Characterization of the interaction between HIV-1 integrase and host dynein intermediate chain 1 protein
, , ,, ,
(1.Department of Histology and Embryology,Zunyi Medical University,Zunyi Guizhou 563099,China; 2.Laboratory of Molecular Human Retrovirology,Department of Medical Microbiology,Max Rady College of Medicine,Rady Faculty of Health Sciences,University of Manitoba,Winnipeg Manitoba R3E0J9,Canada)
[Abstract] Objective To investigate the corelation of HIV-1 integrase (IN) with the dynein intermediate chain 1 (DIC1) during the replication cycle of Human Immunodeficiency Virus type 1 (HIV-1).Methods HIV-1 IN and DIC1 were checked by transfection,Co-Immunoprecipitation (Co-IP),immunofluorescence and western blotting.Results Co-immunoprecipitation approach was performed to reveal that HIV-1 IN was associated with another protein,DIC1of the cytoplasmic dynein motor complex,and the C-terminus of IN was essential for this interaction.The immunofluorescence assay also showed that DIC1 was co-localized with HIV IN in the cytoplasm,but not in the nucleus,suggesting that HIV-1 IN was associated with dynein motor complex in the cytoplasm.Conclusion This study provides evidence that HIV enters cells and correlates with the dynein motor complex through viral protein IN and DIC1 interaction,which facilitates its movement to the nucleus for viral DNA integration.
[Key words] HIV-1; integrase; dynein; dynein intermediate chain 1; interaction
Human Immunodeficiency Virus (HIV) replication is initiated from the proper engagement of Env with CD4+T cell surface receptors and co-receptors,followed by the viral and cellular membrane fusion that results in the release of viral contents into the cytoplasm[1].Uncoating of the incoming virus forms a Reverse Transcription Complex (RTC) where the HIV-1 RNA genome is reverse transcribed into cDNA[2].The newly synthesized HIV-1 cDNA remain associated with viral proteins as a complex named pre-integration complex (PIC),that is transported into the nucleus for the viral DNA integration[3].Although the each step in the early stage of HIV-1 infection is well defined,the detailed molecular mechanisms are still not fully understood.One of the intriguing questions is how HIV-1 RTC/PIC is transported from cell periphery to the perinuclear compartment called microtubule organizing center (MTOC)[4].
The intracellular environment is populated with macromolecules and organelles,which allows the free migration of molecules with a hydrodynamic diameter less than 20 nm,whereas the average diameter of HIV PIC is 56 nm[5],therefore,passive diffusion is unlikely to be the method by which HIV migrates intracellular[6].However,since the transportation of cellular macromolecules and organelles towards the nucleus relies on microtubule network and dynein motor complex,it has been hypothesized that HIV-1 utilizes this intracellular transportation system for its own purpose.An earlier study reported that that HIV-1 PICs are associated with MTOC and concentrated in the area of the MTOC[4].In further studies,it was shown that the disruption of dynein/microtubule-mediated intracellular transportation by microtubule depolymerization or microinjection of anti-dynein antibody inhibited HIV-1 PIC transportation towards MTOC had resulted in the peripheral accumulation of PIC and impaired HIV-1 early stage replication[4,7-8].Collectively,these results suggest that the microtubule network and dynein motor complex may play an important role in HIV-1 intracellular transportation.
As the major component of RTC/PIC,capsid (CA) has been proposed to mediate the HIV-1 association with dynein[9].However,no firm evidence has been provided to support this hypothesis.Given that HIV-1 uncoating partially exposes the capsid packaged viral proteins to the cellular environment,it is possible that the interaction between RTC/PIC and dynein is mediated by viral proteins inside the HIV core[10].Unlike the other viral proteins,HIV-1 integrase (IN) accompanies the HIV-1 genetic material throughout HIV-1 early stage replication,thus making it a candidate of interest[6,11].In fact,our previous study have found that during viral infection,HIV-1 IN interacts with one of the dynein adapter proteins,dynein light chain 1 (DYNLL1),and this interaction has a role in proper uncoating and efficient reverse transcription of HIV-1[12].In order to gain more insight into the mechanism underlying dynein recruitment of HIV-1,we further explore the potential association of HIV-1 IN and dynein intermediate chain 1(DIC1) which is another dynein adapter protein and by Co-IP,we have shown that IN is associated with DIC1.Our mutagenic analysis also identified that the C-terminus of IN is crucial for the association between IN and DIC1.Furthermore,our localization analysis revealed that IN co-localizes with DIC1 in the cytoplasm by immunofluorescence technology.
