Comparison and analysis of the antennal sensilla of morphs of the aphid Schlechtendalia chinensis(Bell) (Hemiptera:Pemphigidae)
SHAO Shu-Xia, YANG Zi-Xiang, CHEN Hang, WANG Chao, WU Hai-Xia, JIANG Bo, CHEN Xiao-Ming*
(1.Research Institute of Resource Insects, Chinese Academy of Forestry, Kunming 650000 China; 2. Southwest Forestry University, Kunming 650000, China; 3. Kunming Institute of Survey and Design, State Forestry and Grassland Administration, Kunming 650000, China)
Abstract: We compared and analyzed the ultrastructure, type, distribution and number of antennal sensilla in morphs of the aphid Schlechtendalia chinensis (Bell), using scanning electron microscopy. The results showed that S.chinensis has four types of antennal sensilla: Trichoid sensilla, primary rhinaria, sensory projections and secondary rhinaria, all of which varied in their distribution and number on the antenna in the different morphs. Trichoid sensilla could be divided into I and II types, and they distributed on all morphs of S.chinensis’antenna. Primary rhinaria were mainly observed on the antenna of fundatrices, apterous fundatrigeniae, nymphal alate fundatrigeniae, males and females. There are two types of PrⅠ and PrⅡ, occurring on the last and penultimate segment, respectively. But there was only PrⅠon the antenna of nymphal alate fundatrigeniae. The sensory projection was only located on the third to fifth segments of the antenna of nymphal alate fundatrigeniae. Secondary rhinaria were only located on the third to fifth segments of the antenna of alate fundatrigeniae and alate sexuparae. And they accounted for approximately two-thirds of the antennal surface. We suggest that these differences are related to the habitat of the particular morph (e.g. internal or external to the gall), its main form of behavior (e.g. feeding, mate selection) and whether the morph is winged (e.g. host or migration).
Key words: Antennal sensilla; ultrastructure; behavior; morphology; Schlechtendalia chinensis
邵淑霞,杨子祥,陈航,王超,吴海霞,姜波,陈晓鸣.角倍蚜Schlechtendaliachinensis(Bell) (Hemiptera: Pemphigidae)各蚜型触角感器的比较分析[J].环境昆虫学报,2020,42(6):1510-1517.
角倍蚜Schlechtendaliachinensis(Bell) (Hemiptera:Pemphigidae)各蚜型触角感器的比较分析
邵淑霞1,杨子祥1,陈 航1,王 超2,吴海霞1,姜 波3,陈晓鸣1*
(1. 中国林业科学研究院资源昆虫研究所,昆明 650000;2. 西南林业大学,昆明 650000;3. 国家林业和草原局昆明勘察设计院,昆明 650000)
摘要:本研究利用扫描电镜对角倍蚜各蚜型触角感器的超微结构、类型、分布和数量进行了比较分析。结果表明,角倍蚜的触角上着生4种类型的感器:毛形感器、原生感觉圈、感觉突及次生感觉圈,它们在各蚜型触角上的分布和数量各不相同。其中,毛形感器有TypeⅠ和TypeⅡ两种类型,各蚜型触角上均有分布;原生感觉圈主要分布于干母、无翅干雌、第3代有翅干雌若蚜以及性蚜的触角上,有两种类型:PrⅠ和PrⅡ,分别位于触角的末节和倒数第2节,其中,第3代有翅干雌若蚜的触角上仅存在PrⅠ,无PrⅡ;感觉突仅出现于第3代有翅干雌若蚜触角的第3~5节;次生感觉圈仅存在于有翅型春迁蚜和秋迁蚜触角的第3~5节,其面积约占触角鞭节面积的2/3。因此,推测各蚜型触角感器的差异可能与蚜虫的生境(瘿内或瘿外)、行为(如取食、交配)以及翅的有无(如寄主选择、迁飞等)有关。
关键词:触角感器;超微结构;行为;形态;角倍蚜
中图分类号:Q968.1;S433文献标识码:A
文章编号:1674-0858(2020)06-1510-08
基金项目:国家重点研发计划课题(2018YFD0600403);国家自然科学基金(31872305,U1402263);云南省基础研究计划项目(202001AT070016);中国林业科学研究院基本科研业务费专项资金项目(CAFYBB2020SY030)
作者简介:邵淑霞,女,1981年生,山东济宁人,博士,助理研究员,研究方向为昆虫生态学,E-mail:shuxiashao@126.com
*通讯作者Author for correspondence:陈晓鸣,男,博士,研究员,研究方向为昆虫生态学,E-mail:cafcxm@139.com
收稿日期Received:2019-10-22; 接受日期Accepted: 2020-05-13
