Bio-characteristic profiling related to clinic:A new technology platform for quality evaluation of Chinese materia medica
Li-Na Wen,Ai-Ting Wang,Na Guo,Yumei Han,Can Yan,Dan Yan*
1Beijing Shijitan Hospital,Capital Medical University,Beijing,China.
2Beijing Key Laboratory of Bio-characteristic Profiling for Evaluation of Rational Drug Use,Beijing,China.
3International Cooperation&Co-construction Laboratory of Bio-characteristic Profiling for Evaluation of Rational Drug Use,Beijing,China.
4Experimental Research Center,ChinaAcademy of Chinese Medical Sciences,Beijing,China.
5Beijing Physical Examination Center,Beijing,China.
6Guangzhou University of Chinese Medicine,Guangzhou,China.
Abstract
Keywords:Bio-characteristic profiling related to clinic,Correlation efficacy,Quality evaluation of Chinese materia medica
Background
Because of the exact curative effect,the irreplaceable role of Chinese materia medica(CMM)in the treatment of diseases has been widely recognized at home and abroad.Especially in the west,CMM has become an important partofalternative medicine.However,the safety problems of CMM frequently appear,and the quality evaluation of CMM,which is closely related to the clinical efficacy of CMM,has been recognized as a bottleneck restricting the modernization and internationalization of CMM.Food and Drug Administration (FDA) and European Medicines Evaluation Agency (EMEA) put forward strict requirements for the access standards of CMM.National Medical Products Administration(NMPA)of the State Administration forMarketRegulation (SAMR)of People's Republic of China has promulgated several systems and taken measures to strengthen and promote the quality evaluation of CMM.It has undergone many important development stages,such as the identification of origin,character identification,microscopy identification,physical and chemical identification,content determination,chemical fingerprinting,biological fingerprinting,bioavailability determination,fingerprint-efficacy correlation analysis,which has significantly improved the quality control level of CMM[1-6].However,due to the characteristics of multi-component,multi-efficacy,multi-target complexity and vulnerability to factors such as origin,harvesting time,transportation routes and processing methods,the existing quality evaluation system or methods of CMM still cannot fully meet the quality control needs of CMM and itspreparations[2-5].How to overcome the shortcomings of the existing quality evaluation system,which is difficult to effectively correlate clinical efficacy,one-sidedness,boundedness,and establish the key technology of real-time,dynamic and overall display of the effectiveness and safety related to CMM,is the core scientific problem to be solved to effectively promote the quality evaluation of CMM.
With the progress of modern science and technology,cross-disciplines and disciplinary integration provide a new opportunity for the development of scientific research oriented to serve human health.In this context,to explore multi-disciplinary cross-cutting technology closely combined with clinical practice for quality monitor of CMM,so as to reflect the clinical effectiveness and/or safety of CMM and ensure clinical efficacy.This bio-characteristic profiling originating from the clinical real world is a new concept put forward under the condition of modern science and technology,and it is a new requirement for quality control of CMM and evaluation of clinical rational drug use.
Establishment of the key technology platform for bio-characteristic profiling
Analytical chemistry technology plat form-Represe nted by metabolo mics technology
Metabolomics,which originates from the field of analytical chemistry,is a new discipline that imitates the research ideas of genomics and proteomics,carries out qualitative and quantitative analysis of all low molecular weight(MW)metabolites of a certain organism or cell at the same time in a specific physiological period,and searches for the relative relationship between metabolites and physiological and pathological changes[7].As shown in Figure 1,as a branch of systems biology,it takes the small molecule metabolites(MW<1000),which are the substrates and products of various metabolic pathways,as the research object,cluster index analysis as the basis,high-throughput detection and data processing as the means,and information modeling and system integration as the goal.The samples are mainly extracts of cell,tissue and body fluid of human,animals and plants.The main technical means are nuclear magnetic resonance(NMR)and chromatography-mass spectrometry(HPLC/GC-MS),especially high resolution mass spectrometry.By detecting the spectra ofa series of samples and combining with pattern recognition method,the level of changes of endogenous small molecules can be confirmed,the pathophysiological state of the organism can be judged,and the biomarker associated with it can be possibly found.It provides a forecasting platform for related early warning signals.Compared with genomics and proteomics,metabolomics has the advantage of rapid and sensitive interpretation of what changes have taken place in the body.It is suitable for monitoring drug toxicity and adverse drug reactions,as well as active substance basis research,and effectively evaluating the clinical safety and effectiveness of CMM[8].

