Molecular and morphological evidence for a new species of the genus Typhlomys (Rodentia:Platacanthomyidae)
DEAR EDITOR,
In this study,we reassessed the taxonomic position ofTyphlomys(Rodentia:Platacanthomyidae) from Huangshan,Anhui, China, based on morphological and molecular evidence.Results suggested thatTyphlomysis comprised of up to six species,including four currently recognized species(Typhlomys cinereus,T.chapensis,T.daloushanensis,andT.nanus),one unconfirmed candidate species,and one new species (Typhlomys huangshanensissp.nov.).Morphological analyses further supported the designation of the Huangshan specimens found at mid-elevations in the southern Huangshan Mountains (600 m to 1 200 m a.s.l.) as a new species.
The family Platacanthomyidae is comprised of two extant genera,PlatacanthomysandTyphlomys(Musser & Carleton,2005).Molecular phylogenetic findings support Platacanthomyidae as a unique lineage and sister to all other Muroidea families,although their external morphology is similar to dormice of the family Gliridae and their molar structure is similar toGymnuromysfrom Madagascar (Abramov et al.,2014; Jansa et al.,2009; Musser and Carleton,2005).
The genusTyphlomys(Milne-Edwards,1877),which means“blind mouse” (Jansa et al.,2009),possesses small shrew-like eyes and is the lesser-known member of Platacanthomyidae.These animals have a long hairy tail,long vibrissae,and prominent ears,with the tip of their tails covered in a tuft of long white hair (Hong,1982).Their long vibrissae are a tactile aid in the capture of prey (Anjum et al.,2006),and their short limbs exhibit scansorial and fossorial advantage in terms of leverage (Rubin & Lanyon, 1984). Such morphological adaptations suggest an arboreal and burrowing lifestyle (Cui et al.,2020; Smith,2008).As an arboreal animal with vestigial eyes,these rodents have developed a form of echolocation for orientation and navigation in complex environments without visual assistance (Panyutina et al.,2017).Although eye degradation (Cheng et al.,2017) is a feature present in other mammals,such as moles (Carmona et al.,2008),it is usually associated with fossoriality and is uncommon among scansorial/arboreal species;Typhlomysis the only currently known taxon.
Despite the unique morphology and life history of this genus,the exact species composition ofTyphlomysis still unclear (Jansa et al.,2009).In the original taxonomic description based on pelage,external measurements,and skull morphometrics,Typhlomyscontained five subspecies,includingTyphlomys cinereus cinereus,T.c.chapensis,T.c.daloushanensis,T.c.guangxiensis,andT.c.jingdongensis(Wang et al.,1996).Subsequently,Abramov et al.(2014)supported the specific status ofT.c.chapensisgiven the significant molecular and skull morphology differences between this subspecies and nominalT.c.cinereus.Recent molecular phylogenetic studies confirmed the monophyly ofTyphlomysand suggested that it is composed of at least five species based on genetic evidence of multiple loci and skull morphology and morphometrics (Cheng et al.,2017).The research of Cheng et al.(2017) determined the species level recognition ofT.cinereus,T. daloushanensis, andT.chapensis,described a new species (T.nanus),and identified a putative new species.
Typhlomysis mainly distributed in montane regions of southern China and northwestern Vietnam (Abramov et al.,2014; Cong et al.,2013).As a mid-high elevation inhabitant(340–2 300 m a.s.l.),the distribution ofTyphlomysis strongly related to complex geographical regions (Abramov et al.,2012).For example,in southwest China,the extremely complex topography and well-developed river systems in the Hengduan Mountain region have led to the allopatric divergence of species within the genusTyphlomys(Chen et al.,2017; Fjeldså et al.,2012; Liu et al.,2012) and may have led to the differentiation of two different genetic populations (T.nanusandT.chapensis,Cheng et al.,2017).In southeast China,there are similar river valley systems (Song et al.,2017),but only one species (T.cinereus) has been discovered to date (Cong et al.,2013; Wang,1990; Wang et al.,1996).Thus,to some extent,the species diversity ofTyphlomysmay be underestimated.
In the present study, we integrated molecular and morphometric approaches to describe a new species from the Huangshan and Qingliangfeng mountains in Anhui Province,China.
