Response of Microcystis aeruginosa FACHB-905 to different nutrient ratios and changes in phosphorus chemistry*
PENG Guotao (彭国涛) Steven W. WILHELM LIN Sijie (林思劼) WANG Xiangrong (王祥荣)
Abstract Cyanobacterial blooms are a global problem, with their occurrence tightly tied to nutrient loading. We cultured Microcystis aeruginosa FACHB-905 in growth medium with either inorganic(orthophosphate) or organic (β-glycerophosphate or polyphosphate) phosphorus and at different N:P ratios with 50:1, 30:1, 16:1, 4:1 and 1:4, serving as the phosphorus source. Fluorescence parameters were measured to determine the response of cellular responses to nutrient stress. Scanning electron microscopy (SEM) and estimates of antioxidant activity were employed to examine potential mechanisms of physical change. The results demonstrate that inorganic phosphorus was more bioavailable to M. aeruginosa relative to organic phosphorus in culture. The highest cell concentration (2.21×10 6 cells/mL), chlorophyll- a (0.39 pg/cell) and phycocyanin (1.57 pg/cell) quotas and high levels of chlorophyll fluorescence parameters ( rETR, E k, α,φPS II and Fv/ Fm) were obtained when phosphorus was supplied as K 2 HPO 4 at a N:P ratio of 16–30. Organic sources of phosphorus (β-glycerophosphate and polyphosphate) were bioavailable to M. aeruginosa. In addition, too concentrated orthophosphate (N:P=1:4) resulted in the oxidative stress and lipid peroxidation of cell membrane (identi fied by the antioxidant system activity), and the photosynthetic activity declined consequently. This study has demonstrated the effects of different phosphorus chemistries and N:P ratios on the cyanobacterial growth, photosynthetic activity and cell physiology, which could be an effective tool for predicting cyanobacterial dominance or N-de ficiency in natural lakes (due to the superior ability of cyanobacteria for dissolved N and fix atmospheric N in some cases).
Keyword: antioxidant system; chlorophyll fluorescence parameters; Microcystis; nutrient stress;photosynthesis
1 INTRODUCTION
Over the last three decades, harmful algal blooms(HABs) have occurred frequently in many large shallow lakes, including Taihu Lake and Dianchi in China as well as Lake Erie in North America (Steffen et al., 2014a; Harke et al., 2016). Harmful algal blooms threaten the economic and recreational uses of lakes, and in some case the health of human beings:indeed HABs are now seen as a threat to global freshwater resources (Peng et al., 2015; Bullerjahn et al., 2016). Microcystis is a widespread cyanobacterial genus that is known to dominate eutrophic lakes experiencing the cyanobacteria blooms (Paerl and Otten, 2013). Like many cyanobacteria, blooms of Microcystis have been associated with warm temperatures (more than 25°C), light intensity, and changes in the availability of nitrogen and phosphorus(Hecky and Kilham, 1988; Harke and Gobler, 2013;Kuniyoshi et al., 2013).
For decades, it has been hypothesized that phosphorus (P) availability is a key factor limiting primary producer biomass in freshwater ecosystems(Wilhelm et al., 2003; Schindler et al., 2008), although recent studies have demonstrated that nitrogen (N)-availability may also in fluence this process (Paerl et al., 2016). P affects the physiology of cells through a range of cellular processes including protein, sugar,and nucleic acid metabolism. Research has indicated that in bloom-forming cyanobacteria such as Microcystis, P-de ficiency results in reduced growth rates and down-regulation of its photosynthetic apparatus (Wang et al., 2010). Assays have demonstrated that bioavailable phosphorus (BAP)includes both dissolved phosphorus (DP) and a fraction of particulate phosphorus (PP) that is suspended in the water column (Okubo et al., 2012).Thus, it is not only the concentration of available phosphorus, but the chemistry of that phosphorus that in fluences cyanobacterial bloom dynamics. Indeed,there are suggestions that an increased proportion of soluble reactive phosphorus (SRP) in Lake Erie in flows has led to the large bloom events observed in recent years (Baker et al., 2014; Kane et al., 2014). A main source of phosphorus in lake systems are orthophosphates, which are substrates for phosphorylation of ADP and thus can have signi ficant effect on the ability of algal cells to generate ATP(Shelly et al., 2005; Yang et al., 2014). However,when dissolved inorganic phosphorus (DIP) is low,Microcystis spp. readily obtain phosphorus from dissolved organic phosphorus (DOP) sources to sustain the growth (Shi et al., 2011).