Figure 1.Schematic diagram of metabolomics technology
Physical chemistry technology platform-Represente d by isothermal titration calorimetry technology
Isothermal titration calorimetry(ITC)dynamic monitoring technology originating from the field of physical chemistry is an important analytical method for the study of biother modyna mics and biodyna mics.Through continuously and accurately monitoring the characteristic curves of heat change when molecules of different substances come into contact by a highly sensitive and automated microcalori meter,the thermodynamic parameters(including binding constants,number of binding sites,molar binding enthalpy,molar binding entropy,molar constant pressure heat capacity)and kinetic parameters (including enzyme activity,enzyme-catalyzed reaction Michaelis constant and enzymeconversion number)wereprovided in-situ,on-line and nondestructively to characterize the characteristic information[8].The tested substance needs not to be immobilized or modified,and suspension,colored sample and viscous sample can also be detected by this technology.It is suitable for monitoring the dynamic characteristic spectra of small molecules and protein,protein and protein,small molecule and enzyme,enzymatic reaction kinetics,small molecule and nucleic acid,biological molecule and cell,drug and DNA/RNA etc.and studying the mechanism of action,so as to provide data support for research of clinical safety and effectiveness of drugs(including MCC)[9,10].
Biophysics technology platform-Represented by dyn amic monitoring technology of live cell
Dynamic monitoring technology of living cells derived from the field of biophysics is a kind of new cell analysis technique that can monitor the dynamic changes of living cells in response to certain external stimuli such as drug intervention automatically and continuously in a non-labeled,non-invasive way.A microelectronic sensor is embedded at the bottom of the microporous electronic board,and the adherence,adhesion and growth situation of living cells on the detection board can be measured by impedance index of microelectrodes,so thatthe time-dependent characteristic profiling of cell effects(even real-time intracellular imaging)can be acquired to reveal the cell biological information such as proliferation,viability,apoptosis and morphological changes of living cells.Avoiding the limitations of traditional endpoint cell analysis,such as breakpoint,labeling and destroying cells,it has the advantages of high accuracy,high repeatability and high throughput information.It is suitable for quality evaluation of CMM with relatively clear target cells,for example,cytotoxicity or apoptosis analysis,cell proliferation analysis,receptor analysis and pharmacological analysis[11,12].
Analytical biochemistry technology platform-Repre sented by biosensor technology
Biosensors developed in the field of analytical biochemistry are analysis tools or detection systems that take the immobilized bio-sensitive materials as identification elements(such asenzymes,antigens,antibodies,microorganisms,cells,tissues,nucleic acids,etc.)and convert the irconcentrationsinto optical,electrical,photoelectric,temperature,magnetic,electromagnetic signals and output through appropriate physical and chemical transducers(such as oxygen electrodes,photosensitive tubes,field effecttubes,piezoelectric crystals,etc.)and the signal amplifier output device[13,14].Biosensor has the function of receiver and converter.With the develop ment of analytical chemistry,biochemistry and molecular biology,material chemistry and polymer science and engineering,the principles,types and functions of biosensors are diversified.
Classified by identification element
According to the classification of identification elements,biosensors can be divided into bacterial/viral sensors,immunosensors (such as antigens,antibodies,immunoglobulins,receptors,etc.),DNA/RNA sensors,adapter sensors,enzyme sensors,cell sensors,small molecule sensors,metal ion sensors and so on,which can be used to detect the corresponding identification objects[15-23].The sensing principle of some biosensors is shown in Figure 2.
