Fluorescent Probes for Mitochondrial Reactive Oxygen Species in Biological Systems
LOU Zhang-Rong LI Peng HAN Ke-Li
Fluorescent Probes for Mitochondrial Reactive Oxygen Species in Biological Systems
LOU Zhang-Rong LI Peng HAN Ke-Li*
()
In addition to being the energy powerhouse of the cell, mitochondria are an important source of reactive oxygen species (ROS) during the process of molecular oxygen metabolism. Mitochondrial ROS are closely associated with normal physiological functions as well as human diseases, and participate in cell signaling, nucleic acid and protein damage, and oxidative stress induction. However, the complicated interplay between mitochondrial ROS and the cellular pathological state has not been fully elucidated. It is expected that research on the mitochondrial ROS undertaking in the molecular pathogenesis of human diseases would benefit from development of efficient tools for the detection of these ROS. In recent years, an increasing number of fluorescent probes for mitochondrial ROS with high sensitivity and selectivity have been developed. Here, we present a review of the recent advances in small molecular fluorescent probes for selective detection of ROS inside the mitochondria. In this review, the design, synthesis, characteristics, and applications of the published fluorescent probes for mitochondrial ROS are discussed in detail.
Fluorescent probe; Mitochondria; Reactive oxygen species; Small molecule;Living cells
1 Introduction
Reactive oxygen species (ROS) is a generic term of a series of reactive species, including superoxide anion (O2·−), hydrogen peroxide (H2O2), singlet oxygen (1O2), hydroxyl radical (OH·), peroxyl radical (ROO·), hypochlorous acid/ hypochlorite (HOCl/−OCl), and hypobromous acid/hypo- bromite (HOBr/−OBr)1,2. Most investigation indicates that the majority of intracellular ROS as the by-product of respiration are produced in the mitochondria3−5. And the potential sources and the simplified metabolism of mitochondrial ROS were outlined in Fig.16−8. The primary ROS generated in the mitochondria is O2·−, which is derived from the mono- electronic reduction of triplet-state molecular oxygen (O2) in the process of electron transport chain. Then the O2·−can be transformed into the relatively stable H2O2by the catalysis of mitochondrial superoxide dismutases (SOD). Similarly, HOCl can be generated from H2O2and chloride ions (Cl−) by the catalysis of myeloperoxidase (MPO). In the presence of iron or copper, H2O2and O2·−can also be converted to the extremely instable hydroxyl radical (·OH) by the Fenton reaction. Increasing studies have demonstrated that mitochondrial ROS were closely associated with the physiological and pathological processes in cells2,6, including participating in cell signaling, damaging nucleic acids and proteins, and inducing oxidative stress5,9. What is more, it is known that oxidative stress is responsible for a range of human diseases, such as cancer, degenerative disorders, and diabetes. However10−14, the complicated interplay between mitochondrial ROS and cellular pathological state has not been fully elucidated. Actually, a better insight into the molecular mechanisms responsible for human diseases will provide possible way to therapeutic intervention and better treatments. It is undoubted that exploring the mitochondrial ROS undertaking in the molecular pathogenesis of human diseases will benefit from obtaining the important spatial and temporal information inside individual microenvironment in living systems. Thus, it is critical to develop new tools to monitor the ROS selectively in the mitochondria.
Fluorescent probes, which have the advantages of high sensitivity and selectivity, convenient operation, real-time imaging and non-invasiveness, providing researchers potentially powerful molecular tools to study ROS and biological processes in living systems15,16. During the past decades, the development of fluorescent probes for the selective detection of ROS in biological systems has attracted considerable interest of researchers from the fields of chemistry and biology7,17−28. Simultaneously, the development of fluorescent probes targeting to mitochondria has become a rapidly growing field29−32, owing to the deeper study of disease states in their pathophysiology will benefit from obtaining the important spatial and temporal information inside individual organelle in living systems. According to the current literatures, a number of reviews have been published on description of the fluorescent probes for biological important ROS in the living cells7,23−27,33. Although Chang7have highlighted the progress in the development of small fluorescent probes for mitochondrial ROS in 2010, Gao34have evaluated the mitochondrial ROS imaging approaches from their proper applications and limitations and Lv35have summarized the strategies in designing fluorescent probes for selectively monitoring mitochondrial ROS and discussed the research trends of these probes, there is no comprehensive review of fluorescent probes targeting to mitochondria for ROS has been published in the recent years. It is believed that lacking of systematic summary for the previously developed fluorescent probes targeting to mitochondria for ROS might limit their applications and hinder the further development of this promising field. In this regard, we will present an overview of the recent advances in the small molecular fluorescent probes for the selective detection of ROS inside the mitochondria. In this review, we summarized the fluorescent probes according to their applications in the specific detection of various ROS, the design, synthesis, characteristics, and applications of the published fluorescent probes were discussed in detail. And we hope this review may provide a favor to those who are interested in this continually growing research field.
Fig.1 Simplified metabolism scheme of mitochondrial ROSa.
aSuperoxide anion (O2·−) in the mitochondria is derived from the one-electron reduction of oxygen (O2) by the electron transport chain (ETC), O2·−can then be converted to hydrogen peroxide (H2O2) catalyzed by superoxide dismutases (SOD).Nitric oxide (NO) is produced by mitochondrial nitric oxide synthases (NOS), which can react with O2·−to produce peroxynitrite (ONOO-). H2O2can either be transformed into H2O under the catalysis of glutathione peroxidases (GPx), thioredoxin (TRX) or catalases, converted to a hydroxyl radical (·OH) by iron or copper-mediated Fenton chemistry, or transformed into hypochlorous acid (HOCl) catalyzed bymyeloperoxidase (MPO).

During the course of developing fluorescent probes for monitoring mitochondrial species, several strategies to delivering fluorescent probes selectively into mitochondria have been proposed7,29,32. One effective approach is to attach a positively charged group onto the fluorescent probes, since the inner mitochondrial membrane has a negative potential of −180 mV36. Thereby the fluorescent probes can pass through the inner mitochondria membrane by taking advantage of proton gradient and subsequently locate in mitochondria. It's worth mentioning that the delocalized lipophilic cations, but not inorganic cations, are effective cations in penetrating the hydrophobic mitochondrial membranes and accumulating in mitochondria. The most common lipophilic cations used as mitochondrial transporters are triphenylphosphonium (TPP) and its derivatives. During the past decades, a range of mitochondrial fluorescent probes for ROS using TPP as cargo transporters have been developed. Another strategy is to use proteins, peptides, and peptide mimics as the mitochondrial targeting moiety, such as manganese superoxide dismutase and mitochondria targeted peptides. And the methods with peptidic mitochondrial targeting compounds have been described in detail in the reported literature36,37, which are not the primary scope that we discussed in this review.
2 Fluorescent probes for mitochondrial H2O2
H2O2is an inevitable by-product of the aerobic organism and serves as an important small signaling molecule in regulating normal biological processes and disease progression38. Through various measurements and calculations, the intracellular concentration of H2O2has been determined to fluctuate ranging from a low of ~0.001 μmol∙L−1to a high of ~0.1 μmol∙L−1 39,40. On the other hand, the aberrant generation of H2O2in the mitochondria leads to implications of oxidative stress, which is closely associated with a variety of diseases41,42. In 2008, inspired by the strategy for delivering cargos into mitochondriaattaching delocalizied lipophilic cations, Chang and co-workers43,44developed a novel fluorescent probe 1 for H2O2in the mitochondria of living cells (Scheme 1). In this probe, TPP is selected as the mitochondrial transporters and boronate is used as the peroxide-responsive moiety. This probe can accumulate in the mitochondria selectively and exhibits a turn-on fluorescence response to H2O2screening to other ROS. In addition, the fluorescent probe 1 is successfully applied to real-time imaging of the changes of mitochondrial H2O2in an oxidative stress model of neurodegenerative disease, see Fig.2. It is believed this probe would provide a new strategy to developing multifunctional fluorescent probes for targeting, activation, and detection in living cells.
Two-photon microscopy (TPM) is a new imaging technique that utilizing two lower energy photons as the excitation wavelength. Compared with one-photon microscopy (OPM), TPM affords the advantages of deeper tissue penetration depth (> 500 μm) and lower photodamage effect30,45. In addition, ratiometric fluorescent probes outputting with the ratio of two excitation or emission wavelengths is capable of eliminating most of the interferences from probe concentrations, laser efficiency and environmental conditions46−48. Utilizing the same design strategy for transporting the fluorescent probe into the mitochondria, Cho49developed aratiometric two-photon fluorescent probe 2 for detecting mitochondrial H2O2in the live cells and intact tissues (Scheme 2). The fluorescent probe 2 contained TPP as the mitochondrial- targeting group, boronate-based carbamate as the H2O2active site, and 6-(benzo[]thiazol-2'-yl)-2-(,-dimethylamino) naphthalene (BTDAN) as the two-photon dye. This probeexhibits the fluorescence maxima at 470 nm. After the addition of H2O2, the electron-withdrawing carbamate linkage was cleaved, a new emission at 545 nm emerged and the fluorescence at 470 nm decreased with the rate constant of 1.0 × 10–3s–1following a pseudo first-order kinetics. The probe 2 has a detection limit of 4.6 μmol∙L−1for H2O2and was non-toxic to cells during the imaging experiments. Moreover, this probe enables ratiometric visualization of the endogenous produced mitochondrial H2O2in living cells selectively, as well as the changes in mitochondrial H2O2levels in living tissues at depths ranging from 100–180 μm through the use of TPM.