The aphidSchlechtendaliachinensis(Hemiptera: Aphidoidea: Pemphigidae) is an important resource insect that can induce horned galls that are rich in tannins on the leaves of the gallnut tree,RhuschinensisMil1 (Zhang, 1987). The life cycle ofS.chinensisis complex. During early April,S.chinensisfundatrices induce horned-gall formation onR.chinensisleaves and produce two generations of apterous fundatrigeniae and one generation of alate fundatrigeniae within the gall. The alate fundatrigeniae emigrate to secondary hosts from late September to early October, where upon they produce the next-generation sexuparae. The nymphal sexuparae feed upon sap from the secondary host and secrete a waxy substance that encloses them over the winter. The nymphal sexuparae then develop into alate sexuparae during the following spring, and fly back to trees ofR.chinensis, where they produce male and female individuals. The sexual morphs have no mouthparts and do not feed. After molting three to four times, the males and females mate and each female just produces one fundatrix, who can crawls to feed on a tender leaf of the gallnut tree and induce a gall, thus completing the whole life cycle. So, the life cycle ofS.chinensisis comprised by six generations (fundatrix, the first-generation apterous fundatrigeniae, second-generation apterous fundatrigeniae, alate fundatrigeniae, alate sexuparae and sexuals) and two migratory phases (alate sexuparae flying from the second host to the primary host, and alate fundatrigeniae flying from the primary host to the second host).
The olfactory sensilla of insects can detect low concentrations of specific odorous substances that can enable them to adjust their behavior accordingly, such as mate seeking, predator avoidance, foraging, and locating a reproduction site. Relative to other insect groups, the olfactory sensilla of aphids are simple, although they are sufficient for the basic needs of the aphids, such as visual sense, flying control, feeling, tactile sense, olfactory sense and so on (Park and Hardie, 2004). Studies have indicated that most of the olfactory sensilla of aphids are mainly distributed on their antennas, and their role in the migration, host plant recognition and location, and pheromone regulation of aphids is well established (Sunetal., 2012; Sunetal., 2013; Banetal., 2015). Although numerous studies on the antennal sensilla of aphids have been performed (Bromelyetal., 1980; Kanturskiaetal., 2017),S.chinensishave not yet been fully examined in this regard, and only the morphological structure of the antenna in alate fundatrigeniaeS.chinensishave been examined (Yangetal., 2009; Bietal., 2010).
Given that antennal sensilla in aphids, and other insects, havevery important biological functions, the variation in their number and types has long-term evolutionary significance (Inayatullahetal., 1991). In the present study, we investigated the type, number and distribution of the antennal sensilla in different morphs ofS.chinensis,aiming to explore the corelation between differences in antennal sensilla and the behavior of the different aphid morphs.This results can provide more details to deeply understand the intricated life circle ofS.chinensis.
1 Materials and methods
1.1 Collecting specimens of Schlechtendalia chinensis
Developing galls were collected in the field in Yanjin Country, Yunnan Province, which is located in the southwest of China. The galls were taken to the lab in Kunming. After opening the galls, the different morphs ofS.chinensiswere collected and transferred into centrifuge tubes with 75% ethanol.
During the following spring, the alate sexuparae were collected in the field when they just emerged from the secondary host. These samples were separated into two groups. One group was directly kept in 75% ethanol, and the second was reared in 5-cm culture dishes until they produced male and female individuals, and then put them in centrifugal tubes with 75% ethanol.
The aphid type and collection time used in the experiment are shown in Table 1.
