonal injury.(A) Brain MR images at 1 and 7 years after onset show no abnormality.(B) Results of diffusion tensor tractography (DTT) for the spinothalamic tract (STT) in both hemispheres. Partial tearing at the subcortical white matter in both STTs is observed on 1-year DTT (sky-blue arrows)and both partially torn STTs show severe degeneration at the subcortical white matter on 7-year DTT (green arrows). A: Anterior; R: right.
Two kinds of neuronal degeneration after primary direct insult to neuronal cells in the central nervous system have been suggested: primary and secondary degeneration (Li et al., 2014).Primary degeneration means the death of neurons and glial cells as an early consequence of the primary pathological insult (Li et al., 2014). By contrast, secondary degeneration indicates the degeneration of neurons and glial cells caused by noxious factors released from neurons or glial cells damaged by the primary direct insult (Li et al., 2014). Secondary degeneration is initiated by pathological factors (calcium dysregulation, excessive free radicals, activation of proteases, overexpression of pro-apoptotic proteins, hydrolytic enzymes, and high-level glutamate) released by tissues damaged by the primary insult (Farkas and Povlishock,2007; Li et al., 2014). Both apoptosis and necrosis are known to be involved in this degeneration (Farkas and Povlishock, 2007;Stoica and Faden, 2010; Li et al., 2014). Differentiation of secondary degeneration from primary degeneration based on the time-points after injury is difficult because there is no absolute time when primary damage evolves into delayed effects after TBI(LaPlaca et al., 2007; Li et al., 2014). On the other hand, Shiozaki et al. (2001) reported delayed neuronal loss which meant sudden appearance of a low-density area in the affected hemisphere at several months post-TBI in 8 out of 17 patients with severe head injury. Considering that the central pain started at 5 years after onset in this patient, we think that secondary or delayed degeneration was caused by the degeneration of the STT.
In conclusion, delayed degeneration of the STT was demonstrated in a patient who developed delayed-onset central pain following DAI. Our results suggest evaluation of the STT using DTT in patients with TBI complaining of delayed central pain having the characteristics of neuropathic pain. This study is limited because it was based on a single case; thus further complementary studies involving larger numbers of patients are required.
This work was supported by the Medical Research Center Program (2015R1A5A2009124) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science,ICT and Future Planning.
Sung Ho Jang, Hyeok Gyu Kwon*
Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daemyungdong, Namku, Daegu,Republic of Korea (Jang SH)
Department of Physical Therapy, College of Health Sciences,
Catholic University of Pusan, Pusan, Republic of Korea (Kwon HG)
*Correspondence to:Hyeok Gyu Kwon, Ph.D.,
khg0715@hanmail.net.
orcid:0000-0002-6654-302X (Hyeok Gyu Kwon)
How to cite this article:Jang SH, Kwon HG (2017) Delayed degeneration of an injured spinothalamic tract in a patient with diffuse axonal injury.Neural Regen Res 12(11):1927-1928.
Author contributions:SHJ was responsible for research design and data acquisition. HGK was in charge of conception and design of this study,acquisition and analysis of data, and manuscript authorization. Both of these two authors approved the final version of this paper.
Conflicts of interest:The authors report no disclosures relevant to the manuscript.
Research ethics:All subjects provided informed consent for participation and the study was approved by the institutional review board of Yeungnam University Hospital (approval No. YUMC 2015-07-064). The study followed the Declaration of Helsinki and relevant ethical principles.
Declaration of patient consent:The authors certify that they have obtained the appropriate patient consent form. In the form, the patient will give his consent for his images and other clinical information to be reported in the journal. The patient understand that his name and initial will not be published and due efforts will be made to conceal their identity,but anonymity cannot be guaranteed.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Adams JH, Graham DI, Murray LS, Scott G (1982) Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 12:557-563.
Alexander MP (1995) Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 45:1253-1260.
Farkas O, Povlishock JT (2007) Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog Brain Res 161:43-59.
Goto T, Saitoh Y, Hashimoto N, Hirata M, Kishima H, Oshino S, Tani N,Hosomi K, Kakigi R, Yoshimine T (2008) Diffusion tensor fiber tracking in patients with central post-stroke pain; correlation with efficacy of repetitive transcranial magnetic stimulation. Pain 140:509-518.