1 Materials and methods
1.1 Plasmids and cells AcGFP-IN,AcGFP-IN1-212 and AcGFP-IN50-288 were described previously[12].Human embryonic kidney cells 293T and HeLa cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM),supplemented with 10% Fetal Bovin Serum (FBS),penicillin at a final concentration of 100 I.U./ml,and streptomycin at a final concentration of 100 μg/ml.Cell transfection was performed following the calcium-phosphate-DNA precipitation method[13]
1.2 Chemicals and antibodies Protease inhibitor cocktail and the detergent NP-40 was obtained from Calbiochem.The Western blot (WB) detection enhanced chemiluminescence (ECL) kit was purchased from PerkinElmer Life Science and 4',6-diamidino-2-phenylindole (DAPI) was obtained from Life technologies Inc.Rabbit anti-DIC1 antibody was obtained from Abcam.Horseradish peroxidase (HRP)-conjugated anti-GFP antibody was purchased from Miltenyi Biotec.Horseradish peroxidase (HRP)-conjugated sheep anti-mouse IgG and donkey anti-rabbit IgG were obtained from Amersham Biosciences.Fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibody and Cyanine 5 (Cy5)-conjugated anti-mouse antibody were purchased from GE Healthcare.
1.3 Co-Immunoprecipitation The interaction between HIV-1 IN and DIC1 was analyzed by Co-Immunoprecipitation (Co-IP) as described previously[14].The CMV-AcGFP-control or CMV-AcGFP-IN wt/mt plasmid[14]5 μg was transfected into 293T cells.At 48 h post-transfection,the cells were harvested and washed with PBS twice.Then,90% of the cells were lysed in lysis buffer (lysis buffer:RPMI medium containing 0.12% NP-40 and protease inhibitor cocktail) on ice for 30 min.To further disrupt the cells,100 uL glass beads were added to each sample,and the mixtures were vortexed three times for ten seconds each.The cell lysates were clarified by centrifugation and the supernatants were incubated with rabbit anti-GFP antibody overnight under rotation at 4 ℃.Then,60 μl of protein A-Sepharose beads was added into the supernatants and incubated under rotation at 4 ℃ for 2 h.The beads were collected by low-speed centrifugation and washed by cold lysis buffer thrice.The immunoprecipitates were eluted into 45 μL Laemmli buffer (Solutioncontains4%SDS,20% glycerol,10% 2-mercaptoethanol,0.004%bromphenolblue and0.125MTrisHCl,pH approx6.8)and subjected to western blot (WB) analysis.The presence of GFP and GFP-IN wt/mut were detected by HRP-conjugated anti-GFP antibody.The IN-bound endogenous DIC were detected by anti-DIC1 antibody.To detect the expression of GFP and GFP-IN wt/mut as well as endogenous DIC1,the 10% of the collected cells were lysed in RIPA buffer and the lysates were subjected to WB analysis with corresponding antibodies.
1.4 Immunofluorescence To determine whether HIV-1 IN is associated with dynein,we performed the protein co-localization analysis by immunofluorescence,as previously described[15].Briefly,HeLa cells were seeded on 12 mm2glass coverslips in 24-well plates at 50% confluence.15 h later,Hela cells were transfected with AcGFP-IN or AcGFP plasmid 2 μg.After 12 h of transfection,cells were rinsed with PBS for 3 times followed by fixation and permeabilization with methanol/acetone (1∶1 ratio) for 30 min at room temperature.The glass coverslips were incubated with rabbit anti-GFP (1∶500) and mouse anti-DIC1 (1∶250) primary antibodies overnight at 4 ℃ followed by washing with PBS three times and incubated with (FITC)-conjugated anti-rabbit (1∶1 000) and (Cy5)-conjugated anti-mouse (1∶1 000) fluorescent conjugated antibodies for 1 h at room temperature.Subsequently,the cells were washed with PBS three times.The cells were visualized under the fluorescent microscope (Axiovert 200 Carl Zeiss microscope).