Recent studies have demonstrated that Microcystis displays variable responses at the biomolecular level when exposed to nutrient sources comprised of different N-containing chemical species and concentrations (Steffen et al., 2014b). For example,results from field work studying Microcystis spp. in the Klamath River have shown an increase in total microcystin (MC) cell quota ( QMC) with increasing river N concentrations (Moisander et al., 2009).Evidence suggests that ratios of N to P, and not concentrations of either alone, are important in modulating physiological response of bloom-forming cyanobacteria. In a review that considered both laboratory and field studies, Hyenstrand et al. (1998)concluded a low N:P ratio (29) to be one of nine principal factors in fluencing the success or failure of cyanobacteria in lake ecosystems. High N:P ratios(~20–50) favored the dominance of green algae(Bulgakov and Levich, 1999) or diatoms in Lake Okeechobee (Florida, USA) and Taihu Lake (China)(McCarthy et al., 2009). However, generalizations of optimum N:P ratios are not universal and con flicting results have been reported. For example, in lab work,the optimal growth of a Microcystis aeruginosa strain isolated from Lake Biwa, Japan, occurred at a N:P ratio of 100:1 and declined at lower ratios(N-de ficiency) or high ratios (P-de ficiency)(Nalewajko and Murphy, 2001).
Despite the clear importance of P in aquatic systems, reports on the in fluence of P chemistries and N:P ratios on Microcystis abundance and physiology are inconsistent. Moreover, few studies have directly evaluated the responses of Microcystis photosynthetic and antioxidant system activity under differing N:P ratios and nutrient chemsitries. In this study,Microcystis aeruginosa FACHB-905 was grown in three chemical forms of P at five N:P molar ratios.Physiological response of the cells to nutrient conditions were monitored by measuring cell concentration, pigment concentration, antioxidant system activity, and fluorescence-based photosynthetic parameters (rETR, Ek, α, φPSIIand Fv/ Fm). In addition,we examined cells with scanning electron microscopy(SEM) to determine integrity. Results of this study improve our understanding of the physiological response of Microcystis under environmentally relevant changes in nutrient chemistry and availability.It may thus provide better insight into ongoing efforts to predict and manage cyanobacterial harmful algal blooms.
2 MATERIAL AND METHOD
2.1 Strain and culture conditions
Microcystis aeruginosa (FACHB-905) was purchased from the Institute of Hydrobiology, Chinese Academy of Sciences (Wuhan, China) and maintained in BG11medium (Rippka et al., 1979). Cells in exponential growth phase were collected by centrifugation at 6 000× g for 10 min and washed three times with P-free BG11medium prior to the onset of the experiment. Subsequently, cultures were starved in P-free medium for 72 h under growth conditions of 25°C, 40 μmol photons (PAR)/(m2·s) photon irradiance(incandescent tubes), and a 12 h:12 h LD cycle. After P starvation, cells were re-inoculated into 500 mL glass flasks at an initial cell concentration of~1×106cells/mL and cultured for 11 days under each condition. NaNO3(17.6 mmol/L) was included in the medium of each treatment: this concentration is consistent with the total N concentration in BG11growth medium. Three chemical forms of P were used as treatment: inorganic phosphorus (as K2HPO4), and two kinds of organic phosphorus (as β-glycerophosphate disodium and sodium polyphosphate). For treatments,the N:P ratio was manipulated by varying the P concentration; N remained at 17.6 mmol/L in all treatments. N and P were supplied at molar ratios of 50:1, 30:1, 16:1, 4:1 and 1:4, with a corresponding P of 0.35, 0.59, 1.1, 4.4 and 70.4 mmol/L (P equivalent)respectively. Cell concentration was determined daily based on optical density measured at 650 nm. 50 mL of cells were collected every three days for the measurement of pigment content. Photosynthetic efficiency of cells in each treatment was determined every two days. Cells in K2HPO4medium were sampled for the investigation of N:P ratio effects on the morphology and antioxidant system activities. All experiments were performed in triplicate.
2.2 Measurement of cell concentration and growth rate
The relationship between absorbance and cell concentration was studied previously (Peng et al.,2016) and fits the linear regression equation:
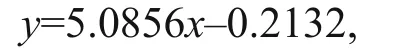
where x is the absorbance at 650 nm and y is the algal cell concentration (×106cells/mL), R²=0.97. OD650was measured daily and converted to an estimated cell concentration using the above equation. The speci fic cell division rate ( μ) of each treatment during exponential growth was then calculated according to the first order rate equation:

where Nt2, Nt1represent the cell concentration at t2and t1, respectively; ln is the natural logarithm of cell number.
2.3 Chlorophyll- a and phycocyanin cell quotas
Chlorophyll- a was extracted into 90% acetone for 24 h in the dark at 4°C (Parsons et al., 1984). A 0.05-mol/L (pH 6.8) phosphate buffer solution (PBS)was used to extract phycocyanin (PC) using the method of Padgett and Krogmann (1987). For extractions, cells were disrupted by three repetitive freezes in liquid N2and thawing in a 4°C water bath. Spectral absorbance at 630, 645, 663 and 750 nm were measured to calculate the concentration of chlorophyll-a according to the formula (Parsons et al., 1984):


where, V is the volume of the sampling cultures(50 mL); V1is the constant volume of the extracted cultures (10 mL); δ is the range of the cuvette(10 mm). For phycocyanin (PC), the concentration was determined spectrophotometrically using the following equation (Padgett and Krogmann, 1987):
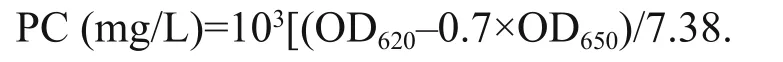
The contents of Chl- a and PC were normalized to cell concentration before statistical analysis.