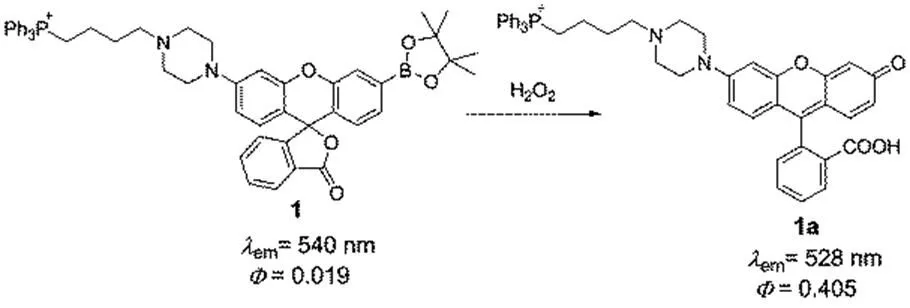
Scheme 1 Structure of the fluorescent probe 1 for detecting H2O2in the mitochondria
Scheme 2 Ratiometric two-photon fluorescent probe 2 for H2O2in the mitochondria.
Adapted from Ref. 49, Copyright 2012 Royal Society of Chemistry.
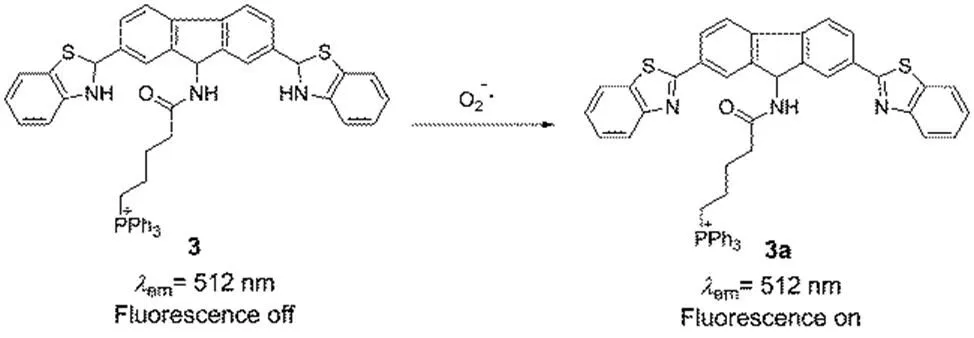
Scheme 3 Two-photon fluorescent probe 3 for O2·− in the mitochondria.
Images displayed represent emission intensities collected in optical windows between 527 and 601 nm upon excitation at 510 nm for probe 1. (a, h) HeLa cells incubated with 5 μmol∙L−1probe 1 for 60 min at 37 °C , (b, e, i, l) Overlay images of MitoTracker Red and Hoechst, (c, f, j, k) Overlay images of probe 1 with MitoTracker Red. (d) HeLa cells incubated with 5 μmol∙L−1probe 1 for 60 min at 37 °C with 100 μmol∙L−1H2O2added for the final 40 min, (g, n) brightfield with 20 μm scale bar. (k) HeLa cells incubated for 24 h with 1 mmol∙L−1paraquat, then washed and incubated with 5 μmol∙L−1probe 1 for 60 min at 37 °C. Reprinted with permission from2008,, 9638. Copyright 2008 American Chemical Society.

Fig.3 TP microscopy images of the mice with LPS-mediated abdomen injury.
(A) O2·−was produced inside the peritoneal cavity of the mice during the LPS-mediated inflammatory response. (B) TP fluorescentimages of mice abdomen incubated with probe 3 (100 μmol∙L−1). Images were acquired using 770 nm TP excitation. TP fluorescent emission windows: 500−550 nm. Reprinted with permission from2013,, 9877Copyright 2013 American Chemical Society.

Scheme 4 Sensing mechanism of fluorescent probe 4 for O2·−.
3 Fluorescent probes for mitochondrial O2·–
As mentioned above, O2·−is the primary ROS produced in the mitochondria. During the electron transport chain, most of oxygen is reduced to water by a four-electron reduction, but a small portion (it is estimated range from 0.1% to as high as 4%) of oxygen is reduced to O2·−by a monoelectronic reduction6,50. Subsequently, O2·−continues to involve in the ROS metabolism and produce a series ROS and RNS. O2·−can be transformed to H2O2,or be oxidized by NO and generate ONOO− 6. It is considered that O2·−plays an important role in a lot of physiological and pathological processes in biological systems51. Consequently, developing efficient fluorescent probes for mitochondrial O2·−in living systems is imperative. Tang52developed a novel reaction-based two-photon fluorescent probe 9-butyltriphenylphosphoniumacylamino-2,7- dibenzothiazolineflurene 3 for monitoring O2·−in the mitochondria (Scheme 3). In this probe, fluorene was utilized as the two-photon fluorophore, benzothiazoline was employed as the O2·−responsive group due to its good selectivity, and TPP was selected as the mitochondria-targeted group. In the presence of O2·−, probe 3 exhibited a fluorescence increase at the maximum wavelength of 512 nm, which was excited with one-photon wavelength of 483 nm and two-photon wavelength of 800 nm. The probe was capable of responding to O2·−selectively screening to other ROS, and the detection limit was 9.5 nmol∙L−1for O2·−. The 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay showed an IC50 value was 783 μmol∙L−1, indicating the probe was low toxicity to live cells under experimental conditions at the concentration of 20 μmol∙L−1. More importantly, probe 3 was applied to visualizing the O2·−fluctuations in living mice through the two-photon fluorescence imaging, see Fig.3.
Choosing fluorescein isothiocyanate (FITC) as the fluorophore and integrating 2,4-dinitrobenzenesulfonyl group as the O2·−responsive moiety into the fluorophore, Zhongsynthesized a fluorescent probe for selectively monitoring the change of O2·−levels (Scheme 4)53. In addition, the mitochondrial locating signal (MLS) peptide (NH2-MSVLTPLLLRGLTGSARRLPVPRAK―COOH) was conjugated to the fluorescent probe 4, which enables it successfully detected the dynamic change of O2·−in the mitochondria. After the addition of O2·−, 2,4-dinitrobenzene- sulfonyl group was cleaved and the fluorescence of the probe was turned “on”. This probe exhibited quick response, superior mitochondria targeting capabilities and monitored successfully the dynamic change of O2·−in living cells induced by chemical stimuli or single-walled carbon nanotubes.
4 Fluorescent probes for mitochondrial HOCl
HOCl is widely used as the disinfectant and bleaching agent in our daily lives. The endogenous HOCl that playing an essential role in the antimicrobial system is generated from H2O2and chloride ions (Cl−)the catalysis of the hemeenzyme myeloperoxidase (MPO)54. On the other hand, the abnormal produced HOCl will lead to oxidative stress through the oxidation or chlorination reaction55. Increasing evidence suggests it is associated with a variety of diseases56,57. As the main source of cellular ROS, mitochondria play a crucial role in the ROS relevant biological processes6,58. As a result, the monitoring of mitochondrial HOCl is equally important to investigate the role of HOCl playing in the pathogenesis. In this section, we summarized a series of fluorescent probes with high performances for the detection of HOCl, and their detection limits towards HOCl were shown in the Table 1.

Table 1 Detection limits of probes for HOCl.
aThe limit of detection (LOD),the unit is nmol∙L−1.
Lin.59designed and synthesized a novel near-infrared fluorescent probe 5 for HOCl in living animals, as shown in Scheme 5. This fluorescent probe is constructed basing on a unique class of near-infrared functional fluorescent dyes, which are developed by Lin's group. These new near-infrared dyes possess superior photophysical properties including excellent absorption extinction coefficients, high fluorescence quantum yields and good photo-stability. In addition, utilizing the quantum chemical calculations, they investigated the structure-optical properties of these unique dyes, which providing potential direction for the developing new near- infrared fluorescent dyes with excellent performances. Importantly, this reported efficient fluorescent probe is capable of imaging the endogenously produced HClO in the mitochondria of living RAW264.7 macrophage cells, as well as in the living animals (Fig.4).
Peng60developed an oxime containing fluorescent probe 6 based on a BODIPY scaffold for mitochondrial HOCl selectively, Scheme 6. In this probe, oxime group was introduced into the 2-position of BODIPY dyes as the active moiety reacting with HOCl, TPP group was integrated into the-position of BODIPY as the mitochondria locating group. This probe emits faint fluorescence due to the non-radiative decay of C=N bonds in the excited state. Upon addition of HOCl, the probe reacts rapidly with HOCl and the C=N moiety is transformed to carboxylic group, resulting in the fluorescence of the probe turned “on”. Furthermore, the probe is applied in living cells for imaging the change of HOCl in mitochondria successfully.

Scheme 5 Mitochondria-targeted fluorescent probe 5 for HOCl.
(a) Negative control, neither LPS nor probe 5 was injected; (b) Saline was injected in the intraperitoneal (i.p.) cavity of mice, followed by i.p. injection of probe 5 (120 nmol∙L−1); (c) LPS was injected into the peritoneal cavity of the mice, followed by i.p. injection of probe 5 (120 nmol∙L−1); (d) Quantification of fluorescence emission intensity from the groups a−c. Images were acquired with an excitation filter 670 nm and an emission range of 690−740 nm. Reprinted with permission from2012,, 1200Copyright 2012 American Chemical Society.

Scheme 6 The mitochondria-targeted fluorescent probe 6 for HOCl.
Adapted from Ref.60, Copyright 2013 Royal Society of Chemistry.
Next, utilizing the oxidization-hydrolysis of benzoyl acetohydrazide, Yu61synthesized two colorimetric and fluorescent probes 7, 8 based on the rhodamine platform for selectively detecting HOCl over other ROS, Scheme 7. In the probe 7, a TPP group is employed as the mitochondria-targeted functional group. And in the other probe 8, a quaternized pyridine moiety conjugating to the rhodamine fluorophore is applied to accumulating in the mitochondria. Herein, the TPP group and the cationic pyridinium moiety not only act as mitochondria targeting groups but also improve the water solubility of these new developed probes. After addition of HOCl, the fluorescence intensity of probe 7 and 8 located at 577 and 575 nm respectively increased gradually. Both of these two fluorescent probes exhibit several advantages in terms of water solubility, selectivity and response time. Interestingly, the standard MTT assay demonstrated that more than 85% of HeLa cells were dead after the addition of 20 μmol∙L−1of 7, while more than 95% of the cells remained alive treated with 20 μmol∙L−1of 8. This result indicates that a pyridinium unit might show a lower toxicity than a TPP moiety. Additionally, probes 7 and 8 were utilized in imaging HOCl in living mice, which were anticipated to apply in a variety of biological processes mediated by HOCl.