Blind mouse specimens were collected from the Huangshan and Qingliangfeng mountains (Anhui Province,China) and consisted of four adult males and six adult females.Tissue samples were extracted,amplified,and sequenced for the cytochromeb(cytb) gene (1 070 bp),interphotoreceptor retinoid-binding protein (IRBP(1 052 bp)) gene,and growth hormone receptor (GHR(774 bp)).All DNA sequences generated are available in GenBank (Supplementary Table S1).Uncorrected pairwise genetic distances of the cytbgene were computed using the APE R package (Paradis et al.,2004).Bayesian phylogenetic analyses were conducted with MrBayes v3.2.2,including each nuclear gene,cytbgene,and concatenated mitochondrial and nuclear datasets (Ronquist et al.,2012).The Poisson tree processes (PTP) model (Zhang et al.,2013) and BPP v3.4 (Yang & Rannala,2010) were run to infer putative species boundaries (see Supplementary Materials and Methods).Divergence times among putative species were estimated with BEAST v1.7.4 based on the concatenated gene (Bouckaert et al.,2014).Specimens collected for morphometric analyses,combined with detailed skull data from Cheng et al.(2017),included twoT.cinereus,29T.daloushanensis,21T.chapensis,threeT.nanus,and nineT.sp.1 specimens.Morphometric data were taken using digital calipers to the nearest 0.01 mm (Table 1),and following the measurements described in Cheng et al. (2017)(Supplementary Materials and Methods).The skull of voucher specimen AE1902HS04 was broken in transit, so its associated morphometric data are missing from this study.Principal component analysis (PCA) and canonical discriminant function analysis (DFA) were used to compare genetically delineated groups within the genusTyphlomys(Supplementary Materials and Methods).
The combined mitochondrial-nuclear gene and nuclear gene phylogenetic reconstructions recovered similar topologies as Cheng et al.(2017) (Figure 1A-b,c,d),and the monophyly of these lineages was strongly supported (Bayesian posterior probability (PP) ≥0.99). Among them, one clade was comprised ofT.chapensis(Clade A) andT.nanus(Clade B);the other clades (C-F) corresponded withT.cinereus,T.daloushanensis,previously identified cryptic species (T.sp.2),and newly identified species (T.sp.1),respectively.Overall,the relationship betweenT.cinereus(clade E) andT.sp.1(clade F) was strongly supported as sister species (PP≥0.99).In the mitochondrial cytbgene tree (Figure 1A-a),the phylogenetic tree still showed the same topological structure with an additionalT.cinereusfrom Guangdong (Lv et al.,2016).
The cytbuncorrected genetic distances among different species ranged from 11.3% to 19.2% (Supplementary Table S4).Considering that species-level divergence in different mammal groups ranges from 2% and 11% (Baker & Bradley,2006),the different lineages ofTyphlomys(A–F,Figure 1A-a)might represent distinct species.Among them,the genetic distance betweenT.sp.1 andT.cinereuswas 14.2%–14.7%(Supplementary Table S4), indicating species-level divergence.We also observed a single triplet insertion (T.chapensis,T.nanus) and single triplet deletion (T.cinereus,T.sp.1,T.sp.2) in the IRBP sequences.
The topology of the species tree resembled the concatenated gene tree,and all relationships were strongly supported (Figure 1B).The time to the most recent common ancestor (MRCA) ofTyphlomyswas estimated to be 16.94 million years ago (Ma) (95% CI=9.70–26.31).Furthermore,the six putative species diverged between 6.90 and 11.49 Ma,andT.sp. 1 andT. cinereusdiverged about 7.72 Ma(95%CI=4.41–11.40).
The Poisson tree processes (PTP) model for species delimitation recognized eight putative species (Figure 1B-a),and the split results supported the specific status ofT.cinereusandT.sp.1,with species partition support of 0.98 and 0.95,respectively (Figure 1B-a).Based on the results of Cheng et al.(2017),we used BPP to test a six species scenario (Figure 1B-b).The results supported the validity of all six species with high posterior probabilities (PP≥0.99,Supplementary Table S5) and the specific status ofT.sp.1(PP≥0.99).