2.4 Estimation of photosynthetic efficiency
Photosynthetic efficiency of cultures was examined by estimating PS II efficiency with a WATER-PAM Chlorophyll Fluorometer (Walz, Effectnich,Germany). Microcystis cultures were dark adapted for 15 min and then exposed to a sequence of increasing actinic irradiance in 8 preset discrete increments ranging from 56 μmol photons/(m2·s) to 915 μmol photons/(m2·s). Each actinic light incubation lasted for 20 s before a saturation pulse of blue light(~1 800 μmol photons/(m2·s), 600 ms) was applied to determine the relative electron transport (rETR) at each irradiance level. rETR was calculated as follows:

where, ( Fm'– F') Fm' is termed the PS II operating efficiency ( φPSII). At a given photosynthetically active photon flux density (PPFD) this parameter provides an estimate of the quantum yield of liner electron flux through PS II (Baker, 2008). The maximal rate of rETR (rETRmax) and photosynthetic efficiency ( α)were determined by fitting the rapid light response curve to an exponential function modi fied from Platt et al. (1980):

where I represents irradiance. The saturation values( Ek) are determined from the interception point of α value with the maximum photosynthetic rate, which follows the equation (Platt et al., 1980):

The maximum quantum photochemical efficiency of PS II was calculated as the following formula (Ting and Owens, 1992):
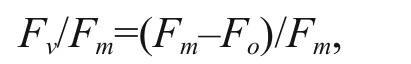
where Fois the fluorescence of dark-adapted cells stimulated by a weak probe light immediately following 15 min of darkness; Fmis the maximum fluorescence signal following the closure of all reaction centers by a 600-ms pulse of saturating irradiance.

Fig.1 Growth curves of M. aeruginosa grown in media with different N:P ratios: K 2 HPO 4 (a), β-glycerophosphate (b) and polyphosphate (c) medium
2.5 SEM
The morphology and integrity of cyanobacterial cells was determined via electron microscopy using a desktop scanning electron microscope (SEM)(Phenom Pro, China). The pretreatment of the samples were as follows: (1) 16 mL of 25% glutaraldehyde and 84 mL of 0.2 mol/L PBS (pH 7.2) were mixed to prepare the preservation solution; (2) 40 mL of cultures were collected every sampling time point,and after centrifugation at 6 000× g, the cells were suspended in the preservation solution for 4 h; (3)after centrifugation to remove the supernatant, the cells were washed with the preservation solution three times; (4) gradient concentration of ethanol at 30%,50%, 70%, 80%, 90%, 100% were used to successively elute the cells; (5) after the last elution, samples were centrifuged to collect cells for the examination by SEM.
2.6 Assessment of antioxidants
Antioxidant system activity of cells was measured with the Malondialdehyde (MDA) assay kit (TBA method) (Placer et al., 1966), the total superoxide dismutase (T-SOD) assay kit (hydroxylamine method)(Winterbourn et al., 1975) and the reduced glutathione(GSH) assay kit (spectrophotometric method,Hafeman et al., 1974), all supplied by the Nanjing Jiancheng Bioengineering Institute, China. Cultures(60 mL) were collected on a Whatman GF/C filter(nominal particle retention of 1.2 μm). The filter membrane was ground with liquid N2then 6 mL of 0.05 mol/L PBS was added to the sample. After centrifugation at 6 000× g for 15 min, the supernatant was assayed for enzyme activity using a spectrophometer (UV-2100, UNICO, Shanghai,China).
2.7 Statistical analysis
All experiments were performed with three biological replicates. Data are reported as the mean±standard deviation. Origin 8.0 (OriginLab, US)was used for one- and two-way analysis of variance(ANOVA) to test for statistical differences between treatments in data relating to cell concentration,growth rate, effective and maximum quantum efficiency of PS II, and enzyme activities. Tukey’s post hoc tests were used to determine differences among means in ANOVA tests generating signi ficant results. An α=0.05 was used to determine statistical signi ficance. T-tests were performed to evaluate the signi ficant differences for each enzyme between day 2 and 4.