All forms ofS.chinensiswere deposited in the Research Institute of Resources Insects (RIRI), Chinese Academy of Forestry (CAF), Kunming, China.
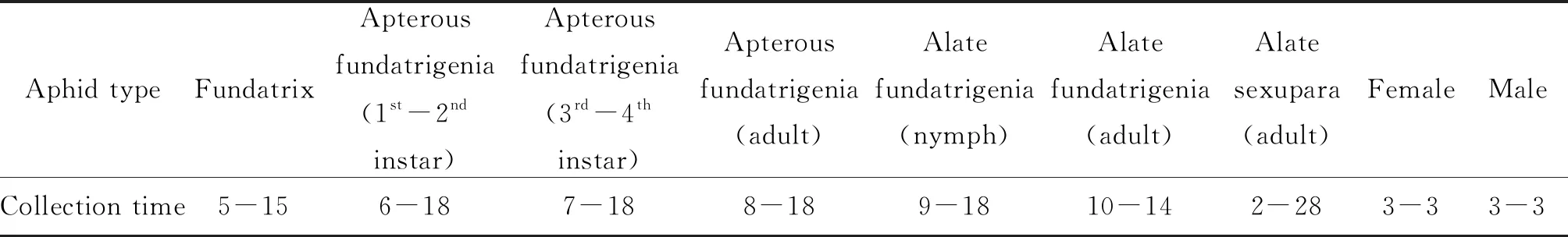
Table 1 Aphid type and collection time used in the experiment
1.2 Scanning electron microscopy(SEM)
The aphid specimens were transferred from the centrifuge tubes to a culture dish. After each specimen had dried out under room temperature, it was put on a flat specimen holder with conductive adhesive. Antennal sensilla were observed with a scanning electron microscope HITACHI TM-3000 at accelerating voltages of 15 kV. The sensilla were classified according to the named system of Zacharuk and Shields (1991). 10 samples per aphid type were observed.
2 Results
Four types of antennal sensilla were found on the antenna of different morphs inS.chinensis, and they were trichoid sensilla, primary rhinaria, sensory projections and secondary rhinaria, respectively.
2.1 Trichoid sensilla
Trichoid sensilla distributed on the antenna of allS.chinensismorphs, and could be divided into two types: I (Fig.1-A)and II (Fig.1-B). Type I sensilla were long and seta-like. They occurred on the antenna of all theS.chinensismorphs, and have difference in number, location and length. ForS.chinensisinhabiting galls, eight Type I sensilla were present on each antenna of the fundatrix and apterous fundatrigeniae, and seven occurred on the antenna of third-generations alate fundatrigeniae. Type I sensilla on the antennae of first or second instar apterous fundatrigeniae were, on average, 8.91±1.03 μm in length, and those on the antennae of nymphal alate fundatrigeniae were 11.72±4.59 μm. There were nine Type I sensilla on the four antennal segments of the male, which were 5.06±1.32 μm in length; by contrast, there were only seven on the antenna of the female and they were 2.99±0.52 μm long. The lowest number of Type I sensilla (six) occurred on five antennal segments from alate fundatrigeniae and alate sexuparae; the sensilla were 5.04±1.19 μm and 9.25±1.76 μm in length, respectively (Table 2).
Type II sensilla occurred at the end of the terminal antenna, which has five short and woolly setae. One seta is located centrally and the other four are located around it. The basal diameter and length of Type II sensilla from all morphs ofS.chinensisare shown in Table 2.
2.2 Primary rhinarium
Primary rhinaria were observed on antenna from all morphs ofS.chinensisexcept for the alate fundatrigeniae and alate sexuparae, and their numbers changed among different morphs. There was only one primary rhinarium on the last antennal segment of nymphal alate fundatrigeniae, whereas there were two on each antenna of the fundatrices, apterous fundatrigeniae, males and females: One occurred on the last segment, and the other on the penultimate segment.
The primary rhinaria located on these different segments also differed in their morphology. The primary rhinarium located marginally on the ventral side of the last segment (Pr I) (Fig. 1-D), was a circular cavity formed by sunken antennal cuticle. Near the inner side of the cavity, there was a board protuberance; on the opposite inner side, there were several short hair-like protuberances surrounding the cavity. These hair-like protuberances are likely to have protective functions, preventing unwanted particles from entering the sensillum. Of all the sensillum forms ofS.chinensis, Pr I on the antenna of nymphal alate fundatrigeniae was the largest (Table 2).