A summary of the descriptive morphometric variables is given in Supplementary Table S2.Based on principal component analysis of the 11 craniodental measurements,the eigenvalues of two factors exceeded 1.0.The first principal component (PC1) explained 63.52% of total variation,with an eigenvalue of 6.987,which was dominated by ZMW,GLS,LUIM, CBL, BL, M1–M1, LNM–FLM, HCV, and UML(descriptions of all morphometric acronyms can be found in Table 1).The second principal component (PC2) explained 14.66% of total variation,with an eigenvalue of 1.613,which was mainly related to IOB and BCH.The two factors diverged along the first principal component,reflecting differences in overall cranial size.The principal component biplot (Figure 1C)showed that theT.daloushanensisspecimens were mostly clustered in the positive region of PC1 and thatT.chapensisoccupied the positive region of PC2,consistent with the results of Cheng et al.(2017).The other three groups,T.nanus,T.cinereus,andT.sp.1,were all in the negative field of PC1 and PC2 but were completely separated.Among them,males ofT.sp.1 had longer and wider skulls overall than females.
Discriminant function analysis of the same variables correctly classified 100% of morphometric distinctions (Figure 1C).Canonical axes 1,2,and 3 explained 59.7%,34.9%,and 4.4%of total variation,respectively.The first canonical axis (CAN1)mainly included ZMW,LNM–FLM,HCV,and UML.The second axis,based on standardized canonical coefficients(CAN2),consisted of GLS,CBL,and M1–M1.
Phylogenetic analysis showed a significant divergence betweenT.sp.1 andT.cinereus(Figure 1A).The genetic distances betweenT.sp.1 and its nearest species (T.cinereus) was greater than 14% (Supplementary Table S4),reflecting species-level divergence (Bradley & Baker,2006).These results indicate thatT.sp.1 is