3 RESULT
3.1 Cell concentration and cell division rate
Cell division curves for each treatment are shown in Fig.1. Speci fic cell division rates ( μ) determined during the exponential growth phase and the corresponding highest cell concentrations are listed in Table 1. Increase in cell concentration fluctuated slightly in the first three days (adaptive phase) and then entered the logarithmic phase at day 3 (Fig.1).For most N:P ratios, the highest cell concentrations in K2HPO4-media were obtained around day 8, about one or two days sooner than cultures grown in either of the organic P-media (Table 1). Cultures in K2HPO4-medium achieved the highest growth rate at each N:P ratio except at 50:1. The highest growth rate of 0.04/d was obtained at an N:P ratio of 4:1 in K2HPO4-medium. There was no signi ficant difference in highest cell concentration (~2.1×106cells/mL) among the 50:1 and 30:1 regardless of P source. Cell concentrations were signi ficantly higher in the cultures grown in K2HPO4media at an N:P ratio of 16:1, 4:1 and 1:4, compared to those grown in organic phosphorus media ( P <0.05). Growth rates and cell concentration of cultures grown in polyphosphatemedia decreased as N:P ratio dropped, with lowest values occurring at the N:P ratio of 1:4 ( P <0.05).

Table 1 The growth rate and maximum cell concentration of the cultures with different phosphorus sources at different N:P ratios

Table 2 The concentration of chlorophyll a and phycobiliprotein pg/cell with different medium at day 6 and day 9
3.2 Pigment concentration
Cell quotas of chlorophyll- a ( Qchl) and phycocyanin( Qphy) during the exponential growth phase (day 6 to day 9) are provided in Table 2. In K2HPO4media,cultures at an N:P ratio of 16:1 had the highest Qchland Qphyof 0.38±0.01 and 1.57±0.01 pg ( P <0.05),respectively, which corresponds to total concentrations of 0.75±0.03 and 3.1±0.1 mg/L (50 mL culture, data not shown). The peak content of PC (3.2±0.1 mg/L,1.55±0.05 pg/cell) appeared at day 9 at an N:P ratio of 30:1. The lowest Chl- a and PC concentrations occurred at a N:P ratio of 1:4.
For cultures grown in organic phosphorus sources,the peak Qchland Qphywere observed at an N:P ratio of 30:1 in β-glycerophosphate medium and occured at day 6 and 9 respectively. Cultures grown in polyphosphate media had peak Qchland Qphyoccurring on day 6 at an N:P ratio of 50:1 and day 9 at a ratio of 4:1 respectively ( P <0.05). At the N:P ratio of 1:4, Qchland Qphydeclined over time in all treatments, probably due to the low growth rate and cell concentration observed (Table 1).
3.3 Photosynthetic activity
3.3.1 Rapid light curves
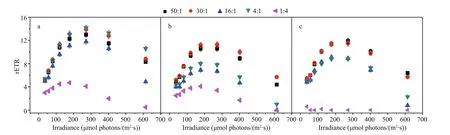
Fig.2 The rapid light curve (RLC) of different N:P ratios: K 2 HPO 4 (a), β-glycerophosphate (b) and polyphosphate (c)medium at day 4

Table 3 The values of rETR max, E k and α with different phosphorus sources
Figure 2 illustrates the rapid light curve (RLC) of samples collected on day 4. Cells displayed healthy photosynthetic activity for each treatment with the exception of those growing in media with an N:P ratio of 1:4 (severely N-limited). Cells grown in media with N:P ratios of 30:1 and 50:1 showed a strong resilience to high light intensity regardless of phosphorus source. In addition, high rETR values were observed at the low N:P ratio of 4:1 if the phosphorus source was inorganic. However, the rETR of the cultures growing in β-glycerophosphate and polyphosphate media fell to nearly 0 in many treatments after day 6 (data not shown). The rETRmax,Ekand α values of each culture at day 4 are described in Table 3. The values of these three parameters were 0 at the N:P ratio of 1:4, since rETR values were too low to fit the model of Platt (Platt et al., 1980). The rETRmaxfor treatment groups were around 14, 11 and 12 μmol photons/(m2·s), which occurred at the N:P ratios of 50:1 and 30:1 in β-glycerophosphate and polyphosphate media and at 4:1 in K2HPO4-medium(one-way ANOVA). The cultures in K2HPO4media had the highest Ekvalues compared to the other phosphorus-source treatments (two-way ANOVA,P <0.05), with the maximum value of 95.83 μmol photons/(m2·s). There was no signi ficant difference among cultures in the organic phosphorus treatments(two-way ANOVA). Photosynthetic efficiency ( α)values of the cultures in K2HPO4-media were signi ficant higher than the other groups at the N:P ratios of 30:1, 16:1, and 4:1 ( P <0.05), suggesting that cells used light energy with higher efficiency when grown with an inorganic phosphate source.