Scheme 7 Structures of mitochondria-targeted fluorescent probe 7 and 8 for HOCl.
Subsequently, on the basis of the coumarin-rhodamine system and TPP moiety utilized as the mitochondria carrier, Yu62developed another two fluorescent probes 10 and 11 for mitochondrial HOCl, Scheme 8. Distinguishing from the intensity-based fluorescent probes 7 and 8, both of probes 10 and 11 are capable of detecting HOCl ratiometrically. Probes 10 and 11 emit the fluorescence of coumarin, which is located at the maximum wavelength of 481 nm. After the addition of various ROS, the dibenzoylhydrazine moiety in the coumarin-rhodamine platform can be selectively oxidized to dibenzoyldiimide by HOCl, which is further decomposed in the nucleophilic solvents and transformed into the compound 12. During this process, the rhodamine fluorophore turn into a spiroring-opening pattern and the fluorescence of rhodamine ranging from the wavelength of 560 nm to 610 nm emerged. These two probes possess the advantages of excellent selectivity, high sensitivity and fast response. More importantly, the probe 11 was applied in quantifying the mitochondrial OCl-in cancer cells for the first time successfully, as well as monitoring the variation of OCl−in living HeLa cells stimulated by CCCP and paraquat.
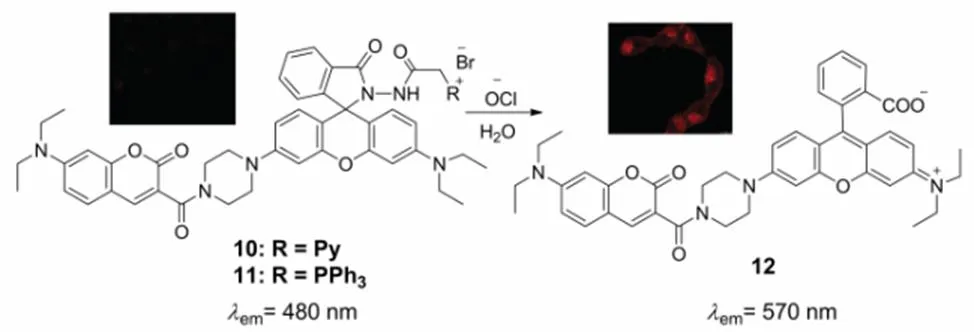
Scheme 8 Structures of mitochondria-targeted fluorescent probe 10 and 11 for HOCl.
Adapted from Ref.62, Copyright 2015 Royal Society of Chemistry.
By changing the cyclometalated ligands, Chao and coworkers63designed and synthesized a series of iridium(III) complexes 13−16 for detecting mitochondrial HOCl with different phosphorescent wavelength ranged from 534 to 598 nm, Scheme 9. Unlike the strategy of other probes to accumulating into the mitochondria, there are no mitochondria targeting groups in these phosphorescent iridium(III) complexes and the cationic iridium(III) complexes can successfully penetrate the mitochondrial membrane and localize in the mitochondria. In these complexes, diaminomaleonitrile moiety can be oxidized by OCl-selectively to form aldehyde or carboxylate and was utilized as the acceptor. These iridium(III) complexes emit weak luminescence resulting from the C=N isomerization process. After the addition of OCl-, the C=N was destructed and the phosphorescence was turned on. All these iridium(III) complexes exhibited high selectivity toward OCl-over other ROS, excellent mitochondrial specificity and low cell toxicity, which were successfully utilized as multicolor phosphorescent probes ranging from green to red for monitoring OCl-in living cells.

Scheme 9 Structures of mitochondria-targeted luminescent probes 13−16 for HOCl.
Scheme 10 Mitochondria-targeted luminescent probe 17 for HOCl.
Soon afterwards, another mitochondrial targeted iridium(III) phosphorescent probe17 for responding to OCl-over ROS was developed by Chao and coworkers, Scheme 1064. In the same way, the accumulation in the mitochondria is attributed to the cationic iridium(III) complexes, and the selectivity towards OCl-with switched on phosphorescence signal is achieved by means of the diaminomaleonitrile group. This phosphorescent probe exhibits a significant two-photon absorption cross- section of 78.1 GM at 750 nm, which enables the probe to detect OCl-in the deeper tissues. Moreover, this probe was employed as two-photon phosphorescent imaging for mitochondrial OCl-in the living cells, as well as the distribution of OCl-stimulated by endotoxin lipopolysaccharide (LPS) in live zebrafish. And the results indicated the endogenous OCl-was mainly generated in the liver of zebrafish during inflammation conditions induced by LPS.
Choosing the well-known two-photon fluorophore Acedan possessing excellent photophysical properties as the fluorescence reporting moiety, Chang65developed a two-photon fluorescent probe 18 for mitochondria HOCl, Scheme 11. In this probe, 2-mercaptoethanol was employed as the protecting group of ketone, which will decrease the “pull” effect of the typical ‘push-pull’ structure and quench the fluorescence of acedan. What is more, the thiol atom could be oxidized to sulfoxide selectively by HOCl over other ROS. As a result, the oxathiolane group undergoes deprotection reaction to reveal the ketone and leads to the fluorescence enhancement of the probe. In order to detect HOCl at subcellular levels, TPP as the mitochondria-targetable group was further introduced to ensure the probe localizing in the mitochondria. This probe exhibits the advantages of fast response (within seconds), excellent sensitivity (<20 nmol∙L−1) and high selectivity toward HOCl. Besides, the probe 18 was successfully applied to two-photon imaging of HOCl in the mitochondria of macrophage cells in an inflamed murine model.
In the meantime, utilizing phenothiazine as the fluorophore and quaternized pyridine moiety as mitochondria-targeted group, Wang and coworkers66prepared a fast-responsive fluorescent probe 19 for imaging mitochondrial HOCl, Scheme 12. Upon the addition of HOCl, the divalent sulphur of probe 19 was oxidized to sulfoxide within a few seconds, which resulted in a significant increase of fluorescence intensity by 40.5-fold at 562 nm. This probe shows high selectivity towards HOCl over various competitive compounds, including other ROS, HSO3-, SCN-, S2-, CN-, F-and thiols. The detection limit of this probe towards HOCl was evaluated to be 17.9 n mol∙L−1, which suggests the excellent sensitivity of probe 19 in monitoring HOCl. In addition, probe 19 was utilized to imaging of endogenous HOCl in the mitochondria of RAW 264.7 cells and living nude mouse.
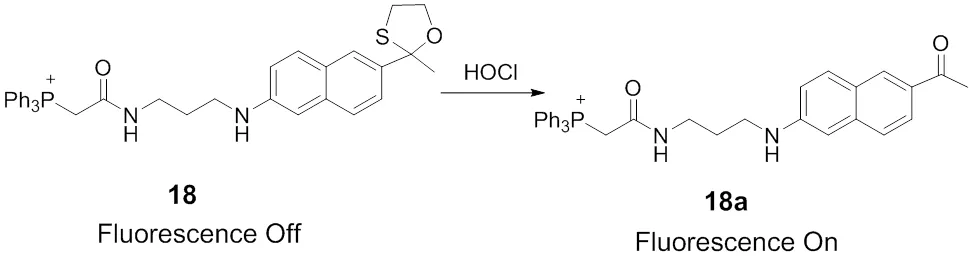
Scheme 11 Mitochondria-targeted fluorescent probe 18 for HOCl.
Another fluorescent probe for monitoring the mitochondrial HOCl based on the phenothiazine skeleton was developed by Wang and coworkers (Scheme 13)67. It is predictable that the carbon–carbon double bond with strong electron-withdrawing groups could be destroyed by HOCl and the conjugate structure would be decreased, thereby the fluorescence of the probe would be shifted. As expected, a great hypochromatic shift of the fluorescence peak from 640 to 522 nm was occurred after the addition of HOCl. The desirable photophysical properties make probe 20 a ratiometric fluorescent probe to overcome the limitations of intensity-based probes through self-calibration of two emission bands. In addition, TPP group was utilized as mitochondria-targeted vector to transport the probe into the mitochondria. Probe 20 was applied successfully in imaging of mitochondrial HOCl in the living RAW264.7 cells, as well as the endogenous HClO in the acute inflammation conditions of nude mouse stimulated by lipopolysaccharide (LPS).
In order to explore whether HOCl can be generated in the mitochondria of macrophages during the bacterial infection, Ma68developed a sensitive fluorescent probe 21 for detecting HOCl targeting to mitochondriaintegrating a typical mitochondrial-targeting moiety of TPP cation. As shown in Scheme 14, the probe was established on the basis of rhodamine thiolactone, which had been proved to only react with Hg2+and HOCl. The probe exhibits more than 200-fold increase in fluorescence emission at 580 nm when responding to HOCl, indicating the thiolactone ring underwent oxidative cleavage and generated spiroring-opening forms of rhodamine triggered by HOCl. There are other advantages of probe 21, including fast response, high selectivity and accurate mitochondrial-targeting ability. All of these properties make this probe undertake as an efficient probe to monitor HOCl at trace levels and investigate the roles of mitochondrial HOCl played in the living systems during bacterial infection conditions. Utilizing the probe 21, the confocal fluorescence imaging of the real-time change of mitochondrial HOCl in RAW264.7 cells as a model infected byare carried out, whichrevealed that mitochondria could also produce HOCl in the case of bacterial infection.