distinct from all recognized species and support its recognition at the species level.PTP and BPP analyses recognized eight and six putative species,respectively,and supportedT.sp.1 andT.cinereusas separate species (Figure 1B).Therefore,T.sp.1 should be treated as a new species,and as sister species toT.cinereus.The divergence ofT.sp.1 andT.cinereusoccurred at least as far back as the Middle Pliocene to Late Miocene.In addition,our research indicated thatTyphlomysdiversification began between the Middle and Late Miocene(Figure 1B),which may be related to the rapid uplift of the Qinghai-Tibetan Plateau and repeated paleoclimatic changes(Cheng et al.,2019; Liu et al.,2017; Wan et al.; 2018).Similar processes have been suggested for the diversification ofUropsilus(Wan et al.,2013),Perognathus(Riddle et al.,2014),andApodemus(Michaux et al.,2002).
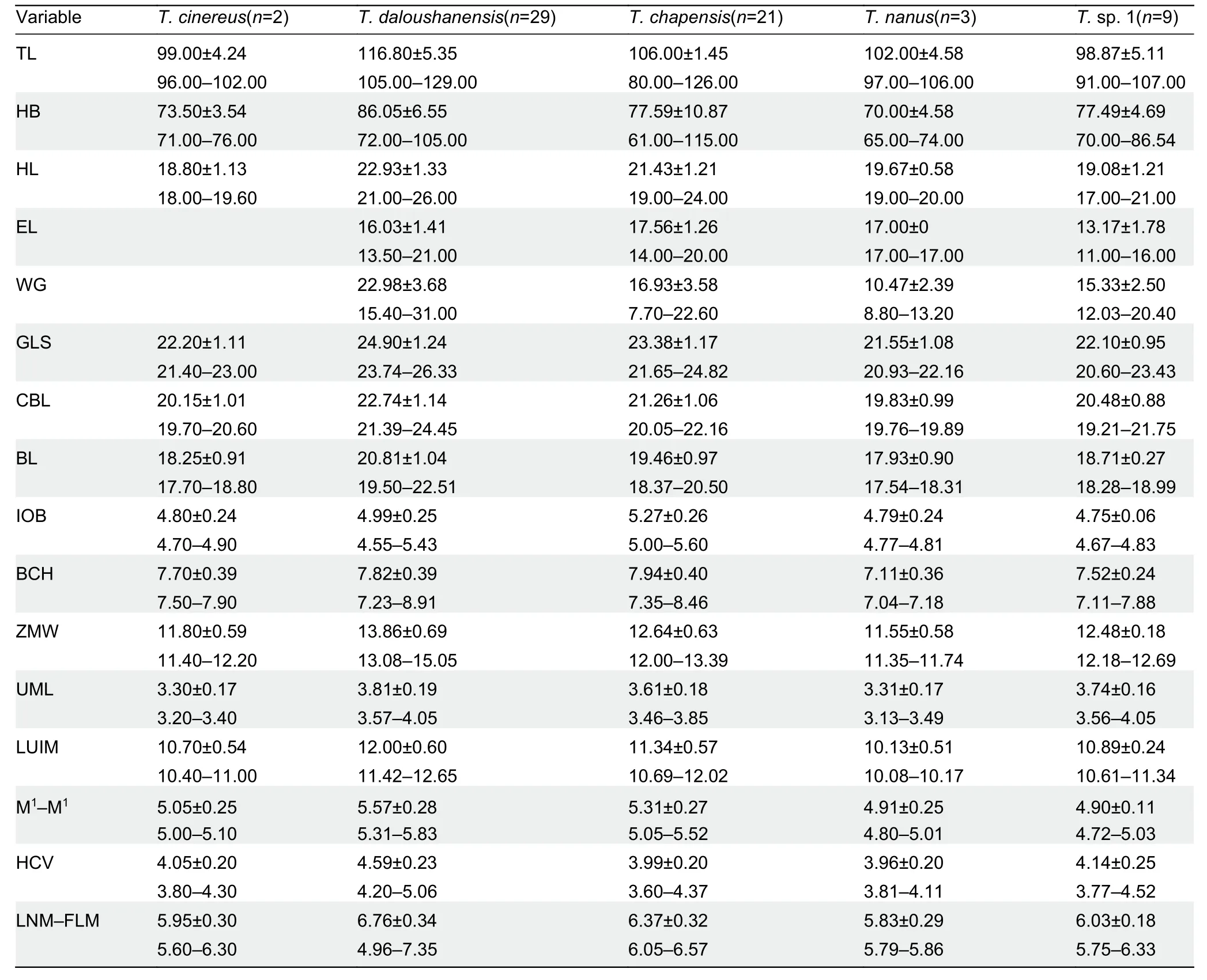
Table 1 External and cranial morphological measurements (mm) of Typhlomys
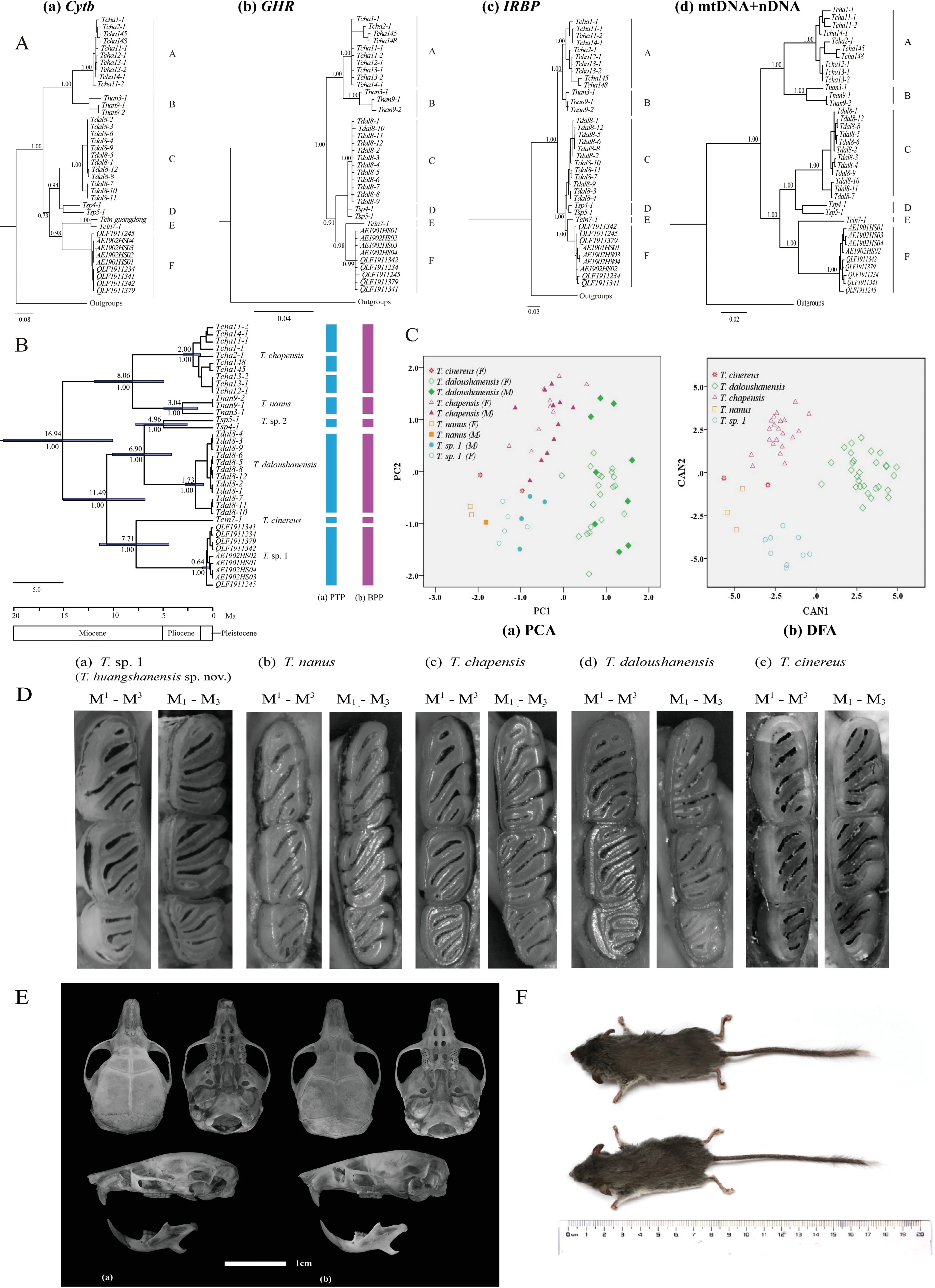
Figure 1 Phylogenetic tree,species delimitation,and principal component and discriminant function analysis of Typhlomys,with skulls and dorsal views of Typhlomys huangshanensis sp.nov.and molar comparisons of five species
The distribution of the six different species showed a distinct geographic pattern (Supplementary Figure S1),which could be partly due to the complex topography,developed river systems,and low dispersal ability of the animals (Cheng et al.,2017; Fu & Zeng,2008; Hinckley et al.,2020; Zhou et al.,2012).On the other hand,in southeast China,scattered mountain ranges with high elevations,such as the Wuyi and Huangshan mountains,form potential sky islands and show allopatry in isolated areas (McCormack et al.,2009).These mountains span a wide altitudinal range that helps to buffer changes in