3.3.2 φPSIIand Fv/ Fm
Effective and maximum quantum efficiency of PS II among N:P ratio treatments are illustrated in Fig.3.In the first four days, high φPSIIvalues were observed from cells grown in K2HPO4-media (Fig.3a) at all N:P ratios except 1:4 ( P <0.05). After day 4, cells grown in K2HPO4-medium at an N:P ratio of 30:1 demonstrated the photosynthetic activity consistently higher than those of other N:P ratios. However, the highest φPSIIvalues were observed in cells grown in β-glycerophosphate-medium at the N:P ratio of 50:1( P <0.05), while those grown at the ratio of 30:1 possessed high φPSIIvalues during the first four days(Fig.3b). In comparison, the highest effective quantum yield of the cultures grown in polyphosphate-media occurred at the ratio of 30:1 and 16:1 ( P <0.05, Fig.3c).
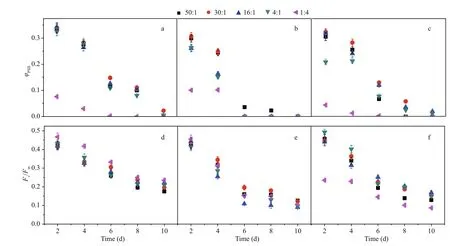
Fig.3 The effective and maximum photochemical efficiency of PS II at different N:P ratios with K 2 HPO 4 (a, d),β-glycerophosphate (b, e) and polyphosphate medium (c, f)

Fig.4 SEM images of M. aeruginosa cells cultured in standard BG11 medium (a) and in K 2 HPO 4 medium with an N:P ratio of 1:4 (b, c) (25 000×)
The maximum quantum efficiency of M. aeruginosa detected after 15 min of dark adaptation re flects potential photosynthetic activity. The values of Fv/ Fmin all cultures were high at the beginning of the experiment, with an average of almost 0.45. There was a rapid decline in Fv/ Fmin cells grown in β-glycerophosphate and polyphosphate media after day 6 (Fig.3d–e). Maximum Fv/ Fmvalues in cells grown in β-glycerophosphate-media were observed at an N:P ratio of 30:1 while maximum Fv/ Fmin cells grown in polyphosphate-media occurred at an N:P ratio of 16:1 and 4:1 during the culture period (oneway ANOVA, P <0.05).
3.4 Cellular ultrastructure
SEM was conducted to determine the morphological changes in M. aeruginosa cells as a results of different N:P ratios in culture media. The image of cells cultured with standard BG11medium (K2HPO4-P) is shown in Fig.4a. M. aeruginosa cells appear in healthy condition, with the smooth surface and oval shape.After being cultured for six days in K2HPO4-medium with a N:P ratio of 1:4, cells appeared deformed,shriveled, and with an accumulation of unidenti fied extracellular substance surrounding the cells (Fig.4b).Near the end of the experiment, there appeared to be an increase in the extracellular substance surrounding the cells. Most of the cells had suffered a flattening or severe distortion leaving them with an aberrant shape,suggesting that many of the cells were dead (Fig.4c).
3.5 Examination of cellular antioxidant systems
MDA, SOD, and GSH contents of Microcystis cells cultured in K2HPO4-media at varied N:P ratios are shown in Fig.5. At day 2, the concentration of MDA fluctuated slightly while the SOD and GSH content increased signi ficantly ( P <0.05), reaching about 0.89 and 1.35 U 10-6cells, respectively.Furthermore, there was a very large increase in GSH between day 2 and day 4 ( P <0.001). The maximum values of GSH (~3.5 U 10-6cells at N:P=50:1 and 30:1, P <0.05), were concurrent with sharp declines in MDA content and SOD activity. SOD activity fell to nearly 0 by day 4.
4 DISCUSSION
Phosphorus is often considered a key element limiting biomass production in lakes (Schindler et al.,2008; Cao et al., 2010). However, P exists in many chemical forms that vary in bioavailability, depending on plankton species. In the current study, we have attempted to determine the relative bioavailability of different chemistries of P to the bloom-forming cyanobacteria Microcystis aeruginosa. Additionally we sought to determine and whether the ratio of available N to P in the system altered the ability of Microcystis to utilize these different forms of P. Our results demonstrated that an inorganic phosphorus source (K2HPO4) was more available to M. aeruginosa during P-replete conditions compared to organic sources of P. M. aeruginosa was also capable of obtaining phosphorus from DOP (β-glycerophosphate and polyphosphate) when DIP was not available. It is important to note that N and P had combined effects and the limitation of P or N occurred when the N:P ratio of external inputs was too great (>50:1) or too small (1:4), and balanced growth was observed at other nutrient ratios. Where intracellular N:P ratios≠~16:1, then cultures are not stoichiometrically balanced. Where intracellular N:P ratios >~16:1 then cultures are more likely to be P-limited and physiological responses will be P dependent. Where intracellular N:P ratios are <~16:1, then cultures are more likely to be N-limited and physiological responses will be N dependent. We consider the physiological responses of M. aeruginosa to nutrient stress in more detail and attempt to show relevance in how these responses might in fluence Microcystis bloom dynamics.
