Synthesis of Ultrafine TbO1.81 and Tb2O3 Powders for Magneto-Optical Application
, Ü , , , , IU
(1.School of Materials Science and Chemical Engineering, Ningbo University, Ningbo 315211, China; 2.Key Laboratory of Optoelectronic Materials Chemistry and Physics, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China; 3.Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou 350108, China; 4.Key Laboratory of Photoelectric Materials and Devices of Zhejiang Province, Ningbo 315211, China)
Abstract:Ultrafine TbO1.81 and Tb2O3 powders were obtained from the pyrolytic precursor prepared via a wet chemical route using ammonium hydrogen carbonate (AHC) as the precipitant. The precipitation precursor has a chemical composition of hydrated terbium carbonate and exhibits one-dimensional nanorod morphology. The average width of the nanorods rises as the increase of AHC concentration. Calcining the precursor in air directly yields a round TbO1.81 nanopowder with an average particle size of ~140 nm through dehydration, decarbonation and particle growth processes. On the other hand, a Tb2O3 powder with a finer particle size of ~85 nm is reduced under flowing hydrogen atmosphere upon heating. The molar ratio of AHC to Tb3+ significantly affects the particle dispersion of final oxide products and the best molar ratio for the synthesis of well dispersed powder is 1∶1. The bandgap energies of TbO1.81 and Tb2O3 are ~1.67 eV and 5.20 eV, respectively.
Key words:ultrafine powder; Tb2O3; TbO1.81; co-precipitation method; magneto-optical effect; morphology
0 Introduction
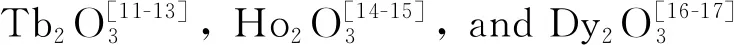
As is well known, the optical qualities of transparent ceramic materials generally depend on the performances of the starting particles, including purity, size, dispersion, morphology, et al. Up to now, the synthesis routes for terbium oxide powder mainly include combustion, sol-gel, chemical precipitation, and solvent extraction[18-20]. Among these methods, the wet chemical method has been proved to be a good way for the processing of readily sinterable particles as the starting materials in pore-free ceramic manufacture[21-23]. Saito et al[24]employed ammonium hydrogen carbonate as the precipitant to prepare the ultrafine Y2O3powder, with which the vacuum-sintered body presented good transparency even though without sintering additive. We synthesized red (Gd,Ln)2O3∶Eu(Ln=Y,Lu) phosphor powders by a co-precipitation method and studied compositional effects on photoluminescence[25]. With these sinterable particles, transparent red-light-emitting ceramics were subsequently fabricated, which exhibit potential application in the optical field[26].
In the present work, ammonium hydrogen carbonate was utilized as the precipitant for the synthesis of ultrafine TbO1.81and Tb2O3particles. The effects of precipitant concentration and calcination atmosphere (air/hydrogen) on the properties of precipitation precursors and oxidation/reduction products were systematically investigated by X-ray diffractometry (XRD), field emission scanning electron microscopy (FE-SEM), Fourier transform infrared spectroscopy (FT-IR), laser diffraction particle size analysis (LDPSA), thermogravimetry (TG), and UV-Vis absorption spectroscopy.
1 Experimental
The raw materials of Tb(NO3)3·6H2O (99.95% purity, Shanghai Diyang Chemical Co., Ltd., Shanghai, China) and ammonium hydrogen carbonate (AHC; AR pure, Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) were respectively dissolved by distilled water to prepare 0.3 mol/L Tb(NO3)3solution as the mother liquor and 1.5 mol/L AHC solution as the precipitant. White precipitation was obtained by dropwise addition of AHC precipitant into Tb(NO3)3mother liquor at a rate of ~4 mL/min under mild stirring at ~25 ℃. The adding AHC content was metered by selected molar ratio (R) of AHC to Tb3+from 1 to 4. After aging for 24 h, the resulting suspension was separated by centrifugation and repeatedly washed using distilled water and absolute ethanol to remove byproducts. The centrifugal product was dried at 90 ℃ over 12 h and was then lightly ground in an agate mortar. For comparison, the obtained precursor powder was calcined in a tubular furnace at 1 000 ℃ for 3 h under flowing air and hydrogen (~150 mL/min), respectively.
The chemical composition of precipitation precursor was qualitatively characterized by Fourier transform infrared spectroscopy (FT-IR; Model Nicolet 6700, Thermo Fisher Scientific, Massachusetts, USA). The phase structures of the products were determined by X-ray diffraction (XRD; Model D8 Advance Davinci, Bruker, Karlsruhe, Germany) using nickel-filtered Cu Kαradiation as the incident X-ray source in the 2θrange of 5°~80°. The precursor sample was filled in a small alumina crucible and subjected to thermogravimetric analysis (TG; Model Diamond, PerkinElmer, Massachusetts, USA) in air at the ramp rate of 10 ℃/min up to 1 200 ℃. A field emission scanning electron microscope (FE-SEM; Model S-4800, Hitachi, Tokyo, Japan) was used to observe the particle morphologies. The dispersion of the calcination products was detected by laser diffraction particle size analysis (LDPSA, Model ZS90, Malvern Instruments, Malvern, UK). The absorption spectra of the calcination products were determined by an UV-Vis spectrophotometer (Model Lambda 950, Perkin-Elmer, Shelton, USA) in the wavelength range from 200 nm to 800 nm.