climate and has provided continuously suitable habitats forTyphlomyssince the early Late Miocene (He et al.,2019; Wu et al.,2013).The complex topography of these mountains may facilitate allopatric speciation over long evolutionary timescales by physical isolation,eventually resulting in the appearance of narrowly distributed endemic species.
With respect to the previous taxonomy ofTyphlomys,dental formulas and skulls are used as keys for species diagnosis.Measurements forTyphlomysare provided in Table 1 and photographs of the skulls and teeth are provided in Figure 1D,E.Based on the PCA and DFA results,T.sp.1 did not aggregate with other species.
Based on the above measurements,T.sp.1 has a shorter and narrower skull (GLS=20.60–23.43,ZMW=12.18–12.69,M1-M1=4.72–5.03) thanT.daloushanensis(GLS=23.74–26.33,ZMW=13.08–15.05,M1–M1=5.31–5.83),although both have flattened braincases. Regarding the upper molars, the anterofossette on M1ofT.daloushanensisis much wider than that ofT.sp.1,and the anterofossette is present on M²,whereas it is absent inT.sp. 1. Furthermore,T.daloushanensishas yellowish-white hair on its hind feet,which differs from the black pelage ofT.sp.1.
In addition,T.sp.1 has a narrower interorbital breadth(IOB=4.67–4.83) thanT.chapensis(IOB=5.00–5.60).The latter has a distinct dome-shaped skull,which is flattened inT.sp.1.The mesofossettid on M1ofT.chapensisis usually open on the buccal side but closed inT.sp.1.The dorsal pelage is yellowish gray inT.chapensis,but brownish gray inT.sp.1.
Typhlomyssp. 1 has a wider zygomatic breadth(ZMW=12.18–12.69) thanT.nanus(ZMW=11.35–11.74).The former also has a flattened braincase,whereasT.nanushas a dome-shaped braincase.Typhlomyssp. 1 has a posterofossettid on M1,which is absent inT.nanus.The mesofossettid on M1is open inT.nanusbut closed inT.sp.1.