Primary rhinaria II (Pr II) were distributed on the penultimate antennal segment, also marginally located on the ventral side, near the last segment, but were smaller than Pr I. The Pr II sensillum had two forms: Pr II-1 (Fig. 1-C and Fig. 1-D) and Pr II-2 (Fig. 1-E). Pr II-1 was a hemispherical protuberance inserted in a cavity, lacking any obvious hair-like protuberances and found only on the antennae of fundatrices and apterous fundatrigeniae. Pr II-2 was a board-like protuberance formed by antennal cuticle, but lacking cavity and only distributed on the antennae of males and females. The sizes of the primary rhinaria located on the antenna of allS.chinensismorphs were detailed in Table 2.
2.3 Sensory projection
The sensory projection was a rod-like protuberance formed by antennal cuticle (Fig.1-F). It was only located on the third to fifth segments of the antenna of nymphal alate fundatrigeniae. The sensory projections were, on average, 3.92±1.12 μm in height, and 2.75±0.33 μm in diameter.
2.4 Secondary rhinarium
Secondary rhinaria were only located on the third to fifth segments of the antenna of alate fundatrigeniae and alate sexuparae. There was a large ring or band projections that spiraled horizontally onto the antennal surface. The secondary rhinaria accounted for approximately two-thirds of the antennal surface (Fig. 1-G).

Fig.1 Type and morphology of antennal sensilla situated on the antenna of the morphs of Schlechtendalia chinensisNote: A, Trichoid sensillumType Ⅰ (TsⅠ). Scale bar=50 μm;B, Trichoid sensillum Type Ⅱ (TsⅡ). Scale bar=30 μm;C, Primary rhinarium situated in penultimate antennal segment (Pr II-1). Scale bar=30 μm;D, Primary rhinarium situated on the final and penultimate segments of the antenna of the fundatrix and apterous fundatrigenia morphs (Pr I and Pr II-2). Scale bar=30 μm;E, Primary rhinarium situated on the final and penultimate segments of the antenna of the male and female morphs (Pr I and Pr II-2). Scale bar=30 μm;F, Sensory projection (Sp). Scale bar=30 μm;G, Secondary rhinarium (Sr). Scale bar=50 μm.
3 Discussion
The antennal sensilla of an aphid can determine its behavior; therefore, the differences in the host selection, habitat and behavior of the different morphs ofS.chinensisare likely to result in differences in the type and number of antennal sensilla. These differences are caused by a series of selection stresses (Chapman, 1982), and have important ecological adaptation significance.
The trichoid sensillum was the most common type of sensillum on allS.chinensismorphs. Studies have shown that there are many differences in the structure and function of Trichoid sensillum I and Trichoid sensillum II (Seliferetal., 1964; Bromelyetal., 1980). Trichoid sensilla I are mainly distributed on the surface of the body and antennae of the aphid and are mechanoreceptors (Bromelyetal., 1980). Other reports have indicated that Trichoid sensilla I can detect contact with other insects, and is used by the insect to defend against attack by natural enemies (Bromelyetal., 1980). In the present study, we found that Trichoid sensilla I were located on antenna of allS.chinensismorphs, with some differences in number, but significant differences in length, with the longest (8.91±1.03 μm) occurring on the antenna of 1stor 2ndinstar apterous fundatrigeniae, which are the smallest morph in the galls, followed by those on the fundatrices (6.25±0.80 μm) and being shortest (4.03±1.24 μm) on the antennae of 3rdor 4thinstar apterous fundatrigeniae. When we observed the behavior ofS.chinensisthat lived in the gall, we found that there were significant differences between the activity levels of the fundatrices and apterous fundatrigeniae. Fundatrices and apterous fundatrigeniae that are older than the 2ndinstar stage rarely moved and continued to eat at the same site. By contrast, 1stor 2ndinstar apterous fundatrigeniae were the most active. After being born, they crawled over the inner layer of the gall or over other aphids until they found an appropriate feeding site. Therefore, longer Trichoid sensilla I are likely more suitable forS.chinensisliving in galls, enabling them to perceive the inner environment of the gall, and to find suitable feeding sites. Moreover, it is likely that the longer Trichoid sensilla I can help the males and females to locate each other for mating. In this study, we found that trichoid sensilla I on the antenna of the males were longer than those on the antenna of the females (5.06±1.32 μm and 2.99±0.52 μm, respectively).