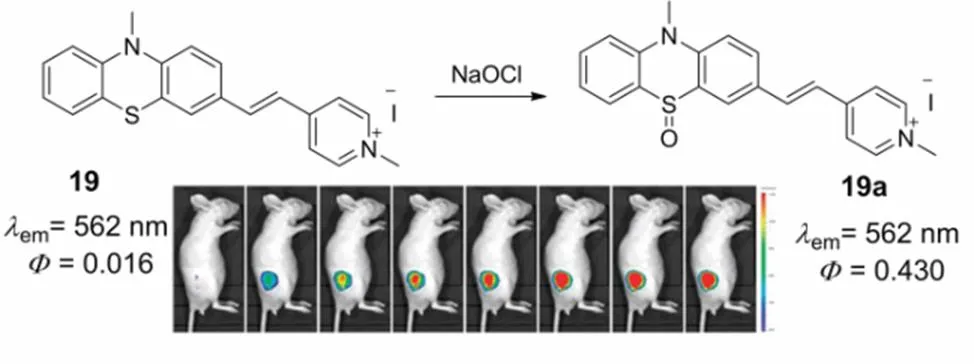
Scheme 12 Mitochondria-targeted fluorescent probe 19 for HOCl.
Adapted from Ref. 66, Copyright 2015 Royal Society of Chemistry.
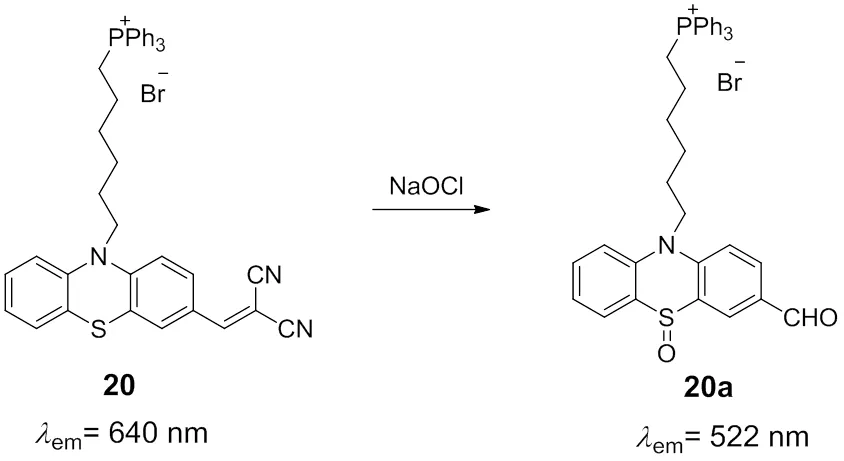
Scheme 13 Mitochondria-targeted fluorescent probe 20 for HOCl.
Bao69reported a mitochondria-targeted near-infrared fluorescent probe 22 for specific monitoring of HOCl in living cells, Scheme 15. In this probe, benzo[e]indolium group was conjugated with 7-diethylaminocoumarin fluorophore by the C=C bond, which could extend the-conjugation and make the probe emit fluorescence in the near-infrared region. Besides, the cationic benzo[e]indolium group could also increase the water-solubility of probe 22 and enable the probe accumulating selectively in mitochondria. After the addition of HOCl, the C=C bond was destroyed and the probe exhibited blue fluorescence from coumarin moiety. All of the advantages of probe 22 in colorimetric and ratiometric fluorescence dual responses to HOCl, high sensitivity, fast response and low detection limit (33 nmol∙L−1) enable the probe being utilized to visualizing the mitochondrial HOCl by ratiometric fluorescence imaging.

Scheme 15 Mitochondria-targeted fluorescent probe 22 for HOCl.
Scheme 14 Mitochondria-targeted fluorescent probe 21 for HOCl.
5 Fluorescent probes for mitochondrial hROS
As mentioned above, mitochondria are the major compartment for the production of intracellular ROS. Highly reactive oxygen species (hROS) containing ·OH, ONOO-, HOCl could be generated through the precursors of O2-· and H2O270. Owing to their strong oxidizing properties, hROS can directly oxidize nucleic acids, proteins, and lipids. On the other hand, hROS are likely to play specific roles in living cells71.
In consequence, it is necessary to develop efficient probes for mitochondrial hROS to explore their precise roles.Nagano and coworkers designed and synthesized the fluorescent probe 23 for the real time detection of hROS generated in the mitochondria71. As shown in Scheme 16, rhodamine dye was utilized as a fluorophore, which is positively charged at physiological pH and accumulates selectively in the mitochondria. 4-amino-phenyl ether moiety as the electron donor was integrated into rhodamine and quenched the fluorescence of rhodamine by photoinduced electron transfer (PET) process. In addition, hROS can specifically react with the electron donor moiety within a few seconds and switch on the high fluorescence of probe 23. This probe is capable of sensitively detecting different levels of mitochondrial hROS in the different cell types induced by H2O2stress. It is worth to mention that although this probe is responsive towards hROS rather than one single reactive oxygen species, the probe is still significant in a range of biological and pathological investigations.
Peng72developed a new fluorescent probe 24 for detecting mitochondrial hROS on the basis of hybrid cyanine- phenothiazine platform that containing two responsive sites, Scheme 17. The sulfur atom in this probe with rich electron density can be oxidized to sulfoxide and sulfone derivatives by hROS, and at the same time, the C=C bond connected between phenothiazine and cyanine can also be destructed. As a result, the probe displayed a ratiometric signal in absorption spectra, as well as a fluorescence “off-on” output when responded to HOCl. Additionally, the cyanine group with positive charge will help this probe accumulate in the mitochondria. The intensities at 595 nm of probe 24 show a linear response to 1−10 μmol∙L−1NaClO with a detection limit of 0.836 μmol∙L−1. The probe is an efficient sensor to detect endogenous hROS production with high selectivity and good sensitivity in the mitochondria of living cells in real time.
6 Fluorescent probes for mitochondrial ozone
Ozone in the stratosphere can prevent both plants and animals from being damaged by the ultraviolet light. Ozone is recognized a powerful oxidant harmful to human health and can damage the respiratory tract. However, ozone can be produced endogenously in human atherosclerotic arteries and antibacterial responses during inflammation73. It is found that the cholesterol ozonolysis products existed in clinical brain samples might accelerate amyloidogenesis dramatically74. Tang75developed a near-infrared fluorescent probe 25 with a large stokes shift about 140 nm for imaging of endogenous ozone in the living cells. As shown in Scheme 18, the L-tryptophan was chosen as the ozone recognition site, the cationic near-infrared fluorescent dye tricarbocyanine was employed as the signal transducer, helping the probe accumulate in the mitochondria selectively. Probe 25 is constructed based on the intramolecular charge transfer (TICT) mechanism and works in a fluorescence “off and on” manner. The probe exhibits a rapid fluorescence response to mitochondrial ozone and the limit of detection is determined to be 17 nmol∙L−1.
7 Fluorescent probes for mitochondrial redox changes
Although ROS are produced endogenously in the mitochondria during the electron transport process, cells possess their own antioxidant defense system to regulate the internal redox balance76,77.However, there are a variety of factors intra and extracellular may increase the generation of ROS and break the equilibrium of cellular redox states. The cellular redox homeostasis plays an essential role in biological processes and accounts for numerous of human diseases2,76.
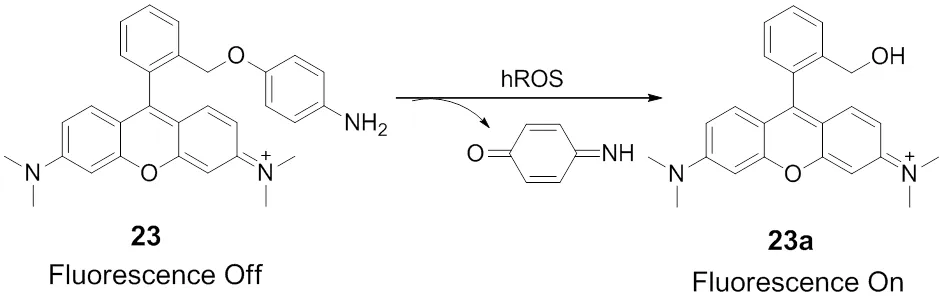
Scheme 16 Mitochondria-targeted fluorescent probe 23 for hROS.
Scheme 17 Mitochondria-targeted fluorescent probe 24 for hROS.
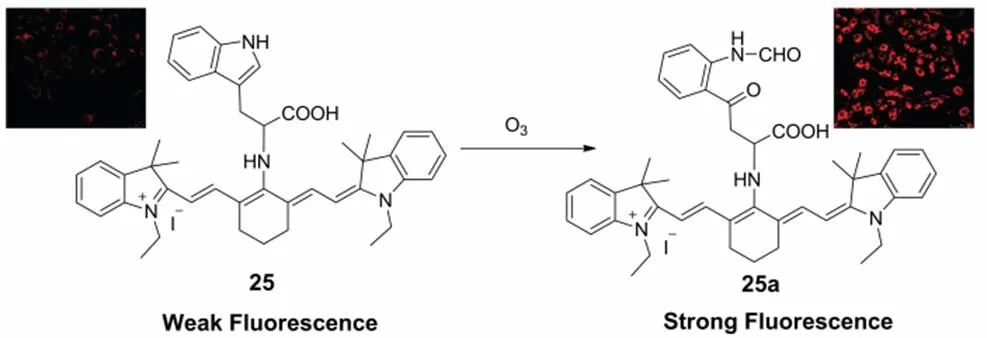
Scheme 18 Mitochondria-targeted fluorescent probe 25 for O3.
Adapted from Ref. 75, Copyright 2012 Royal Society of Chemistry.