Hong JH, Jang SH (2010) Degeneration of cingulum and fornix in a patient with traumatic brain injury: diffuse tensor tractography study. J Rehabil Med 42:979-981.
Hong JH, Kwon HG, Jang SH (2011) Probabilistic somatotopy of the spinothalamic pathway at the ventroposterolateral nucleus of the thalamus in the human brain. AJNR Am J Neuroradiol 32:1358-1362.
Hong JH, Choi BY, Chang CH, Kim SH, Jung YJ, Lee DG, Kwon YH, Jang SH(2012) The prevalence of central poststroke pain according to the integrity of the spino-thalamo-cortical pathway. Eur Neurol 67:12-17.
Jang SH, Kwon HG (2013) Anatomical location of the medial lemniscus and spinothalamic tract at the pons in the human brain: a diffusion tensor tractography study. Somatosens Mot Res 30:206-209.
Jang SH, Seo JP (2015) Differences of the medial lemniscus and spinothalamic tract according to the cortical termination areas: A diffusion tensor tractography study. Somatosens Mot Res 32:67-71.
Kim JH, Ahn SH, Cho YW, Kim SH, Jang SH (2015) The relation between injury of the spinothalamocortical tract and central pain in chronic patients With Mild Traumatic Brain Injury. J Head Trauma Rehabil 30:E40-46.
Kwon HG, Jang SH (2014) Delayed gait disturbance due to injury of the corticoreticular pathway in a patient with mild traumatic brain injury. Brain Inj 28:511-514.
LaPlaca MC, Simon CM, Prado GR, Cullen DK (2007) CNS injury biomechanics and experimental models. Prog Brain Res 161:13-26.
Li HY, Ruan YW, Ren CR, Cui Q, So KF (2014) Mechanisms of secondary degeneration after partial optic nerve transection. Neural Regen Res 9:565-574.
Lincoln NB, Jackson JM, Adams SA (1998) Reliability and Revision of the Nottingham Sensory Assessment for Stroke Patients. Physiotherapy 84:358-365.
Ofek H, Defrin R (2007) The characteristics of chronic central pain after traumatic brain injury. Pain 131:330-340.
Parizel PM, Ozsarlak, Van Goethem JW, van den Hauwe L, Dillen C, Verlooy J, Cosyns P, De Schepper AM (1998) Imaging findings in diffuse axonal injury after closed head trauma. Eur Radiol 8:960-965.
Seo JP, Jang SH (2013) Traumatic thalamic injury demonstrated by diffusion tensor tractography of the spinothalamic pathway. Brain Inj 27:749-753.
Seo JP, Jang SH (2014) Injury of the spinothalamic tract in a patient with mild traumatic brain injury: diffusion tensor tractography study. J Rehabil Med 46:374-377.
Shiozaki T, Akai H, Taneda M, Hayakata T, Aoki M, Oda J, Tanaka H, Hiraide A, Shimazu T, Sugimoto H (2001) Delayed hemispheric neuronal loss in severely head-injured patients. J Neurotrauma 18:665-674.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK,Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM(2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208-219.
Stoica BA, Faden AI (2010) Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics 7:3-12.
Tomaiuolo F, Worsley KJ, Lerch J, Di Paola M, Carlesimo GA, Bonanni R,Caltagirone C, Paus T (2005) Changes in white matter in long-term survivors of severe non-missile traumatic brain injury: a computational analysis of magnetic resonance images. J Neurotrauma 22:76-82.
- 中国神经再生研究(英文版)的其它文章
- The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction
- Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogenactivated protein kinase and nuclear factor kappa B signaling pathways
- Taking out the garbage: cathepsin D and calcineurin in neurodegeneration
- MicroRNAs as diagnostic markers and therapeutic targets for traumatic brain injury
- Interferon regulatory factor 2 binding protein 2: a new player of the innate immune response for stroke recovery
- Endogenous retinal neural stem cell reprogramming for neuronal regeneration