Classified by active materials
Active materials are the core carriers of biosensors.According to the classification of active materials,biosensors can be divided into semiconductor sensors(such as quantum dots,metal nanoparticles),dye sensors,semiconductor- semiconductor sensors,semiconductor-dye sensors,semiconductor-metal sensors,semiconductor-carbon sensors and so on[24-27].
Classified by principle of signalgeneration and transduction
According to the mode of signal output, it can be divided into chemiluminescence sensor, photoelectrochemical sensor, electrochemical sensor, surface plasmon resonance (SPR) sensor, magnetic sensor, electromagnetic sensor and so on. Among them, photoelectroche mical sensor technology is more widely used than others in recent years because of its simplicity, economy and higher sensitivity [28].
In practical applications, biosensors are often constructed by a variety of principle simultaneously, such as electrochemical cell sensor, photoelectroche mical enzyme sensor, magnetic immunosensor and so on. Compared with the traditional chemical and biological detection methods, biosensors can be customized according to the detected objects, with various forms, wide application, sensitivity and rapidity and strong practicability. Due to the variety of identification elements, biosensors are especially suitable for the analysis of heavy metal residues, allergic reactions and quality fluctuations based on validity and trace detection of CMM.
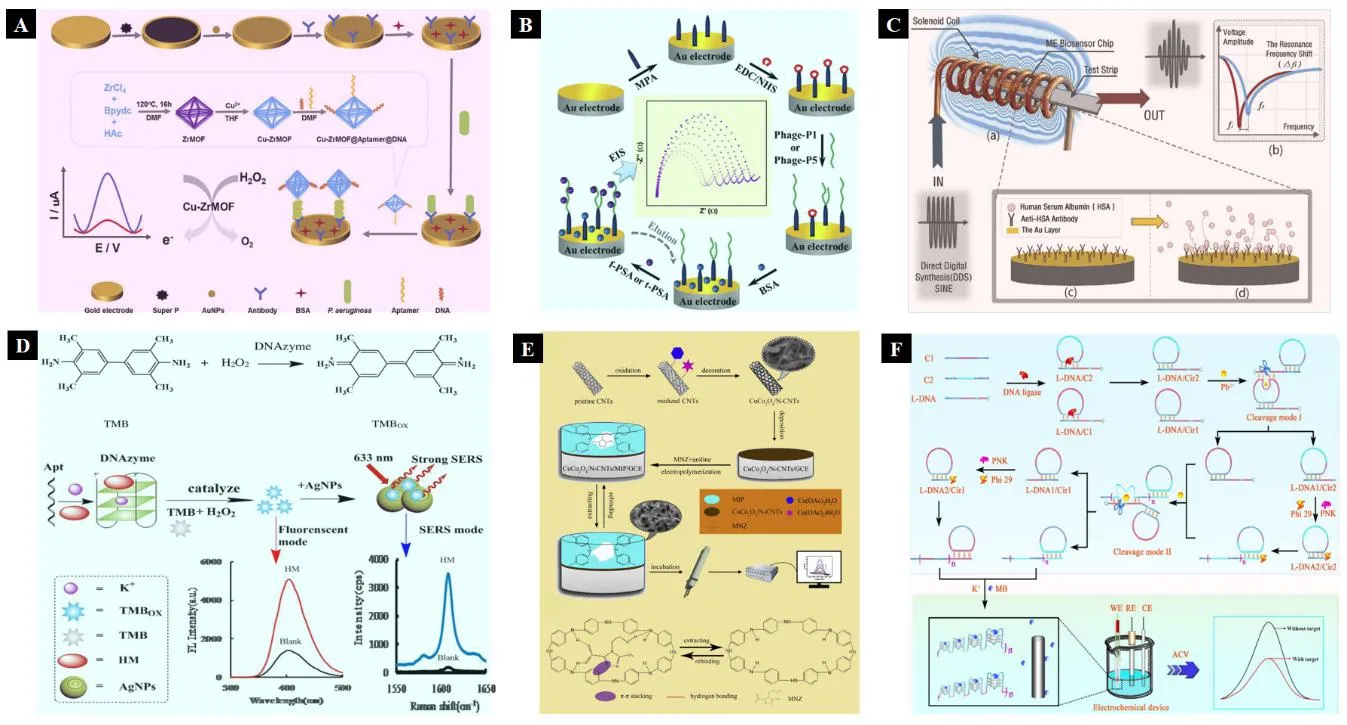
Figure 2.Scheme of some biosensors
Tissue engineering technology platform-Technology of constructing model organisms related to clinic
Tissue engineering originated from the field of biomedicalengineering isa new discipline which combines cell biology with material science to construct tissues or organs in vitro or in vivo.The four elements include seed cells,biomaterials,the integration of cells and biomaterials,as well as the integration of implants and microenvironment in vivo.The basic principle is to obtain a small amount of living tissues from the body,isolate cells(also known as seed cells)from the tissues by special enzymes or other methods for further culture and expansion in vitro,and then put the expanded cells into biomaterials(scaffolds)with good biocompatibility,biodegradability and absorbability,make cells adhere to biomaterials to form cell-material complexes;culture the complexes in biomimetic environment in vitro to form tissue or organ regeneration orimplant them into pathologically injured tissues or organ lesions in vivo[29].With biomaterials gradually degraded and being absorbed in vivo,implanted cells continue to grow in vivo and secrete extracellular matrix to form corresponding tissues or organs eventually,so as to achieve the purpose of repairing trauma and reconstructing function.The three-dimensional structure