Takeoka and coworkers78designed and synthesized a fluorescent probe 26 for direct monitoring the mitochondrial redox reactions, Scheme 19. In this probe, coumarin 343 localizing in the mitochondria is chosen as the fluorophore, a nitroxide radical 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO) moiety suitable for biological specimens is integrated into the fluorophore scaffold and is utilized as the redox reactions acceptor. Probe 26 emits faint fluorescence due to the electron transfer or exchange between the free radical and fluorophore. Once the TEMPO acceptor was reduced to hydroxylamine, the electron transfer process was blocked and fluorescence of probe 26 was restored. The versatile TEMPO moiety makes 26 as an efficient fluorescent probe to monitor the reversible redox reaction in living cells. Additionally, the probe was successfully applied in direct observation of the electron transport chain blocked by treating with rotenone in the mitochondria, providing an efficient tool to study the dynamic changes of respiratory chain.
Bi79developed two mitochondrial-targeting fluorescent probes for the detection of oxidative stress, similarly taking advantage of the nitroxide radical TEMPO as the reactive group. As shown in Scheme 20, the probes 27-28 are constructed on the basis of the rhodamine B dye containing cationic residues, which can pass through the mitochondrial membranes. The probes are successfully utilized for the real-time visualization of mitochondrial oxidative stress stimulated by phorbol 12-myristate 13-acetate (PMA) and the changes of mitochondrial morphology under oxidative stress in the living cells.
Glutathione peroxidase (GPx) is a major selenoenzyme with selenocysteine in the active site, and serves as an antioxidant to protect various organisms from oxidative damage80. Recently, a number of selenium and tellurium compounds as the GPx mimics capable of catalyzing the reduction of H2O2by GSH have been reported80,81. Based on the redox features of tellurium moiety, Han and coworkers22developed a near-infrared fluorescent probe 29 for the dynamic detection of redox change in the mitochondria, shown in Scheme 21. The near-infrared dye heptamethine cyanine with positive charge acts as the signal transducer and assists the probe accumulating in the mitochondria. Fluorescence of probe 29 is quenchedthe photoinduced electron transfer (PET) process between Te moiety and dye in the excited state. Once the tellurium group of 29 is oxidized by ONOO-, the PET process will be blocked, resulting in an enhancement of fluorescence. The probe was sensitive to ONOO-and the detection limit was determined to be 0.92 μmol∙L−1. In addition, the MTT assay suggested the probe was low toxicity to live cells under experimental conditions with an IC50 value of 160 μmol∙L−1. As an excellent fluorescent probe responsive to redox change, 29 can be utilized to imaging the dynamic changes of redox cycles between the oxidative stress of ONOO−and reductive repair of GSH in living cells and animal, see Fig.5. Moreover, the PET mechanism of probe 29 was fully rationalized by the DFT and TDDFT calculations.
Similarly, utilizing selenium with unique redox property as the fluorescent modulator and choosing heptamethine cyanine as the near-infrared fluorophore scaffold and mitochondrial vector, Tang and co-workers82synthesized a reversible fluorescent probe 30 targeting to mitochondria for redox changes between ONOO-and reduced ascorbate in the living cells, Scheme 22. Subsequently, another reversible fluorescent probe 31 based on heptamethine cyanine for monitoring the redox changes of GSH/H2O2cycles was developed (Scheme 22)83. After addition of GSH, the five-membered ring of ebselen was cleaved and the fluorescence of 31a was quenched due to the PET process. However, when the reduced probe was treated with H2O2, the fluorescence of the probe was restored. This probe can be utilizing in imaging the GSH/H2O2redox changes in HepG2 and HL7702 cells during the apoptosis induced by L-buthionine sulphoximine (BSO), as well as the changes of H2O2at the wound margin in zebrafish larvae.
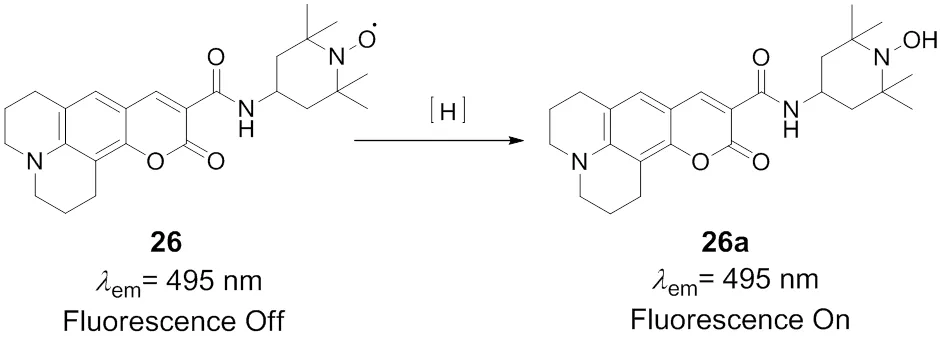
Scheme 19 Fluorescent probe 26 for mitochondrial redox changes.
Scheme 20 Structures of fluorescent probes 27 and 28 for mitochondrial redox changes.
Flavins are typically fluorescent in the oxidized form and non-fluorescent upon reduction84. According to this, Newdesigned and synthesized a flavin-containing fluorescent probe for detecting the redox changes targeting to the mitochondria, Scheme 2385. In this probe, the modified flavin is used as the redox-responsive moiety, the positively-charged TPP group capable of passing through the mitochondrial membranes was incorporated at the end of an aliphatic side chain at-8 position of naphthalimide. As expected, the probe 32 exhibits an emission maximum of 545 nm (= 0.26) similar to that of the previously reported redox probe86. After treated with by a range of reductants, fluorescence of the reduced form of probe 32 was decreased by 115-fold. However, re-oxidation of the probe was occurred rapidly after the addition of oxidants, restoring its initial fluorescence. These properties enable probe 32 serving as a reversible fluorescence response to the status of oxidation and red uction. The cytotoxicity of probe 32 in the HeLa cells was revealed an IC50 value of 65 μmol∙L−1for 24 h determined by the MTT assay. In addition, the probe can be employed to image variations in oxidative capacity of the haematopoietic cells in bone marrow, thymus and spleen.

Scheme 21 Fluorescent probe 29 for mitochondrial redox changes induced by ONOO- and GSH.
(a) Probe 29 (1 μmol∙L−1) and L-cysteine (1 mmol∙L−1); (b) probe 29 (1 μmol∙L−1);(c) LPS (10 μg∙mL−1) and IFN-γ (200 ng∙mL−1) for 4 h and then with PMA (100 nmol∙L−1) for 0.5 h, then 1 μmol∙L−1probe 29 for 30 min; (d) Mice treated as (c) described, then injected i.p. with AG (1 mmol∙L−1), GST (250 units∙mL−1), and L-cysteine (1 mmol∙L−1); (e) Mice treated as d) described, then injected i.p. with SIN-1(1 mmol∙L−1) for 20 min; (f) Quantification of total photon flux from each mouse (a−e).The total number of photons from the entire peritoneal cavity of the mice (a−e) was integrated. Images constructed from 790 to 860 nm fluorescence collection window,ex= 735 nm. Reprinted with permission from2013,, 7674Copyright 2013 American Chemical Society.
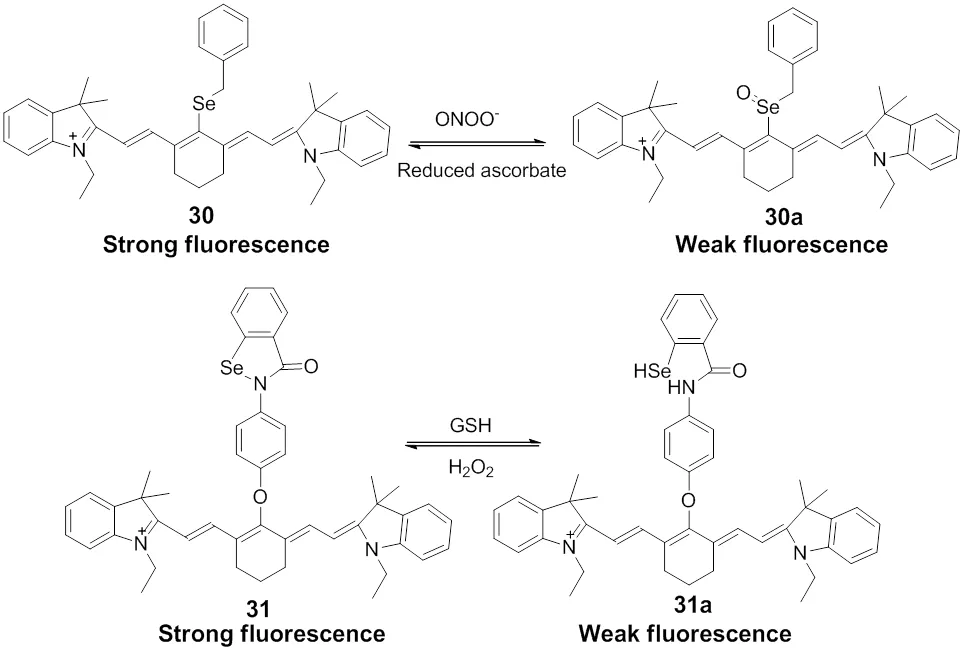
Scheme 22 Fluorescent probes 30 and 31 for mitochondrial redox changes
Scheme 23 Fluorescent probe 32 for mitochondrial redox changes.
8 Fluorescent probes for mitochondrial nitric oxide
Nitric oxide (NO) has been recognized to be a messenger for cellular signaling, which involves in the regulation of both physiological and psychological processes in the living systems87. There is evidence that suggest the physiological NO concentration range to be 100 pmol∙L−1(or below) up to ~5 nmol∙L−1 88. The mitochondria is the primary organelle where NO is generated during the process of L-arginine converting to L-citrulline catalyzed by nitric oxide synthase89. Hence, developing methods for visualizing mitochondrial NO is meaningful, which provides possibility in exploring the NO biological functions and pathogenesis at the molecular level.