2 Results and discussion

Fig.1 FT-IR spectrum of the as-synthesized precipitation precursor
Fig.1 displays the FT-IR spectrum of the as-synthesized precipitation precursor prepared atR=1 with an aging time of 24 h. This sample possesses characteristic hydrated carbonate structure. The broad peak at ~3 400 cm-1and the narrow absorption band at ~1 630 cm-1both arise from molecular water. The former derives from symmetricv1 and antisymmetricv3 vibrations and the latter is attributable to bending modev2. A group of absorption bands at ~1 500 cm-1, 1 420 cm-1, 1 090 cm-1, 850 cm-1, 760 cm-1, and 690 cm-1are diagnostic carbonate anion, among which the peaks at ~1 500+1 420 cm-1, ~1 090 cm-1, ~850 cm-1, and ~760+690 cm-1are caused by thev3,v1,v2, andv4 vibration modes, respectively[27].
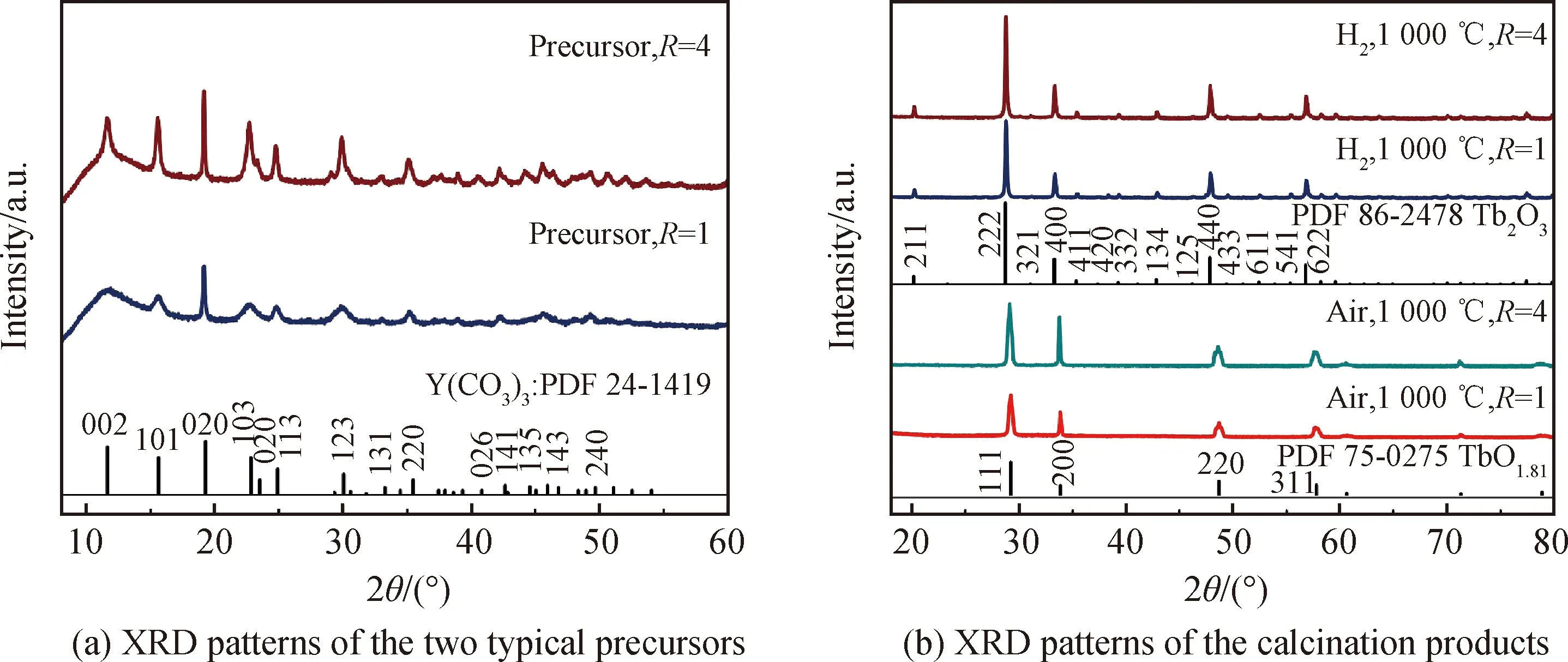
Fig.2 XRD patterns of the two typical precursors synthesized at R=1 and 4, and their calcination products calcined at 1 000 ℃ in air or hydrogen atmosphere
Fig.2(a) shows X-ray diffraction patterns of the two precipitation precursors synthesized atR=1 and 4. The diffraction peak ofR=4 precursor is sharper than that ofR=1 counterpart, since a high AHC concentration would readily lead to better crystallinity. Their diffraction peaks can be both indexed to the orthorhombic rare-earth carbonate of Y2(CO3)3·nH2O (JCPDS No.24-1419) in accordance with the FT-IR result. The (202) diffraction of the precipitation precursor slightly shifts towards to the low angle side compared with the XRD standard card (2θ=19.18° for the precursor and 2θ=19.32° for the standard card), since the ionic radius of Tb3+(0.923 nm for CN=6) is larger than that of Y3+(0.090 0 nm for CN=6). Therefore, the chemical compositions of the two precursors can be both expressed as Tb(CO3)3·3.3H2O, where the number of crystal water in the precursor is determined from TG analysis.