Typhlomyssp.1 (GLS=20.60–23.43) andT.cinereus(GLS=21.40–23.00) have a similar skull size and domeshaped braincase,butT.sp.1 has a longer upper molar(UML=3.56–4.05) thanT.cinereus(UML=3.20–3.40).The anterofossettes on M1inT.sp.1 are narrower than those inT.cinereus.Furthermore,T.sp.1 has inconspicuous M2anterofossettids,which are wide and very well developed inT.cinereus.
Taxonomic account
TyphlomyshuangshanensisHu & Zhang,sp.nov.
Holotype:AE1902HS03.Dried skin (Figure 1F) and cleaned skull of an adult male,collected on 12 February 2019 by Lie Yu.Type locality:Monkey Valley of Huangshan Mountains(N30.119,E118.306,710 m a.s.l.),Tangkou Township,Huangshan City,Anhui Province,China.The specimen was deposited in the Biological Museum of Anhui University,Anhui,China.
Paratypes:AE1901HS01 (Figure 1F) and AE1901HS02,two adult females,collected on 10 January 2019 by Lie Yu from Monkey Valley,Tangkou Township,Huangshan City,Anhui Province, China (N30.119, E118.306, 710 m a.s.l.).QLF1911245 and QLF1911341,two adult males,collected on 20 November 2019 by Zhongzheng Chen from Qingliangfeng Mountains,Xuancheng City,Anhui Province,China (N30.130,E118.845,1 303 m a.s.l.; N30.138,E118.825,1 040 m a.s.l.).QLF1911244,QLF1911234,QLF1911379,and QLF1911342,collected on 25 November 2019 by Zhong-Zheng Chen from Qingliangfeng Mountains,Xuancheng City,Anhui Province,China (N30.130,E118.844,1 228 m a.s.l.).The above specimens were prepared as dried skins with cleaned skulls.Measurements of holotype (mm):AE1901HS03.Weight=20.40 g; head and body length=86.54; tail length=98.30; hind foot length=18.13; ear length=11.46; greatest length of skull=21.05; condylobasal length=19.85; basal length=18.34; interorbital breadth=4.83; braincase height=7.81; zygomatic width=12.97; length between upper incisor and molar=10.98;upper molar row length=4.05; crown breadth of 1stupper molars=5.10; height of coronoid valley=5.01; length between backmost notch point of mandibular and front of lower molars=6.22.
Diagnosis:The new species can be distinguished by a flattened braincase rather than the domed braincases ofT.nanusandT.chapensis.It has a shorter skull thanT.daloushanensisand a narrower interorbital breadth thanT.chapensis.Its hind feet are covered with black hair,differing from the yellowish-white hair ofT.daloushanensis.It has a longer upper molar thanT.cinereus.In the new species,the anterofossettes on M1are narrower than those inT.cinereus,and the anterofossettid of M2is missing or inconspicuous.
Description:A slightly smaller species in the genusTyphlomys(HB=77.49±4.69; GLS=22.10±0.95).The dorsal and ventral pelage have two distinct colors:the dorsal pelage is brownish gray,while the ventral pelage is gray and covered with creamy white hair.The dorsal surfaces of the hind feet are covered with black hair.The braincase is flattened,and its height is relatively low.The palatal holes are relatively developed and usually have 2–3 columns.Upper molars are wide; with M2anterofossettes missing; M3lacks the anterofossette and posterofossette.The M1anterofossettid is shorter than the mesofossettid,which is closed,and the metalophid is long and developed,with an anterior extra ridge present, which merges with the metalophid at the anterolophid.The posterofossettid is complete.The M2anterofossettid is almost inconspicuous, and the posterofossettid is absent on M3.
Ecology and habitat:The specimens were captured from a mixed evergreen and deciduous broad-leaved forest belt.Other sympatric small mammals includedApodemus agrarius,Apodemus draco,Callosciurus erythraeus,Micromys minutus,andCrocidura anhuiensis(Wang,1990; Zhang et al.,2019).
Distribution:Typhlomys huangshanensissp. nov.is currently known from the Huangshan and Qingliangfeng mountains,Anhui Province,China.The known elevational range is 710–1 303 m a.s.l..It might occur in other mountain areas in southern Anhui and western Zhejiang.