Xiao and coworkers90reported the first mitochondria targeting fluorescent probe 33 for monitoring the exogenous and endogenous NO in the living cells, Scheme 24. Inspired by the reported work of NO can trigger rhodamine leuco form undergoing a ring-opening reaction91, the “-phenylenediamine- locked” rhodamine spirolactam is utilized as a NO responsive moiety in probe 33. Additionally, the cationic TPP group that is able to selectively accumulate in mitochondria was attached to the probe. When probe 33 responding towards NO, a significant fluorescence enhancement of the maximum wavelength locating at 585 nm is observed and the detection limit is determined to be 4.0 nmol∙L−1. The fluorescence imaging experiment has confirmed the probe could visualize the endogenous and exogenous NO in mitochondria with high sensitivity and good selectivity without interference from other reactive oxygen and nitrogen species (Fig.6).
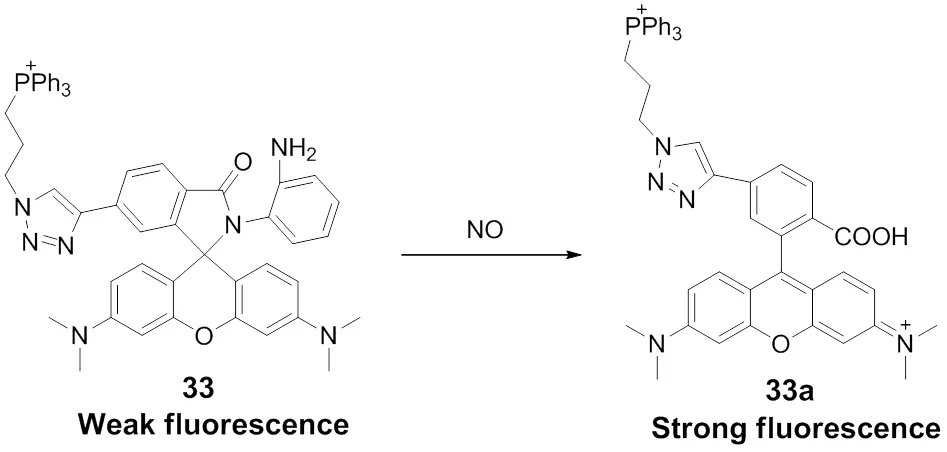
Scheme 24 Mitochondria-targeted fluorescent probe 33 for NO.
MCF-7 was stained with (a) Rh 123 (1.0 μmol∙L−1),ex= 488 nm;em= 500−540 nm) and (b) Probe 33 (1.0 μmol∙L−1, Channel 2 (Ch2),ex= 559 nm;em= 565−610 nm) with NO solution (20 equiv) for 5 min. (c) Overlay of a and b with the DIC image. (d) Intensity profile of region of interest 1 cross the MCF-7 cell. (e) Intensity scatter plot of Ch1 and Ch2. (f) Simulated image (the white pixels represented by the scatter plot (e) was highlighted by selecting the points with a red rectangular selection). Reprinted with permission from2013,, 7076.Copyright 2013 American Chemical Society.
On the basis of-phenylenediamine capable of responding to NO specifically, Guo92chose the pyronin dye integrating to one of the amino groups of-phenylenediamine and developed a fluorescent probe for mitochondrial NO in a dual-channel mode. As shown in Scheme 25, probe 34 possesses delocalized positive charge and can selectively localize in the mitochondria. The probedisplays no fluorescence (= 0.0003) due to a PET process from-phenylenediamine to the pyronin dye. Upon adding 100 equiv. of NO, the PET process is blocked and a fluorescence enhancement of 2743-fold at the wavelength of 616 nm was observed (= 0.2500). Probe 34 exhibits high sensitivity to NO and the detection limit is estimated to be 12 nmol∙L−1. Besides, the probe can selectively monitor NO over dehydroascorbic acid (DHA), ascorbic acid (AA), and methylglyoxal (MGO), as well as other ROS. Notably, the intermediate triazole 34a could be further converted into green-emission aminopyronin 34b (max= 536 nm) and red-emission thiopyronin 34c (max= 618 nm) in the presence of Cys and GSH. Consequently, probe 34 was able to monitor the exogenous and endogenous NO in the mitochondria of living cellstwo distinct emission channels in the assistance of Cys and GSH, see Fig.7.

Scheme 25 Sensing mechanisms of mitochondria-targeted fluorescent probe 34 for NO, Cys and GSH.
Recently, focusing on iridium(III) complexes with excellent photochemical properties and mitochondria-targetable properties, Chao and coworkers93developed a two-photon phosphorescent probe for the detection of NO in the mitochondria. As shown in Scheme 26, Chaosynthesized a series of iridium(III) complex35-38 containing 1,10-phenanthroline-5,6-diamine and explored their abilities in phosphorescent response towards NO. However, only the complex 37 displays an “off-on” phosphorescent response for NO specifically. The probe 37 showed a number of advantages, including wide phosphorescence emission range, low cytotoxicity and mitochondrial targeting, as well as fast and specific two-photon phosphorescence response. In addition, this probe was applied in real-time imaging mitochondrial NO in living cells, 3D multicellular spheroids and zebrafish.
9 Fluorescent probes for mitochondrial ONOO-
Peroxynitrite (ONOO-) is a powerful oxidation and nitration agent, which is derived from nitric oxide and superoxide with no enzyme catalysis94. The physiological concentration of peroxynitrite is assessed to be in the nanomolar scale range for hours′ time22. However, when against invading microorganisms (e.g., Chagas′ disease and bacterial infection), the endogenous abnormally concentration of ONOO-has been estimated up to 100 μmol∙L−1per min in immune system cells22., although ONOO-plays an essential role in physiological processes, the overproduction of ONOO-has been implicated in a range of diseases, including diabetes, cancer, and neurodegenerative disorders95.

Fig.7 (A1−A4) Fluorescence images of B16 cells stained by probe 34 (2 μmol∙L−1, 30 min) only. (B1−B4) Fluorescence images of B16 cells stained by probe 34 (2 μmol∙L−1, 30 min) and then DEA·NONOate (200 μmol∙L−1).
Emission was collected at 480−550 nm for green channel (excited at 458 nm) and at 580−630 nm for red channel (excited at 543 nm). Scale bar: 20 μm. Reprinted with permission from2014,, 12520.Copyright 2014 American Chemical Society.
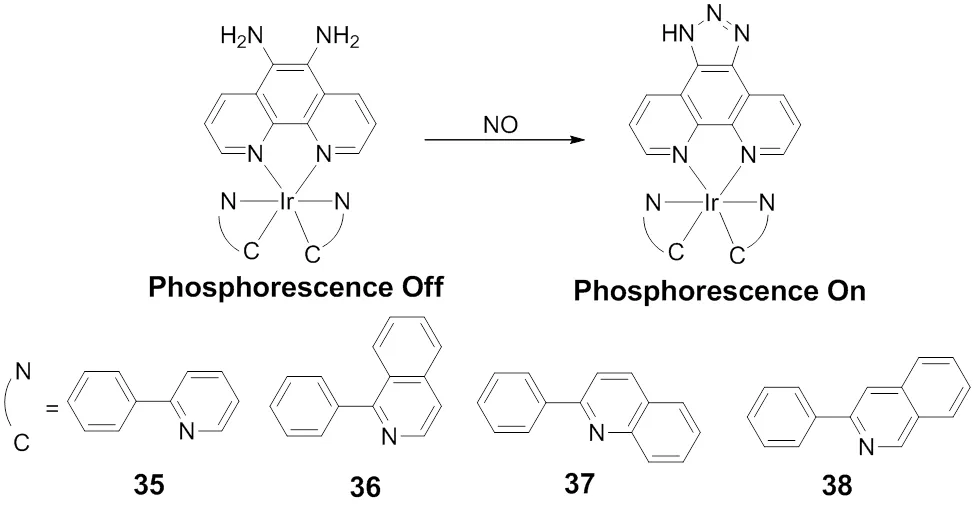
Scheme 26 Structures of iridium(III) complex 35−38 for NO.
Scheme 27 Mitochondria-targeted fluorescent probe 39 for ONOO-.
Guo and coworkers96reported a mitochondrial-targeting fluorescent probe for monitoring the exogenous and endogenous ONOO-in the living cells, which was proved to possess some advantages in terms of selectivity, sensitivity and fast response. In this probe 39, the electron-rich methyl(4- hydroxyphenyl)amino group that was selectively responsive to ONOO-over other ROS was employed as a fluorescence modulator, and the electropositive pyronin specifically accumulating in the mitochondria was chosen as a fluorescence reporter, Scheme 27. Probe 39 emits faint fluorescence due to therapid photoinduced electron transfer process (PET) from the modulator to the excited fluorophore. However, the PET process is blocked and the fluorescence increased by 85-flod dramatically upon the two-electron oxidation of methyl(4- hydroxyphenyl)amino group by ONOO-. In addition, the probe exhibited high sensitivity to ONOO-with a detection limit as low as 10 nmol∙L−1.
10 Conclusions
In this review, we present an overview of small molecular fluorescent probes specifically targeting to mitochondria for monitoring ROS in the biological systems. Generally, the effective approach to delivering probes selectively into mitochondria is to attach a positively charged group onto the fluorescent probes due to a negative potential of the inner mitochondrial membrane. There is no doubt that the development of mitochondrial targeting fluorescent probes for ROS may facilitate specifically exploring the significant roles of ROS performed in the mitochondria. However, in order to design ideal fluorescent probes with high performances for imaging ROS in the mitochondria, besides selectivity, sensitivity, water solubility and cell permeability, the following factors should be taken into account. First, ROS possessing characteristics of short lifetime and high reactivity, the probes should be capable of monitoring mitochondrial ROS in real time. Second, NIR or two-photon fluorescent probes with deeper tissue penetration, less photo- damage and lower auto-fluorescence should be developed. At last, the fluorescent probes for mitochondrial ROSattaching a positively charged group are always membrane potential-dependent, developing neutral fluorescent probes is an alternative strategy. In addition, exploring the fluorescence sensing mechanism is crucial to develop high- performance fluorescent probes33. The DFT/TDDFT calculations proved to be capable of giving a clear picture of the photophysical processes in the excited states97-105help us enlarge the understanding in the fluorescence sensing mechanism, as well as provide a basis for developing more efficient fluorescent probes106,107. In summary, the ongoing research in developing fluorescent probes for ROS in the mitochondria with excellent properties will provide an effective tool for investigating the complex interplay between mitochondrial ROS and human disease.