of biomaterial scaffolds provides a good environment for cells to obtain nutrition,growth and metabolism.Tissue regeneration technology provided by the development of tissue engineering provides a platform for the construction of model organisms associated with clinical efficacy.This research model of quality evaluation system based on human bionic model is obviously superior to that based on cell model or animal model.It can shorten the evaluation cycle and ensure the reliability of the study results,which will strongly promote the rapid and effective development of quality evaluation of CMM.
Application of the new technical platform of bio-characteristic profiling related to clinic for quality evaluation of CMM
Safety(toxicity)evaluation
Anaphylaxis monitoring of CMM Injections based on mast cell model
Adverse reaction is not only the core factor restricting the industrialization development of CMM Injections,but also a difficultproblem the whole pharmaceutical industry of CMM faces.Among all the adverse reactions of CMM in China,about 50%was caused by CMM Injection.Allergy is the main type of adverse reactions of CMM Injections.Shuanghuanglian Injection has been listed in the top 10 annual rankings of adverse drug reactions of CMM Injections in China for many years.Mast cells are important mediators of anaphylaxis or similar anaphylaxis.Based on this model organism,a monitoring method for allergic reaction of Shuanghuanglian Injection was established by our research group,which was based on the dynamic monitoring technology of living cells[12].The normal samples and samples that can cause adverse reactions can be effectively distinguished through the growth behavior of cells reflected by real-time cell dynamic analyzer after blind numbering.It makes up for the limitation of previous chemical fingerprint and biological fingerprint in identifying adverse reaction samples comprehensively,and improves the safety evaluation ability of CMM.
Anaphylaxis monitoring of CMM based on immunosensor
Immunosensor assay(ISA)is a method detecting target analytes with high specificity and high accuracy based on rapid and specific antigen (Ag)-antibody (Ab)recognition reaction.The specific and sensitive detection of various substances(such as prostate cancer antigen)in complex biologicalsystems has been successfully achieved.These techniques play an important role in early diagnosis and treatment of diseases.At present,the developed immunosensors include SPR immunosensor[25],fluorescent immunosensor [30],photoelectroche mical immunosensor[31]and so on.In view of the successful application of immunosensors in disease diagnosis and treatment,it is feasible and practical to develop biosensors for specific detection of immunoglobulin IgG,IgA and IgM related to allergic reactions and apply them to the monitoring of allergic reactions induced by CMM.