2 Results
2.1 HIV-1 IN is associated with DIC1 Since dynein adaptor proteins recruit cargo to the dynein complex through the interaction with DIC1/2 (Fig 1A),we tested whether HIV-1 IN interacts with DIC1 by using co-immunoprecipitation (CO-IP) method,as described in Materials and Methods.Briefly,An HIV IN expressing plasmid,pAcGFP-IN wt was transfected into 293T.Meanwhile pAcGFP was used as control.At 48 hof post-transfection,the cells were collected and lysed in 0.12% NP40 lysis buffer.The lysates were clarified and then subjected to Co-IP using the rabbit anti-GFP antibody.The immunoprecipitations were resolved in 12% SDS-PAGE.The co-immunoprecipitated DIC1 were analyzed by using mouse anti-DIC antibody.The result revealed that the IN is associated with endogenous DIC1 (Figure 1B,upper panel).The middle and lower panel of Figure 1B showed the expressions of endogenous DIC1,GFP and GFP-IN.

A:Schematic diagram of Dynein complex structure and dynein components; B:HIV-1 IN is associated with DIC1.AcGFP or AcGFP-IN plasmid was transfected into 293T cells.After 48h of transfection,cells were lysed and cell lysates were subjected to Co-IP using anti-GFP antibody.The co-pulled down DIC1 were detected by WB using anti-DIC1 antibody (upper panel).Meanwhile,1/10th of the cells lysates were run onto SDS-PAGE,and the expression of GFP,GFP-IN or endogenous DIC1 were detected by WB using respective antibodies (Middle,and lower panels).Fig 1 HIV-1 IN is associated with DIC1
2.2 HIV-1 IN C-terminus is required for IN/DIC1 association The HIV-1 IN contains three functionally distinct domains,N-terminal domain (1-49aa.),catalytic domain (20-211aa.),and the C-terminal domain (212-288aa.) (Fig 2A)[15].Therefore,we further investigated which region of IN is required for the association of IN with intracellular DIC1.Each of AcGFP,AcGFP-INwt,AcGFP-IN1-212,or AcGFP-IN5-288 plasmid was transfected into 293T cells separately.The co-immunoprecipitated DIC1 was analyzed by using the anti-DIC1 antibody.The result revealed that both INwt and IN 50-288 mutant were able to pull down DIC1 (Fig 2B,lane 2 and 4).However,even though the higher level of IN 1-212 mutant was expressed in the cells (Fig 2B,lane 7),no IN 1-212/DIC1 interaction could be detected (Fig 2B,lane 3).Together these results indicated that the C-terminal domain of IN is required for IN/DIC1 Association.

A:Schematic diagram of HIV-1 INwt and HIV-1 IN mut (AcGFP-IN50-288,and AcGFP-IN1-212) expressors used for transfection and Co-IP analysis; B:HIV-1 IN C-terminal domain is required for IN/DIC1 association.AcGFP-,AcGFP-INwt,AcGFP-IN1-212 or AcGFP-IN50-288 plasmid was transfected into 293T cells.After 48h of transfection,cells were lysed and cell lysates were subjected to Co-IP using anti-GFP antibody.The co-pulled down DIC1 were detected by WB using anti-DIC1 antibodies.Meanwhile,1/10th of each transfected cells lysates were run onto SDS-PAGE,and the expression of GFP,GFP-INwt,GFP-IN mutants or endogenous DIC1 were detected by WB with corresponding antibodies.Fig 2 HIV-1 IN C-terminus is required for IN/DIC1 association
2.3 HIV-1 IN co-localizes with DIC1 in the cytoplasm To confirm the association of HIV-1 IN with DIC1,we performed the co-localization assay.Briefly,HeLa cells cultured on glass coverslips were transfected with AcGFP-IN or AcGFP plasmid.After 24 h of transfection,cells were fixed and permeated.Due to the lower expression levels of AcGFP-IN in HeLa cells,the indirect immunoflourescence method was used to detect the localization of AcGFP-IN and AcGFP in the cells.We found that the AcGFP-IN showed two different distribution patterns:distributed in the cytoplasm (Fig 3,upper panel),and inside the nucleus (Fig 3,middle panel).It is worth to note that GFP-IN wasco-localized with DIC1 when AcGFP-IN was located in the cytoplasm.However,when GFP-IN was translocated into the nucleus,it lost co-localization with DIC1 since DIC1 is still located in the cytoplasm (Fig 3 middle panel).When AcGFP was expressed in the HeLa cells,the GFP protein and DIC1 are distributed throughout the cell,since no clear co-localization was observed (Fig 3,Lower panel).Collectively,these results indicate that AcGFP-IN,but not AcGFP,is associated with DIC1 in the cytoplasm before GFP-IN nuclear import.