(1) Smith, L. L.2004,, 318. doi: 10.1016/j.freeadbiomed.2004.04.024.
(2) Dröge, W.2002,, 47. doi: 10.1152/physrev.00018.2001.
(3) Figueira, T. R.; Barros, M. H.; Camargo, A. A.; Castilho, R. F.; Ferreira, J. C. B.; Kowaltowski, A. J.; Sluse, F. E.; Souza-Pinto, N. C.; Vercesi, A. E.2013,, 2029. doi: 10.1089/ars.2012.4729.
(4) Lenaz, G.2001,, 159. doi: 10.1080/15216540152845957.
(5) Inoue, M.; Sato, E. F.; Nishikawa, M.; Park, A. M.; Kira, Y.; Imada, I.; Utsumi, K.2003,, 2495. doi: 10.2174/0929867033456477..
(6) Kowaltowski, A. J.; de Souza-Pinto, N. C.; Castilho, R. F.; Vercesi, A. E.2009,, 333. doi: 10.1016/j.freeradbiomed.2009.05.004.
(7) Dickinson, B. C.; Srikun, D.; Chang, C. J.2010,, 50. doi: 10.1016/j.cbpa.2009.10.014.
(8) Weinberg, S. E.; Chandel, N. S.2015,, 9. doi: 10.1038/.nchembio.1712.
(9) Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J. I.2008,, 661. doi: 10.1126/science.1156906.
(10) Kals, J.; Kampus, P.; Kals, M.; Zilmer, K.; Kullisaar, T.; Teesalu, R.; Pulges, A.; Zilmer, M.2006,, 902. doi: 10.1016/j.amjhyper.2006.02.003.
(11) Wisløff, U.; Najjar, S. M.; Ellingsen, Ø.; Haram, P. M.; Swoap, S.; Al-Share, Q.; Fernström, M.; Rezaei, K.; Lee, S. J.; Koch, L. G.; Britton, S. L.2005,, 418. doi: 10.1126/science.1108177.
(12) Van Dam, P. S.2002,, 176. doi: 10.1002/dmrr.287.
(13) Newsholme, P.; Haber, E. P.; Hirabara, S. M.; Rebelato, E. L. O.; Procopi, J.; Morgan, D.; Oliveira-Emilio, H. C.; Carpinelli, A. R.; Curi, R.2007,, 9. doi: 10.1113/jphysiol.2007.135871.
(14) Dhalla, N. S.; Temsah, R. M.; Netticadan, T.2000,, 655. doi: 10.1097/00004872-200018060-00002.
(15) Li, X.; Gao, X.; Shi, W.; Ma, H.2014,, 590. doi: 10.1021/cr300508p.
(16) Yang, Y.; Zhao, Q.; Feng, W.; Li, F.2013,, 192. doi: 10.1021/cr2004103.
(17) Lou, Z.; Li, P.; Song, P.; Han, K.2013,, 6291. doi: 10.1039/c3an00198a.
(18) Lou, Z.; Li, P.; Pan, Q.; Han, K.2013,, 2445. doi: 10.1039/c3cc39269d.
(19) Wang, B.; Li, P.; Yu, F.; Chen, J.; Qu, Z.; Han, K.2013,, 5790. doi: 10.1039/c3cc42313a.
(20) Wang, B.; Li, P.; Yu, F.; Song, P.; Sun, X.; Yang, S.; Lou, Z.; Han, K.2013,, 1014. doi: 10.1039/c2cc37803e.
(21) Yu, F.; Li, P.; Li, G.; Zhao, G.; Chu, T.; Han, K.2011,, 11030. doi: 10.1021/ja202582x.
(22) Yu, F.; Li, P.; Wang, B.; Han, K.2013,, 7674. doi: 10.1021/ja401360a.
(23) Soh, N.2006,, 532. doi: 10.1007/s00216-006-0366-9.
(24) Chen, X.; Tian, X.; Shin, I.; Yoon, J.2011,, 4783. doi: 10.1039/c1cs15037e.
(25) McQuade, L. E.; Lippard, S. J.2010,, 43. doi: 10.1016/j.cbpa.2009.10.004.
(26) Miller, E. W.; Chang, C. J.2007,, 620. doi: 10.1016/j.cbpa.2007.09.018.
(27) Gomes, A.; Fernandes, E.; Lima, J.2005,, 45. doi: 10.1016/j.jbbm.2005.10.003.
(28) Lou, Z.; Li, P.; Han, K.: Selenium as a Versatile Center in Fluorescence Probe for the Redox Cycle Between HClO Oxidative Stress and H2S Repair. In; Armstrong, D., Ed., 2015; Vol. 1208; pp 97−110.
(29) Xu, Z.; Xu, L.2016,, 1094. doi: 10.1039/c5cc09248e.
(30) Kim, H. M.; Cho, B. R.2013, 323619. doi: 10.1155/2013/323619.
(31) Zhu, H.; Fan, J.; Du, J.; Peng, X.2016,, 2115. doi: 10.1021/acs.accounts.6b00292.
(32) Xu, W.; Zeng, Z.; Jiang, J. H.; Chang, Y. T.; Yuan, L.2016,, 13658. doi: 10.1002/anie.201510721.
(33) Lou, Z.; Li, P.; Han, K.2015,, 1358. doi: 10.1021/acs.accounts.5b00009.
(34) Zhang, X.; Gao, F.2015,, 374. doi: 10.3109/10715762.2015.1014813.
(35) Xiao Y., Chen L., Lv W., Nan F.2016,, 538. doi: 10.13488/j.smhx.20160420.
(36) Hoye, A. T.; Davoren, J. E.; Wipf, P.; Fink, M. P.; Kagan, V. E.2008,, 87. doi: 10.1021/ar700135m.
(37) Yousif, L. F.; Stewart, K. M.; Kelley, S. O.2009,, 1939. doi: 10.1002/cbic.200900185.
(38) Rhee, S. G.2006,, 1882. doi: 10.1126/science.1130481.
(39) Stone, J. R.; Yang, S.2006,, 243. doi: 10.1089/ars.2006.8.243.
(40) Dickinson, B. C.; Peltier, J.; Stone, D.; Schaffer, D. V.; Chang, C. J.2011,, 106. doi: 10.1038/nchembio.497.
(41) Opazo, C.; Huang, X. D.; Cherny, R. A.; Moir, R. D.; Roher, A. E.; White, A. R.; Cappai, R.; Masters, C. L.; Tanzi, R. E.; Inestrosa, N. C.; Bush, A. I.2002,, 40302. doi: 10.1074/jbc.M206428200.
(42) Van Camp, W.; Van Montagu, M.; Inze, D.1998,, 330. doi: 10.1016/s1360-1385(98)01297-7.
(43) Dickinson, B. C.; Chang, C. J.2008,, 9638. doi: 10.1021/ja802355u.
(44) Dickinson, B. C.; Lin, V. S.; Chang, C. J.2013,, 1249. doi: 10.1038/nprot.2013.064.
(45) Kim, H. M.; Cho, B. R.2015,, 5014. doi: 10.1021/cr5004425.
(46) Yu, H.; Xiao, Y.; Guo, H.; Qian, X.2011,, 3179. doi: 10.1002/chem.201002498.
(47) Komatsu, K.; Urano, Y.; Kojima, H.; Nagano, T.2007,, 13447. doi: 10.1021/ja072432g.
(48) Srikun, D.; Miller, E. W.; Domaille, D. W.; Chang, C. J.2008,, 4596. doi: 10.1021/ja711480f.
(49) Masanta, G.; Heo, C. H.; Lim, C. S.; Bae, S. K.; Cho, B. R.; Kim, H. M.2012,, 3518. doi: 10.1039/c2cc00034b.
(50) Robinson, K. M.; Janes, M. S.; Pehar, M.; Monette, J. S.; Ross, M. F.; Hagen, T. M.; Murphy, M. P.; Beckman, J. S.2006,, 15038. doi: 10.1073/pnas.0601945103.
(51) McCord, J. M.; Roy, R. S.1982,, 1346.
(52) Li, P.; Zhang, W.; Li, K.; Liu, X.; Xiao, H.; Zhang, W.; Tang, B.2013,, 9877. doi: 10.1021/ac402409m.
(53) Si, F.; Liu, Y.; Yan, K.; Zhong, W.2015,, 7931. doi: 10.1039/c5cc01075f.
(54) Hampton, M. B.; Kettle, A. J.; Winterbourn, C. C.1998,, 3007.
(55) Yap, Y. W.; Whiteman, M.; Cheung, N. S.2007,, 219. doi: 10.1016/j.cellsig.2006.06.013.
(56) Sugiyama, S.; Kugiyama, K.; Aikawa, M.; Nakamura, S.; Ogawa, H.; Libby, P.2004,, 1309. doi: 10.1161/01.ATV.0000131784.50633.4f.
(57) Weitzman, S. A.; Gordon, L. I.1990,, 655.
(58) Chan, D. C.2006,, 1241. doi: 10.1016/j.cell.2006.06.010.
(59) Yuan, L.; Lin, W.; Yang, Y.; Chen, H.2012,, 1200. doi: 10.1021/ja209292b.
(60) Cheng, G.; Fan, J.; Sun, W.; Sui, K.; Jin, X.; Wang, J.; Peng, X.2013,, 6091. doi: 10.1039/c3an01152f.