4.1 Changes in cell concentration
In nature, dissolved inorganic phosphorus,interchangeably referred to as soluble reactive phosphorus (SRP) (Wang et al., 2010), is thought to be preferentially absorbed and utilized by phytoplankton. The absorption and assimilation of organic phosphorus by algal cells is commonly thought to require extracellular phosphatase activity(e.g., alkaline phosphatase, APA) to hydrolyze the organic P substrate (Chróst and Overbeck, 1987),making these sources less desirable and more energetically expensive for growth. Yet, dissolved organic phosphorus comprises a signi ficant portion of the total dissolved phosphorus pools and plays an important role in modulating algal biomass and community structure. This P fraction and its effects on cyanobacterial physiology remain poorly characterized, largely due to the diversity and complexity of the compounds present (Shun et al.,1994; Shi et al., 2011).
In the current study, we observed that growth rate was tied to the differential bioavailability of P based on chemistry. High P concentrations (N:P ratios of 16:1 or 4:1) might contribute to the growth rates in either P form. Cells were not able to grow at the low N:P ratio of 1:4, when N might become the limiting factor, especially in polyphosphate-media. However,there were no signi ficant differences in maximum cell concentration in cultures supplied either inorganic or organic P when N:P ratios were high (50:1 and 30:1),and signi ficant decrease was seen in organic media at an N:P ratio of 1:4. It has been reported that P de ficiency induces high APA activity, which enhances the availability of P (Ding et al., 2016) and the peak cell concentration as a result. Therefore, we might be tempted to conclude that the equivalent maximum cell concentration at the N:P ratio of 50:1 and 30:1(molarity of P were 0.35 and 0.59 mmol/L) for all P sources was achieved by the activity of APA. When P is the limiting factor, P forms did not affect the cell growth. Recently, Steffen and colleagues (Steffen et al., 2014b) demonstrated that APAse activity may not be linked entirely to P availability; they showed that growth on urea increased APAse activity in M.aeruginosa in lab cultures. This blurs the utility of this conventional marker of P-stress in algae. Other factors, like the light density, light to dark ratio, and the initial inoculation cell concentration might become the potential limiting factors (Wiedner et al.,2003).
The N:P ratio can indicate which of these two nutrients may serve as the true limiter of cyanobacterial growth, and can be a better indicator than the absolute concentration of either nutrient itself. Strict N-limitation typically occurs where the external N:P is less than 20:1 (Guildford and Hecky, 2000). The decreased growth rate and cell concentration at low N:P ratio treatment (N:P=1:4) in this study is likely due to N limitation. Our results might contribute to the nutrient inputs management in an effort to control cyanobacterial blooms.
4.2 Photosynthetic health
QChl-ais frequently used as an index of abundance of all light-harvesting pigments, which is highly regulated and responds in predictable ways to variations of irradiance, nutrient supply, and temperature (Geider et al., 1997). Lower Chl- a to biovolume ratio has been suggested to be correlated with a poorer ability for algal cells to harvest light under lower light condition while higher Chl- a to biovolume ratio represented a physiological advantage by improving light harvesting at different wavelengths(Pierangelini et al., 2014). In addition, in cyanobacteria, phycocyanin is a pigment-protein complex and, among its other roles, it can serve as an antioxidant, protecting the cell from light damage(Collier and Grossman, 1994). A higher PC content leads to an increase in light-harvesting capacity,which plays an important role in funneling light energy to the underlying PS II reaction centers (Prasad and Dubey, 2011), and subsequently growth rate.Measurement of both pigments can supply insight into changes of photosynthetic health in response to nutrient stress. The Qchlmaxand Qphymaxin M. aeruginosa varied with N:P levels in all phosphorus sources. In K2HPO4-media, the optimal N:P ratio for production of both pigments was 16:1, which is consistent with the optimal nutrient balance predicted by Red field’s Ratio (Goldman, 1986). In contrast, in organic P-media, pigment quota was highest at the P-limiting ratios of 50:1 and 30:1 and was consistent with the growth rate results (Ding et al., 2013).
The rapid light curve, in which the linear electron transport rate through PS II is plotted against irradiance, is commonly used to characterize photosynthetic health. rETR is related to the overall photosynthetic performance of microalgae (Juneau et al., 2005) while rETRmaxis considered a sensitive indicator of environmental stress. Ekis the irradiance value where photosynthesis switches from a lightlimited to a light-saturated rate; thus Ekre flects the endurance to light of Microcystis (White et al., 2011).Highest rETRmaxand Ekvalues were obtained when cells were grown in K2HPO4-media (orthophosphate)and did not vary by N:P ratio in the first four days except at the extreme P-limiting ratio of 1:4. This suggests that photosynthetic activity is not signi ficantly affected by N:P ratios when cells are growing on orthophosphate. Pysiological factors including photosynthetic pigments, photoprotective pigments, and Chl- a:C effect light-limited photosynthetic rates (MacIntyre et al., 2002). The high quota of pigments was likely the reason for the observed high photosynthetic efficiencies when cell were grown on orthophosphate. However, the values of rETR started to decline rapidly at the photon irradiance of 600 μmol photons/(m2·s) in most treatments, indicating that photoinhibition was occurring.