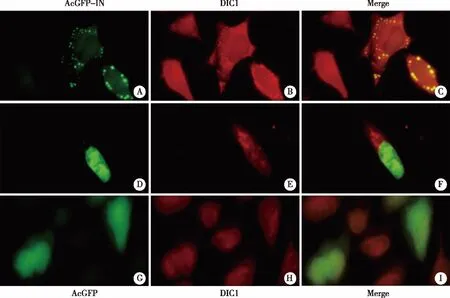
AcGFP or AcGFP-IN plasmid was transfected into HeLa cells cultured on glass coverslips.After 24 h of transfection,cells were fixed and incubated with rabbit anti-GFP (Figure 3A,3D,3G) and mouse anti-DIC1 primary antibodies (Figure 3B,3E,3H) overnight at 4°C.Then,the cells were washed and incubated with (FITC)-conjugated anti-rabbit and (Cy5)-conjugated anti-mouse antibodies.The cells were visualized under the fluorescent microscope with a 40×objective lens (Axiovert 200 Carl Zeiss microscope).Figure 3C is the merging of A and B,Figure 3F is the merging of D and E,Figure 3I is the merging of G and H.Fig 3 HIV-1 IN co-localizes with DIC1 in the cytoplasm
3 Discussion
In the crowded intracellular environment,the free migration of macromolecules is highly restricted[16].To enable the long distance intercellular transportation of functional cargoes,the cells have evolved an organized microtubule transportation system[17].Along the microtubules,dynein and kinesin complexes move towards opposite directions that allow retrograde and anterograde transportation respectively.Several previous studies suggest that HIV-1 RTC/PIC utilizes the dynein-dependent microtubule transportation system for migration from cell periphery to the nucleus[4,6].However,to date,the mechanism underlying the HIV-1 recruitment of dynein is elusive.The previous investigation in our laboratory has demonstrated the interaction of HIV-1 IN with DYNLL1,one of the dynein cargo adaptors[12].However,the functional analysis shows the interaction between HIV-1 IN and DYNLL1 is not involved in the retrograde transportation of HIV-1 because the DYNLL1 knockdown had no effect on HIV-1 nuclear import[12].Since DIC1 is the key structural component in dynein complex,we further explore the potential association of HIV-1 IN with DIC1 in order to gain more insight into the mechanism underlying dynein recruitment of HIV-1.
Through a cell-based co-immunoprecipitation analysis performed by overexpression of AcGFP-IN in 293T cells,we demonstrated the association of GFP-IN with endogenous DIC1.Moreover,we found that GFP-IN is co-localized with DIC1 in HeLa cells through the immunofluorescence analysis.Together,these results suggest that HIV IN may be associated with dynein complex.To gain more insight for IN/DIC interaction,subsequent delete mutation analysis revealed that the C-terminus of HIV-1 IN is important for the association between HIV IN and DIC1.Previous work in our laboratory has demonstrated that the HIV IN 212-288 is essential for multistep in HIV-1 early stage replication including HIV-1 reverse transcription and nuclear import[15].This study thus further suggests that the association between HIV-1 IN and DIC1 might play a functional role during the early stage of HIV-1 replication,especially for the intracellular transportation of HIV RTC/PIC.Altogether,in agreement with our previous study,this study provided more evidence that HIV is able to highjack cellular dynein complex through its IN binding to host DIC1 and efficiently transported from the cell periphery to perinuclear region for nuclear import.Further investigation on this virus/host interaction and its function relevance during HIV replication may contribute to our better understanding of HIV biology.