Toxic substances monitoring of CMM basedon Metabolomics and model biology
Metabolomics can explain the changes of endogenous smallmolecules after the body received external stimulation.Pharmacopoeia clearly limits the content of toxicco mponents of CMM.However,duetothe vulnerability to factors such as source,origin,harvesting time,transportation routes,processing methods and so on,and the quality controlstrategy of CMM toxic components by content determination alone is obviously not perfect.The monitoring method of CMM toxic substances based on metabolomics technology and tissue and organ regeneration technology in vitro such as liver and kidney,can not only reveal the products of drugs after metabolism of liver and kidney as well as the changes of endogenous small molecules of liver and kidney after drug action,but also simulate the human internal environment in vitro to the maximum extent,improving the reliability of quality evaluation results of CMM[7].This strategy will have broad application prospects.
Excessive heavy metals monitoring of CMM based on metal ion sensor
Excessive heavy metal is a common factor for the poor quality of CMM.Long-term exposure or excessive intake of Pb2+can cause neurological,cardiovascular,reproductive and developmental diseases [32].Monitoring the level of Pb2+,especially the content of trace Pb2+,has become an important aspect of CMM quality evaluation.AtomicAbsorption Spectrometry(AAS),Atomic Fluorescence Spectrometry(AFS)and Inductively Coupled Plasma Mass Spectrometry(ICP-MS)are the traditional methods for the determination of Pb2+[33].Although these methods are sensitive enough,they are complex.Relatively speaking,the Pb2+sensor is fast,simple,sensitive,space-friendly,controllable and simple to pretreat,which has been widely used in recent years(as shown in Figure 3).The introduction of this method to the monitoring of CMM heavy metals will greatly improve the quality of CMM.

Figure 3.Pb2+based on electrochemical sensing system
Effectiveness evaluation
Effectiveness evaluation of CMM for promoting blood circulation and removing blood stasisbased on thrombin activity detection
Xueshuantong Freeze-dried Powder for Injection has the effect of activating blood circulation and removing blood stasis as well as activating blood vessels and collaterals.Its pharmacological action is to dilate blood vessels and improve blood circulation.The inadequate effect of activating blood circulation is not conducive to its effectiveness while the excessive effect may lead to clinical adverse reactions such as skin itching,fever,flushing complexion,elevated blood pressure,dizziness,subcutaneous hemorrhage,purpura and others.According to its pharmacological mechanism,a method for evaluating the effectiveness of Xueshuantong Freeze-dried Powder for Injection based on dynamic monitoring of thro mbin activity was established[34].Through real-time monitoring of thrombin activity,the normal samples and the samples with excessive or insufficient effects were effectively identified after blind numbering.Drawing lessons from similarresearch concepts and ideas,the research group has also expanded its application for the quality evaluation of horned animal drugs[35,36],Isatis Radix[37],enriching and developing the quality evaluation system of CMM based on the dynamic monitoring technology of effectiveness.
Evaluation of antibacterial and antiviral effectiveness of CMM Based on bacteria and virus sensors
Bacteria and viruses are important pathogenic factors.Timely diagnosis and correct treatment of related diseases based on laboratory test results are effective strategies to eradicate them.At present,conventional methods for detecting pathogenic microorganisms including plate culture,polymerase chain reaction(PCR)and mass spectrometry.As a gold standard,plate culture method is accurate but time-consuming.Quantitative detection can be carried out by using PCR technology,but the requirement for operators is quite high.In addition,mass spectrometry analysis requires expensive large instruments.Relatively speaking,bacterial and viral sensor analysis is a fast,simple and sensitive method for microbial detection.At present,electrochemical sensors for detection of Pseudomonas aeruginosa(as shown in Figure 4),Escherichia coli,Seka virus(as shown in Figure 5) and hand-foot-mouth virus have been established and successfully applied to the detection of human serum samples.Developing biosensors for specificpathogenic microorganismsto evaluate the antimicrobial and antiviral efficacy of CMM,it is a great complement and improvement to the quality evaluation system of CMM.