φPSIIand Fv/ Fmvalues decline signi ficantly if microalgae are under stress (Kromkamp et al., 1989).The φPSIIvalues in all the treatments declined dramatically after day 6, which could be attributed to a rapid decreasing Fm' or an increasing proportion of closed PS II reaction centers (Wang et al., 2010).Light can be absorbed by pigments not associated with photosynthesis, which leads to excitation energy being dissipated as heat in the light-harvesting antennae or the PS II reaction centers (Anderson et al., 1995), which affect the functional state of the PS II reaction centers consequently. The marked decline of Fv/ Fm(after day 6) observed in the organic phosphorus treatments indicates loss of potential photosynthetic gain. This could have caused a decrease in efficiency in electron transport from the primary quinone-type acceptor (QA-) to QB (Antal et al., 2009; Petrou et al., 2012), or a decline of the D1protein (a key protein in PS II) under nutrient de ficient conditions (Steglich et al., 2001). In freshwater systems, there exists a high possibility of bloom formation when potential photosynthetic activity is high, even when cell biomass is low (Demmig-Adams and Adams, 1992). This condition seems likely to exist at an N:P ratio of 1:4 in K2HPO4-media as shown in this study.
That signi ficant decline in electron transfer rate( φPSII) and Fv/ Fmoccurring at the N:P ratio of 1:4 in all phosphorus sources suggests either a phosphorus overplus response or a severe N limitation response.Considerable evidence demonstrates that Microcystis will engage in luxury uptake of P to form polyphosphate bodies in orthophosphate-rich environments, known as the overplus response (Jacobson and Halmann,1982; Baldia et al., 2007). The concentration of soluble orthophosphate in fresh waters is generally quite low, ~0.01 mg/L or less (Healey, 1973). The 1:4-ratio medium has an orthophosphate concentration of 70.4 mmol/L that is more than sufficient to maintain growth, representing an orthophosphate-rich condition.Perhaps excess orthophosphate is being stored as polyphosphate in cells. The synthesis of polyphosphate needs one ATP for extending P-P bond (Shi et al.,2003), which competes for energy with the growth of M. aeruginosa as well as other physiological needs.
4.3 Cellular antioxidant response
It is well known that the free radical content (O2ˉ,H2O2, OH-etc.) increases in phototrophs during environmental stress, resulting in lipid peroxidation and degradation of the chloroplast (Hu et al., 2005; Li et al., 2009). Lipid peroxidation is considered to be the major mechanism by which oxyradicals disrupt the physicochemical properties of cell membranes,which can cause a decrease of photosynthetic activity(Huang et al., 2015). MDA is the principal product of lipid peroxidation and has been measured as a marker of oxyradical damage in algal cells (Chen et al.,2015). To cope with reactive oxygen species,phototrophs rely on antioxidant systems, including SOD and GSH (Qian et al., 2012; Singh et al., 2012;Huang et al., 2013). SOD acts as a catalyst in the reaction converting O2ˉ to the relative low cytotoxic H2O2(Hu et al., 2015; Liu et al., 2015). GSH can removes free radicals directly as well as act as the substrate for other antioxidants (GSH-Px and GST)(Li et al., 2003; Hu et al., 2015).
Our this study, an increase of MDA was seen at day 2, especially in groups with N limitation (N:P<20),suggesting that oxidative stress co-occurred under nutrient stress. In general, SOD content increases and decreases rapidly over short periods of time, which is consistent with our observations (Fig.5). A signi ficant increase in GSH occurred by day 4, suggesting that the antioxidant system had responded to an oxidative cell environment presumably caused by nutrient stress. Peak GSH content, occurring on day 4, was accompanied by a signi ficant reduction in MDA content and a dramatic drop in SOD activity. From this, we speculate that the cells prioritize SOD production during periods when the lipid peroxidation reactions occur. Membrane damage was a possible reason for changes in pigment content and damage to the ultrastructure of the photosynthetic apparatus resulting in a decrease of photosynthetic activity(N:P=1:4) (Da Costa and Sharma, 2016).