(61) Hou, J. T.; Wu, M. Y.; Li, K.; Yang, J.; Yu, K. K.; Xie, Y. M.; Yu, X. Q.2014,, 8640. doi: 10.1039/c4cc02673j.
(62) Hou, J. T.; Li, K.; Yang, J.; Yu, K. K.; Liao, Y. X.; Ran, Y. Z.; Liu, Y. H.; Zhou, X. D.; Yu, X. Q.2015,, 6781. doi: 10.1039/c5cc01217a.
(63) Li, G.; Lin, Q.; Ji, L.; Chao, H.2014,, 7918. doi: 10.1039/c4tb01251h.
(64) Li, G.; Lin, Q.; Sun, L.; Feng, C.; Zhang, P.; Yu, B.; Chen, Y.; Wen, Y.; Wang, H.; Ji, L.; Chao, H.2015,, 285. doi: 10.1016/j.biomaterials.2015.02.106.
(65) Yuan, L.; Wang, L.; Agrawalla, B. K.; Park, S. J.; Zhu, H.; Sivaraman, B.; Peng, J.; Xu, Q. H.; Chang, Y. T.2015,, 5930. doi: 10.1021/jacs.5b00042.
(66) Xiao, H.; Xin, K.; Dou, H.; Yin, G.; Quan, Y.; Wang, R.2015,, 1442. doi: 10.1039/c4cc07411d.
(67) Xiao, H.; Li, J.; Zhao, J.; Yin, G.; Quan, Y.; Wang, J.; Wang, R.2015,, 1633. doi: 10.1039/c4tb02003k.
(68) Zhou, J.; Li, L.; Shi, W.; Gao, X.; Li, X.; Ma, H.2015, 6, 4884. doi: 10.1039/c5sc01562f.
(69) Xu, J.; Yuan, H.; Qin, C.; Zeng, L.; Bao, G.-M.2016,, 107525. doi: 10.1039/c6ra22868b.
(70) Freinbichler, W.; Colivicchi, M. A.; Stefanini, C.; Bianchi, L.; Ballini, C.; Misini, B.; Weinberger, P.; Linert, W.; Vareslija, D.; Tipton, K. F.; Della Corte, L.2011,, 2067. doi: 10.1007/s00018-011-0682-x.
(71) Koide, Y.; Urano, Y.; Kenmoku, S.; Kojima, H.; Nagano, T.2007,, 10324. doi: 10.1021/ja073220m.
(72) Liu, F.; Wu, T.; Cao, J.; Zhang, H.; Hu, M.; Sun, S.; Song, F.; Fan, J.; Wang, J.; Peng, X.2013,, 775. doi: 10.1039/c2an36030f.
(73) Garner, A. L.; St Croix, C. M.; Pitt, B. R.; Leikauf, G. D.; Ando, S.; Koide, K.2009,, 316. doi: 10.1038/nchem.240.
(74) Zhang, Q. H.; Powers, E. T.; Nieva, J.; Huff, M. E.; Dendle, M. A.; Bieschke, J.; Glabe, C. G.; Eschenmoser, A.; Wentworth, P.; Lerner, R. A.; Kelly, J. W.2004,, 4752. doi: 10.1073/pnas.0400924101.
(75) Xu, K.; Sun, S.; Li, J.; Li, L.; Qiang, M.; Tang, B.2012,, 684. doi: 10.1039/c1cc15844a.
(76) Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M. T. D.; Mazur, M.; Telser, J.2007,, 44. doi: 10.1016/j.biocel.2006.07.001.
(77) Nordberg, J.; Arner, E. S. J.2001,, 1287. doi: 10.1016/s0891-5849(01)00724-9.
(78) Hirosawa, S.; Arai, S.; Takeoka, S.2012,, 4845. doi: 10.1039/c2cc30603d.
(79) Yapici, N. B.; Mandalapu, S.; Gibson, K. M.; Bi, L.2015,, 3476. doi: 10.1016/j.bmcl.2015.07.011.
(80) Bhabak, K. P.; Mugesh, G.2010,, 1408. doi: 10.1021/ar100059g.
(81) Mugesh, G.; du Mont, W.-W.; Sies, H.2001,, 2125. doi: 10.1021/cr000426w.
(82) Xu, K.; Chen, H.; Tian, J.; Ding, B.; Xie, Y.; Qiang, M.; Tang, B.2011,, 9468. doi: 10.1039/c1cc12994e.
(83) Xu, K.; Qiang, M.; Gao, W.; Su, R.; Li, N.; Gao, Y.; Xie, Y.; Kong, F.; Tang, B.2013,, 1079. doi: 10.1039/c2sc22076h.
(84) Visser, A.; Ghisla, S.; Massey, V.; Muller, F.; Veeger, C.1979,, 13. doi: 10.1111/j.1432-1033.1979.tb04210.x.
(85) Kaur, A.; Brigden, K. W. L.; Cashman, T. F.; Fraser, S. T.; New, E. J.2015,, 6686. doi: 10.1039/c5ob00928f.
(86) Yeow, J.; Kaur, A.; Anscomb, M. D.; New, E. J.2014,, 8181. doi: 10.1039/c4cc03464c.
(87) Snyder, S. H.1992,, 494. doi: 10.1126/science.1353273.
(88) Hall, C. N.; Garthwaite, J.2009,, 92. doi: 10.1016/j.niox.2009.07.002.
(89) Wang, P. G.; Xian, M.; Tang, X. P.; Wu, X. J.; Wen, Z.; Cai, T. W.; Janczuk, A. J.2002,, 1091. doi: 10.1021/cr000040l.
(90) Yu, H.; Zhang, X.; Xiao, Y.; Zou, W.; Wang, L.; Jin, L.2013,, 7076. doi: 10.1021/ac401916z.
(91) Zheng, H.; Shang, G. Q.; Yang, S. Y.; Gao, X.; Xu, J. G.2008,, 2357. doi: 10.1021/ol800206x.
(92) Sun, Y. Q.; Liu, J.; Zhang, H.; Huo, Y.; Lv, X.; Shi, Y.; Guo, W.2014,, 12520. doi: 10.1021/ja504156a.
(93) Chen, X.; Sun, L.; Chen, Y.; Cheng, X.; Wu, W.; Ji, L.; Chao, H.2015,, 72. doi: 10.1016/j.biomaterials.2015.04.012.
(94) Koppenol, W. H.; Moreno, J. J.; Pryor, W. A.; Ischiropoulos, H.; Beckman, J. S.1992,, 834. doi: 10.1021/tx00030a017.
(95) Pacher, P.; Beckman, J. S.; Liaudet, L.2007,, 315. doi: 10.1152/physrev.00029.2006.
(96) Zhang, H.; Liu, J.; Sun, Y. Q.; Huo, Y.; Li, Y.; Liu, W.; Wu, X.; Zhu, N.; Shi, Y.; Guo, W.2015,, 2721. doi: 10.1039/c4cc09122a.
(97) Chen, J. S.; Zhao, G. J.; Cook, T. R.; Han, K. L.; Stang, P. J.2013,, 6694. doi: 10.1021/ja402421w.
(98) Yang, S.; Liu, J.; Zhou, P.; He, G.2011,, 10692. doi: 10.1021/jp2044288.
(99) Zhao, G. J.; Han, K. L.2012,, 404. doi: 10.1021/ar200135h.
(100) Zhou, P.; Liu, J.; Yang, S.; Chen, J.; Han, K.; He, G.2012,, 15191. doi: 10.1039/c2cp42167d.
(101) Kowalczyk, T.; Lin, Z.; Van Voorhis, T.2010,, 10427. doi: 10.1021/jp103153a.
(102) Li, G. Y.; Song, P.; He, G. Z.2011,, 305. doi: 10.1088/1674-0068/24/03/305-310.
(103) Li, G. Y.; Zhao, G. J.; Han, K. L.; He, G. Z.2011,, 668. doi: 10.1002/jcc.21651.
(104) Li, G. Y.; Zhao, G. J.; Liu, Y. H.; Han, K. L.; He, G. Z.2010,, 1759. doi: 10.1002/jcc.21466.
(105) Li, G. Y.; Chu, T. S.2011,, 20766. doi: 10.1039/c1cp21470e.
(106) Guo, H.; Jing, Y.; Yuan, X.; Ji, S.; Zhao, J.; Li, X.; Kan, Y.2011,, 3844. doi: 10.1039/c0ob00910e.
(107) Shao, J.; Sun, H.; Guo, H.; Ji, S.; Zhao, J.; Wu, W.; Yuan, X.; Zhang, C.; James, T. D.2012,, 1049. doi: 10.1039/c2sc00762b.
用于检测生物体系中线粒体活性氧的荧光探针
楼张蓉 李 鹏 韩克利*
(中国科学院大连化学物理研究所,分子反应动力学国家重点实验室,辽宁 大连 116023)
线粒体不仅是细胞的能量单元,还是重要的活性氧产生场所。线粒体内的活性氧与正常的生理功能和人类疾病具有紧密的联系,包括细胞信号传导,损伤核酸蛋白质以及诱导氧化应激。然而,线粒体活性氧和细胞病态之间的复杂联系还研究得不够透彻。有效检测线粒体内活性氧的手段有助于研究线粒体内活性氧在各种人类疾病中所起的作用。近年来,发展了许多具有高灵敏度和选择性的荧光探针用于检测线粒体内活性氧。基于这一点,我们综述了用于选择性检测线粒体内活性氧的小分子荧光探针的研究进展,并详细讨论了荧光探针的设计、合成、特点及其应用。
荧光探针;线粒体;活性氧;小分子;活细胞
O641
10.3866/PKU.WHXB201704284
February 22, 2017;
April 13, 2017;
April 28, 2017.
Corresponding author. Email: klhan@dicp.ac.cn; Fax: +86-411-84675584.
The project was supported by the National Natural Science Foundation of China (21503224, 21673237).
国家自然科学基金(21503224, 21673237)资助项目