Etymology:The specific name refers to its type locality,i.e.,Huangshan Mountains,Anhui Province,China.We suggest the English common name as “Huangshan blind mouse” and the Chinese common name as “黄山猪尾鼠”.
Key to species of Typhlomys
1a) Dome-shaped braincase,narrow molars,mesofossettid on M1open buccally....................................................................2
1b) Flattened braincase,wide molars,mesofossettid on M1closed…………….....................................................................3
2a) GLS>21.6 mm,LUIM>10.6 mm,IOB>5.0 mm,posterofossettid and posterolophid on M1present........T.chapensis
2b) GLS<22.2 mm,LUIM<10.2 mm,IOB<4.9 mm,posterofossettid on M1absent.................................................T.nanus
3a) GLS<23.5 mm,BL< 19.0 mm,LUIM<11.4 mm,dorsal surface of hind feet black......................................................4
3b) GLS>23.7 mm,BL>19.5 mm,LUIM>11.4 mm,dorsal surface of hind feet yellowish white............T.daloushanensis
4a) UML<3.4 mm,anterofossette on M1relatively wide,anterofossettid on M2wide and very well developed.........................................................................................T.cinereus
4b) UML>3.5 mm,anterofossette on M1narrow,anterofossettid on M2absent or almost disappeared...........................................................................................T. huangshanensis
Comments
For a long time,only a single species of the genusTyphlomys(T.cinereus) was described in southeastern China (Wang,1990; Wang et al.,1996).In the present study,the molecular and morphological results clearly suggest thatTyphlomys huangshanensissp.nov.is an new species and differs from all other congeners within the genus.This takes the number of species within the genusTyphlomysto six.All specimens of the new species were obtained from the Huangshan and Qingliangfeng mountain areas,but we believe the new species likely has a larger distribution,including at least southern Anhui and western Zhejiang.We do not yet know whetherTyphlomys huangshanensissp.nov.is sympatric withT.cinereusor not,and we do not know the distribution boundary between them.Based on the reported description of the distribution ofTyphlomyssp.in southeastern China(southern Anhui,Zhejiang,and Fujian,Cheng et al.,2017;Wang,1990; Wang et al.,1996),the distribution ofTyphlomys huangshanensissp.nov.is worthy of further investigation.
NOMENCLATURAL ACTS REGISTRATION
The electronic version of this article in portable document format represents a published work according to the International Commission on Zoological Nomenclature (ICZN),and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone (see Articles 8.5–8.6 of the Code).This published work and the nomenclatural acts it contains have been registered in ZooBank,the online registration system for the ICZN.The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefixhttp://zoobank.org/.
Publication LSID:
urn:lsid:zoobank.org:pub:5B150064-C727-4A77-BB5C-6D6FE 3509C7D.
Typhlomys huangshanensisLSID:
urn:lsid:zoobank.org:act:778976E1-1 556-4BE9-939E-EFF85B DF113F.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Permission for field surveys in Huangshan City,Anhui Province was granted by the Management Office of Huangshan Scenic Area.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
B.W.Z.conceived and designed the study.T.L.H.performed the experiments,analyzed the data,and prepared the manuscript.F.C.provided and helped analyze the data.Z.X.,Z.Z.C.,L.Y.,Q.B.,C.L.L.,and T.P.collected materials.All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Mr.Heng Zhang and Ms.Gui-You Wu for support and assistance in molecular analyses.
- Zoological Research的其它文章
- Multilocus phylogeny suggests a distinct species status for the Nepal population of Assam macaques (Macaca assamensis):implications for evolution and conservation
- Dmrt1 regulates the immune response by repressing the TLR4 signaling pathway in goat male germline stem cells
- Fluoxetine ameliorates depressive symptoms by regulating lncRNA expression in the mouse hippocampus
- Revisiting the evolutionary history of domestic and wild ducks based on genomic analyses
- Dynamic evolution of transposable elements,demographic history,and gene content of paleognathous birds
- Contribution to the taxonomy of the genus Lycodon H.Boie in Fitzinger,1827 (Reptilia:Squamata:Colubridae) in China,with description of two new species and resurrection and elevation of Dinodon septentrionale chapaense Angel,Bourret,1933