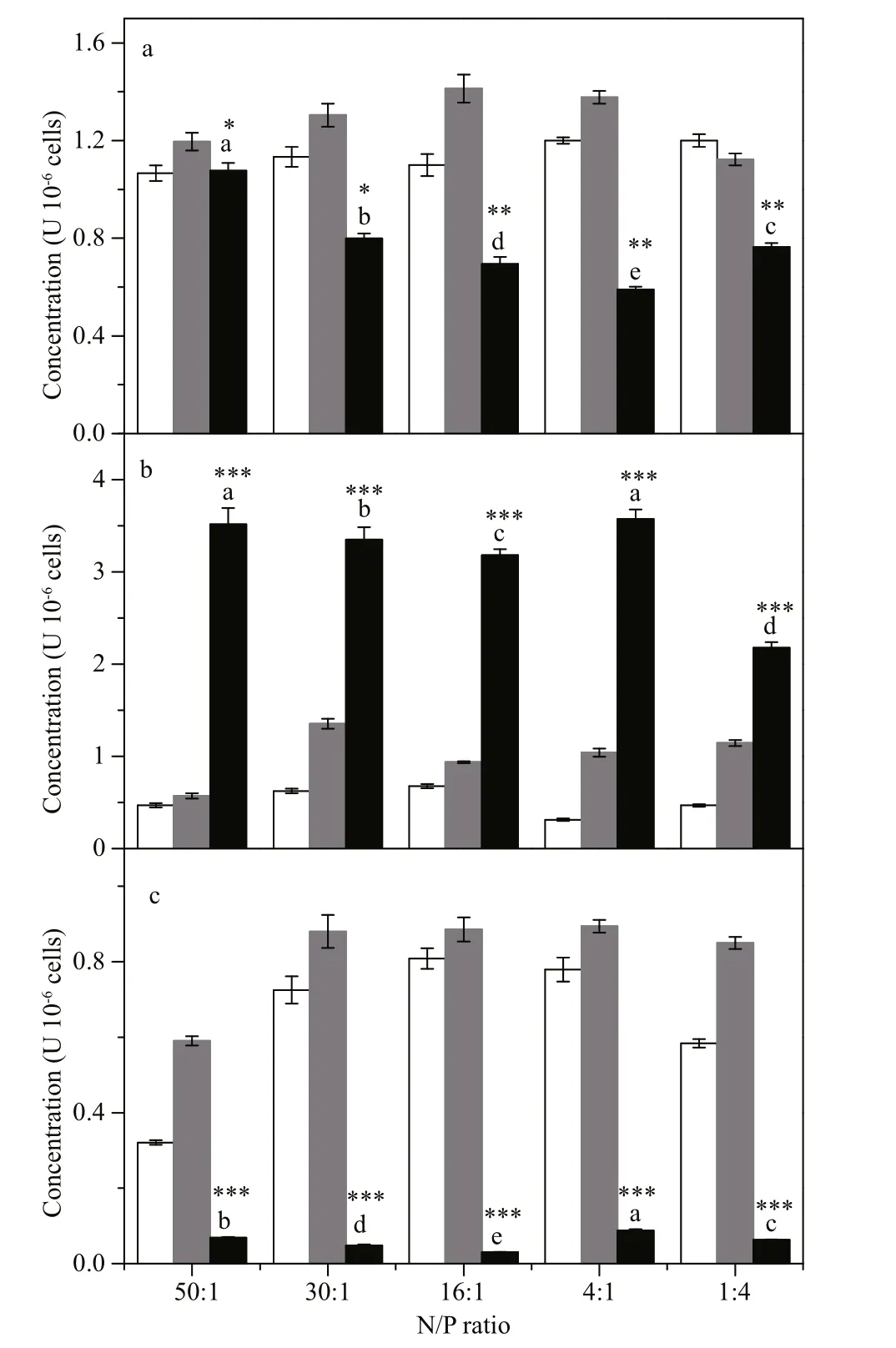
Fig.5 The concentration of malondialdehyde (MDA, a),glutathione (GSH, b) and superoxide dismutase(SOD, c) of M. aeruginosa cultured with K 2 HPO 4 medium at different N:P ratios
This study provides empirical data demonstrating that the chemical species of phosphorus along with the nitrogen to phosphorus ratios are important factors constraining cyanobacterial growth and photosynthetic activity. While the continued control of phosphorus into lakes cannot be ignored, inputs of nitrogen seem likely to interact synergistically with phosphorus to in fluence the physiology of bloom-forming cyanobacteria. Thus our observations add to the pool of data that can help underpin our understanding of how nutrient interactions promote or inhibit cyanobacterial bloom formation.
5 DATA AVAILABILITY STATEMENT
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
6 ACKNOWLEDGEMENT
The authors thank Robbie M. Martin (University of Tennessee) for editing the language of this manuscript.
 Journal of Oceanology and Limnology2018年4期
Journal of Oceanology and Limnology2018年4期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Recent insights into physiological responses to nutrients by the cylindrospermopsin producing cyanobacterium,Cylindrospermopsis raciborskii*
- In fluence of light availability on the speci fic density, size and sinking loss of Anabaena flos- aquae and Scenedesmus obliquus*
- Application of first order rate kinetics to explain changes in bloom toxicity—the importance of understanding cell toxin quotas*
- Regime shift in Lake Dianchi (China) during the last 50 years*
- The effects of electrochemical oxidation on in-vivo fluorescence and toxin content in Microcystis aeruginosa culture*