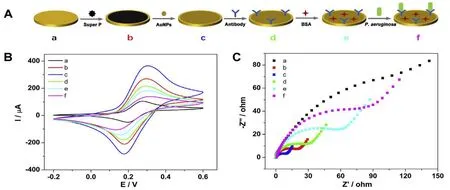
Figure 4.Bacteria based on an electrochemical biosensor

Figure 5.Zika Virus based on an electrochemical biosensor
Stability(Quality fluctuation)evaluation
Stability or quality fluctuation is one of the important indexes for quality evaluation of CMM.The quality of CMM can be effectively controlled by taking the representative pharmacological action of drugs or the bottleneck factors that determine the quality of drugs as the evaluation index of quality stability.Using the research method of multidisciplinary crossing,the research group have successively established a series of key techniques for quality fluctuation evaluation,such as electroche micaldynamic monitoring forYiqifumai(freeze-drying)Injection,living cell dynamic monitoring combining with chemical footprint for Shuanghuanglian Injection[12],and the activity test of thrombin and profibril for Xueshuantong Injection[35]and so on,all of which have achieved industrialization transfer.
Drug combination evaluation
According to the clinical needs of disease diagnosis and treatment,we often encounter the situation of combined application of CMM and western medicine.Intermolecular interaction analysis based on ITC technology plays an important role in clinical rational drug use evaluation.The research group successfully established a compatibility evaluation method based on ITC of Yiqifumai Injection(freeze-dried)combining with 5%Glucose Injection and Vitamin C Injection,which effectively judged the rationality of drug combination[9].On this basis,the evaluation systems of Qingkailing Injection[10]and Shuanghuanglian Injection[12]were further established.
Conclusions and outlook
It can be said that the key technologies described above involved in many interdisciplines come from many related research fields,but we find that they share a common feature-“real-time,dynamic,online detection,associated with activity”.Therefore,we put forward a new academic concept,“bio-characteristic profiling related to clinic,BPRC”of CMM,namely inspired by clinical diagnostic techniques such as electrocardiogram and electroencephalogram can be used to monitor human health and disease outcome,to monitor clinical effectiveness and/or safety-related bioactivity responses between pharmaceuticals and specific model organisms under certain conditions through biomimetic simulation of clinical microenvironment,characterize the characteristic profiling between the response parameters and the reaction time of the system or the drug concentration,and set the discriminant threshold value or function relationship according to the different sources of CMM and related clinical activities,in order to be used forquality fluctuation evaluation ofCMM,drug interaction monitoring and early warning of adverse reactions,etc.[1].It has the characteristics of“real-time,dynamic and full-range monitoring”,and can be associated with clinical safety and/or efficacy of CMM.
The BPRC and its series of key technologies of CMM are proposed and established under the current background,that is taking the identification of primordial,character and physicochemical characteristics as the main quality evaluation model,aiming atpromoting the solution of the“difficult to control and difficult to evaluate”problem of quality in the complex systems of CMM.It not only conforms to the“Guiding Principles for the determination of biological activity of traditional Chinese medicine”,but also reflects the relationship with the clinical safety and/or efficacy of CMM.Compared with conventional chemical fingerprint,BPRC and its series of key technologies pay more attention to the evaluation of overall biological activity related to clinical efficacy,rather than sticking to the search for common peaks in the complex system of CMM,identifying the component information contained in common peaks,and explaining relationship between common peaks and clinical effectiveness and/or safety.It also takes into account the characteristics of multi-component,multi-target and whole regulation of CMM.At the same time,compared with bioassay,BPRC and its series of key technologies pay more attention to the overall dynamic information of biochemical reaction process between CMM and model organisms,rather than the break-point determination of biological activity at a certain point in the reaction process.Therefore,the new system for quality evaluation of CMM,dominated by the academic concept of“BPRC”and the series of interdisciplinary key technologies,is a useful complement to the current quality evaluation model and technology,which also provides a valuable strategy for promoting the stable clinical efficacy of CMM.