Fig.3 FE-SEM images of the precipitation precursors synthesized at R=1 and 4, and their corresponding calcination products obtained at 1 000 ℃ in air or hydrogen atmosphere
As is well known, the hard agglomerates in ceramic particles is detrimental to sintering densification and frequently induce serious defects in the sintered body, such as big-size pores, crack-like cavities, white dots, and so forth. Particle size distribution in differential volume was found to be a good tool to effectively detect the agglomeration[30]. Fig.4 exhibits the LDPSA results of the oxidation and reduction products obtained fromR=1 and 4 precursors. The LDPSA curves of theR=1 and 4 oxidation products as well as theR=4 reduction product all show bimodal distribution with significantly large-size agglomerates. These particles may be hard to be densified into fully dense bulks by pressureless sintering, because this sintering technology frequently requires highly sinterable starting powder. However, theR=1 reduction product exhibits unimodal distribution and has an average particle size of ~331 nm, implying its relatively high sinterablity. With this excellent powder, the pore-free ceramic may be produced by advanced sintering technique (pressureless sintering plus hot isostatic pressure may be a good choice).
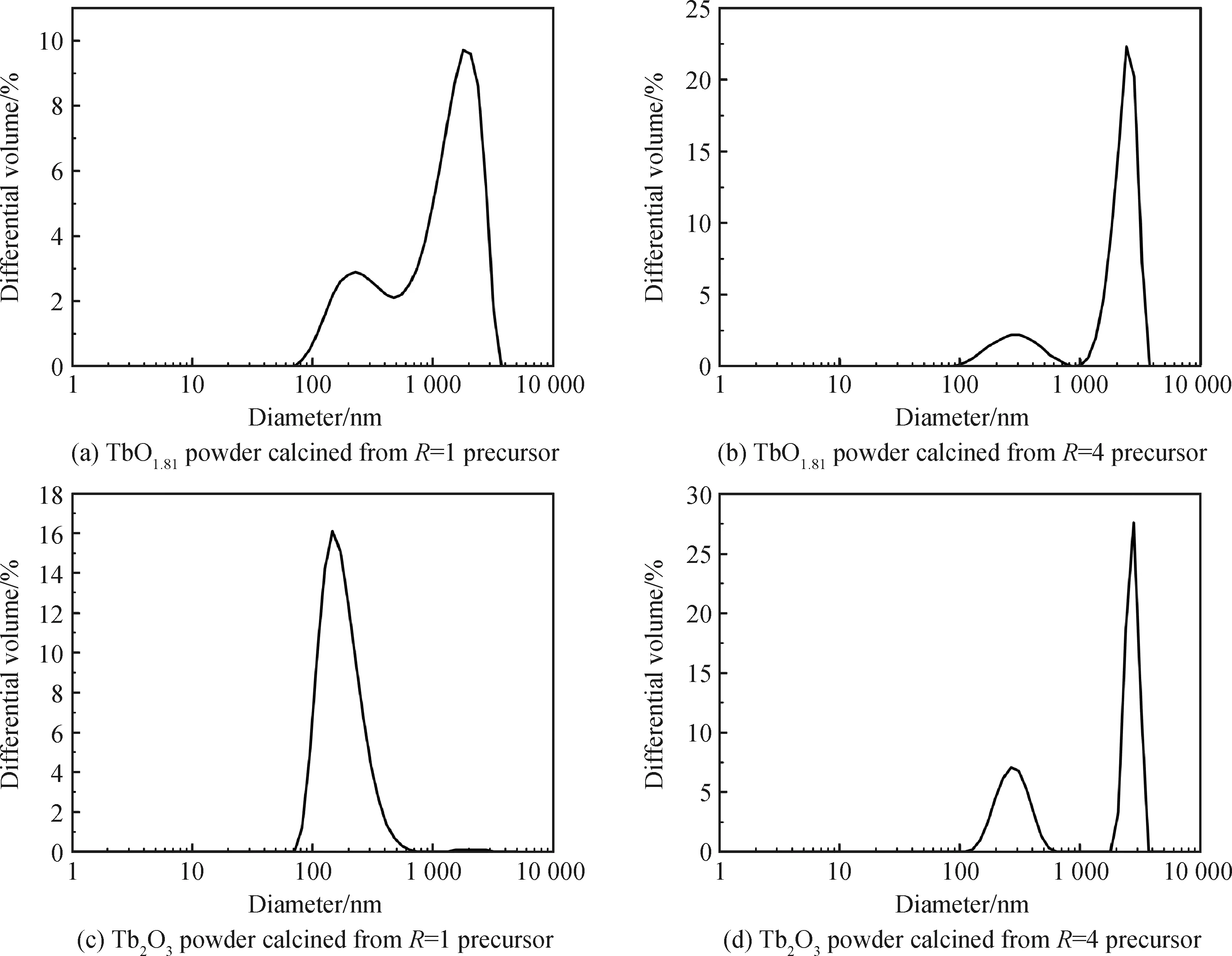
Fig.4 LDPSA results of the calcination products in air and hydrogen atmosphere from R=1 and 4 precursors

Fig.5 TG curve for the Tb(CO3)3·3.3H2O precursor
Fig.5 shows the thermal decomposition processes of Tb(CO3)3·3.3H2O precursor. It can be seen from the TG curve that the carbonate converts into oxide via three main stages. The first step (below ~260 ℃) is primarily due to dehydration with a weight loss of -10.5%, which is close to the calculated weight loss of -10.6%. In the second stage (260~530 ℃), a weight loss of -19.5% (theoretical value: -20.5%) is assigned to decarbonation to yield the Tb2O2CO3and TbO1.81products. The final stage occurs above ~530 ℃, on which the pure TbO1.81product is produced by full decarbonation together with particle growth. The detailed thermal decomposition processes of the precursor can be described as follows:
Optical properties of the TbO1.81and Tb2O3powders were studied by UV-Vis absorption spectra and the results are shown in Fig.6(a) and (b). A broad absorption band from 250 nm to 550 nm for the TbO1.81product was observed corresponding its brownish particle color to the naked eyes (inset in Fig.6(a)). On the other hand, the significant absorption band from 250~300 nm on Tb2O3absorption curve derives from 4f8→4f75d1transition of Tb3+caused by crystal-field interaction and spin-orbit coupling, while that from 350~400 nm originates from7F6→5D2,3transitions of Tb3+. The Tb2O3powder significantly absorbs the component of blue light in the visible spectrum and thus appears yellowish color (inset in Fig.6(b)).
Fig.6 UV-Vis absorption spectra for the TbO1.81 and Tb2O3 powders and the plots of hν against (Ahν)2 obtained from their respective UV-Vis absorption spectra
The relationship between the bandgap energy (Eg) and the absorption coefficient (α) can be expressed from Equation(1):
αhν=B(hν-Eg)1/2
(1)
whereBis the absorption constant andhνis the incident photon energy. Hence a plot of (Ahν)2againsthνfrom the absorption spectra would result inEgvalue by extrapolation of the linear part of the curve to thex-axis. The estimated bandgap energies of TbO1.81and Tb2O3are ~1.67 and 5.20 eV, respectively (Fig. 6(c) and (d)). The bandgap value of Tb2O3powder is close to those of Ho2O3(~5.31 eV) and Er2O3(~5.29) particles[14,31]. Horoz et al[32]calculated the band structure of Tb2O3based on the density functional theory and estimated its energy gap to be ~3.82 eV. The mismatch between their computational result and our experimental value may ascribe to their underestimation on the size of the energy band gap in their adopted GGA (generalized gradient approximation) exchange-correlation function.
3 Conclusion
Ammonium hydrogen carbonate (AHC) is used as the precipitant for the synthesis of hydrated terbium carbonate precursor via a chemical precipitation route. A low AHC concentration leads to a precipitation precursor with clustered one-dimensional nanorod shape, while a higher AHC content results in dispersed nanorods with a broader width. After calcination in air, the precursor decomposes into a round TbO1.81powder via dehydration, decarbonation, and particle growth processes. On the other hand, the well dispersed Tb2O3powder can be reduced from theR=1 precursor upon heating under flowing hydrogen atmosphere. The bandgap energies of TbO1.81and Tb2O3are determined to be ~1.67 eV and 5.20 eV, respectively. The two oxide particles, especially the well-dispersed Tb2O3sample made in this work, may be utilized for the production of transparent Tb2O3ceramics for magneto-optical applications.