Trichoid sensilla II were mainly located at the end of antennae, and usually numbered four to five (Zhang and Zhang, 2000). We found that there were five Trichoid sensilla II on the antenna of allS.chinensismorphs. Studies have shown that this sensillum also acts as a mechanoreceptor, used by the aphid to detect the plant surface (Bromelyetal., 1980). Other studies have shown that these sensilla can help the aphid determine whether the feeding site is suitable (Powelletal., 1995), and as such, these receptors also appear to have gustatory functions (Wensler, 1977).
Our SEM results showed that primary rhinaria were distributed on the antenna of winglessS.chinensis, but did not occur on the antenna of the alate fundatrigeniae and sexuparae, and that there was only one primary rhinarium on the antenna of nymphal alate fundatrigeniae. Therefore, the exist of primary rhinaria may be to meet some needs of wingless aphids or aphids with undeveloped wings, and have the similar function. Numerous studies have shown that, for many species of aphids, primary rhinaria can detect alarm pheromones and volatile odors (Zhao and Ban, 2011). In addition, although there were two primary rhinaria distributed on the antenna of fundatrices, apterous fundatrigeniae, males and females, the morphology of the Primary rhinarium II located on the penultimate antennal segment differed between these morphs. Receptors situated on the antennae of the fundatrices and apterous fundatrigeniae possessed a cavity, whereas those on the antenna of the males and females did not. This difference might be the result of the different habitats occupied by these morphs. Previous studies indicated that sunken cavity sensilla can detect changes in temperature, humidity, water vapor and carbon dioxide (Bruce and Cork, 2001). Therefore, it is possible that the Primary rhinarium II on the antennae of the fundatrices and apterous fundatrigeniae perceive changes in the microenvironment inside the gall, enabling the apterous fundatrigeniae to determine whether living conditions inside the gall are still suitable; or whether it is time for the alate fundatrigeniae to emerge and migrate to a secondary host.
The most significant difference in the different morph antenna ofS.chinensisoccurred in the secondary rhinaria: These occurred on the antenna of alate fundatrigeniae and alate sexuparae, but not on winglessS.chinensismorphs. Therefore, we propose that secondary rhinaria have a function in host recognition and location. One study showed that the secondary rhinaria of winged aphids are important for migration from one host plant to another (Pickettetal., 1992). There are only two types of sensilla on the antennae of alate fundatrigeniae and alate sexuparae: Trichoid sensilla and secondary rhinaria. The secondary rhinaria are numerous, and account for approximately two-thirds of the antennal surface. Therefore, it might not have a single function. Alate fundatrigeniae and alate sexuparae both live outside the gall, and their food and habitat are different from those morphs ofS.chinensisinhabiting the inside of the gall. We suggest that secondary rhinaria have a role in host selection or defense against natural enemies. Some studies have shown that secondary rhinaria have the ability to detect plant odors and alarm pheromones (Pettersson, 1973). Furthermore, Pettersson (1970, 1971) and Dawsonetal. (1990) both indicated that secondary rhinaria distributed on the antenna of the males can detect sex pheromones and have an important role in mating. Our study also found that there were many sensory projections on the third to fifth antennal segments of nymphal alate fundatrigeniae, in addition to seven Trichoid sensilla I, five trichoid sensilla II and one primary rhinarium. We suggest that the sensory projection is a transitional stage in the development of secondary rhinaria.
The present study observed the type and distribution of the antennal sensilla present in the different morphs ofS.chinensis. Future work should further elucidate the function and molecular mechanisms involved in the perception of chemical information by these sensilla.