How to organize and integrate the different technologies of BPRC platform mentioned above for overall quality control?When evaluating the quality of CMM depending on BPRC technology platform,the appropriate technology should be adopted focusing on the characteristics of CMM(including physical,chemical and biological characteristics) according to “sequential strategy”.That is from the source of Chinese medicinal materials to the production process of CMM preparations and then to their safety and effectiveness evaluation,reasonable and effective chemical,biological or cross disciplinary analysis methods should be applied by each link in turn.In the implementation of this sequential strategy,the correlation between the test results and the clinical safety or efficacy of CMM should be considered,as well as the methodology(repeatability,sensitivity,etc.)and universality(instrument availability,economy,etc.)of the technology.For example,in the source of Chinese medicinal materials,metal ions biosensor technology would be applied to detect and control heavy metal exceeding standard;during the production process of CMM preparations,chemical fingerprinting based on high resolution mass spectrometry would be applied to detect effective components as well as isothermal titration calorimetry technology and dynamic monitoring technology of live cell would be applied to monitor preparation quality fluctuation;as for safety and effectiveness evaluation,in addition to cell-level bioassay,demic tissue-engineered organoid would be used for toxicity and pharmacodynamic assessment.Our team has evaluated the quality of Safflower Injection and Xueshuantong Injection with thehelp ofchemical fingerprinting and cell-level bioassay and the samples with adverse reactions were distinguished significantly[11,34].Therefore,we believe that BPRC could promote the ability of quality control of CMM.
In addition,we realize that our review in this article is only a specific window.There are shortcomings of the BPRC technology system because each of the technologies listed in this review has a certain appropriate scope and application conditions,which should be selected according to different characteristics of CMM.The development direction in the future may be that BPRC technology is all-inclusive,and it is believed to be constantly innovated with the emergence of multidisciplinary technologies,especially in biological signal induction,biological information transmission,artificialintelligence and otherresearch fields.In conclusion,BPRC is an open and compatible academic concept,and its key technologies are more interdisciplinary and integrated.With the development of interdisciplinary technology,analytical methods with clinical monitoring advantages or spectral characteristics can be used as potential key technologies for the research and development of BPRC of CMM.
Acknowledgment
This work was supported by the National Natural Science Foundation of China(grant number 81773891),the National Great New Drugs Development Project of China(grantnumber2017ZX09301-040),and the Beijing Municipal Science and Technology Commission(grant numberXMLX201704,2018-2-2242,7194280),the Open Research Fund of the State Key Laboratory Breeding Base of Systematic Research,Development and Utilization of Chinese Medicinal Resources.The funding sources were not involved in study design,data collection or analysis,writing of the manuscript or the decision to publish the results.
 TMR Modern Herbal Medicine2019年4期
TMR Modern Herbal Medicine2019年4期
- TMR Modern Herbal Medicine的其它文章
- Compatible stability study of XingNaoJing injection based on physical-chemical properties analysis
- Similarity measurement of Chinese medicine ingredients for cold-hot nature identificatio n
- Identification of prognostic markers by integrating the genome and transcri ptomics in ovarian cancer
- Effect of Xuebijing injection on hematopoietic homeostasis of LPS induced sepsis in mice
- Network pharmacology based method for mechanistic investigation of the Compound Xintahua in the treatment of atherosclerosis
