东北地区传统发酵食品中两株植物乳杆菌的安全性评价
Victoria Yosea Mliga,杨 柳, ,马 赫,孙 畅,Yohana James Mgale,任大勇,
(1.吉林农业大学经济管理学院,吉林长春 130118;2.吉林农业大学食品科学与工程学院,吉林长春 130118)
The increase in the demand for a healthier lifestyle and the development of products with functional characteristics have led to an increase in the demand for probiotic foods[1].Probiotics are defined as living microorganisms that,when administered in adequate amounts,confer health benefits to the host[2].The effects resulting from the consumption of these microorganisms includes the maintenance of the intestinal flora,reduction of cholesterol levels,immunoregulatory properties,prevention of diarrhea,increased tolerance to foods containing lactose and,possibly,prevention of colon cance[3−4].According to these microorganisms must-have characteristics such as survival to stomach acidity,multiplication capacity and adhesion to the intestinal epithelium;ability to withstand adverse conditions,such as the presence of bile salts,gastric,pancreatic and enteric juice;have an antagonistic effect to harmful bacteria;and not be toxigenic or pathogenic to the host.Currently,lactic acid bacteria (LAB) or bifidobacteria are the main probiotic microorganisms used in the food industry[5].
Many strains of LAB are typically regarded as safe due to their long history of use.However,new isolated probiotic strains cannot be assumed to share the historical safety of traditional strains[6].These new strains must be free of virulence and resistance to antimicrobial factors,ensuring food safety,as the ingestion and use of new strains with specific benefits are directly linked to public health[7].Even though the probiotics industry has developed rapidly in recent years with a large space and an exciting development dynamic,the potential risks of lactic acid bacteria have aroused widespread concern in the academic world.There have been increasing reports of local or systemic infections that may be caused by the intake of lactic acid bacteria[8].Various countries,such as Japan,Germany,Canada and the European Union have established guidelines for the preparation of probiotics and their functional products.However,there is no standard guideline for assessing the safety of lactic acid bacteria in China.
TheLactobacillus plantarumL12 and L20 strains,used in the present study,were isolated from spicy cabbage and hot sauce respectively.Spicy cabbage and hot sauce are very popular traditional food products,mainly in the northeastern China,where production and consumption are highest.These strains were obtained from research previously carried out by the research group,which identified physiological,biochemical,and functional characteristics[9].In the existing research of our group,we found that L12 and L20 strains exhibited the potential for high cholesterol-lowering capacity,strong antioxidant capacity and resistance to bile salts[9].However,in order to verify the primary safety information of these isolated strains and the safety of their consumption when applied to products in the future,it is essential and necessary to carry out safety assessments.Thus,this study was undertaken to evaluate antibiotic resistance assess,to the safety of twoLactobacillus plantarumstrains (L12 and L20) bothin vitroandin vivo,different enzymatic activities,acute oral toxicity,and possible pathogenesis of the aforesaid strains.
1 Materials and Methods
1.1 Bacterial Strains
The bacterial strains were recovered from the collection of the Food Toxicology Safety Laboratory at the College of Food Science and Technology of the Jilin Agricultural University,China.Originally,obtained from samples of spicy cabbage and hot sauces from Northeast China,the strains underwent the stapes of physiological and biochemical identification and functional characteristics.Among the many strains,two strains of relatively strong functionalLactobacillus PlantarumL12 and L20 were selected.In comparison to L12,L20 was observed to have a high cholesterol-lowering capacity with clearance rate was above 61.83%.The survival rate of L12 and L20 in the environment of pH2.0 was greater than 69.73%and 60.93%,and the survival rate in the environment containing 0.3% bile salt was greater than 84.35% and 97.39%,respectively.
1.2 In vitro Toxicity Study
1.2.1 Preparation Bacterial Samples The two bacterial strains L12 and L20 were streaked and inoculated into De Man,Rogosa,and Sharpe agar (MRS) solid medium for inoculation at 37 ℃ for 38 hours,and then single colonies were picked and inoculated in sterile MRS liquid medium for 24 hours at 37 ℃.Thereafter,0.7 g of agarose plus 100 mL of TAE running buffer was dissolved and cooled to 60 ℃,then 0.5 μL of nucleic acid dye were added to the mixture to prepare an agarose gel.
1.2.2 Antibiotic Susceptibility The susceptibility of the probiotic strains to different antibiotic was determined on MRS agar,by disc diffusion method,based on the national standard WS/T 639-2018“China Health Industry Standards-Technical Require ments for Antimicrobial Susceptibility Test”.The procedure involved;mix the test bacterial solution with a vortex shaker,spread 100 μL of the solution evenly on the sterile MRS solid medium at a distance of 2 to 3 cm between drug-sensitive papers,and finally incubated in an incubator at 37 ℃ for 24 hours after the solution being absorbed for 30 minutes.The zones of inhibition (mm) were measured after incubation,and the strains were classified for resistance,according to the CLSI 2010 parameters.
1.2.3 Bile Salt Hydrolase Assays The enzymatic activities of the LAB strains were evaluated using the enzyme-linked immunoassay (ELISA) kit.The bacterial strains were coated on MRS solid medium and cultured at 37 ℃ for 72 h,then single colonies were picked and inoculated with sterile MRS liquid medium,and the medium was incubated at 37 ℃ for 20 h,repeated three times.Thereafter,the two bacterial solutions were centrifuged at 3000 r/min for 20 min to take the supernatant,and then diluted the bacterial suspension with PBS to a concentration of 1×106CFU/mL.After two different repetitions,the microbial bile salt hydrolyses was measured using Enzyme Linked Biotechnology’s microbial bile salt hydrolyses (BSH) enzyme-linked immunoassay (ELISA)kit.By taking the concentration of the standard on the abscissa and the value OD on the ordinate,a standard curve was plotted,and the corresponding concentration (U/L) was found from the standard curve as a function of the OD value of the sample multiplied by the dilution factor.MRS liquid medium was used as blank control.
1.2.4 D-lactic Acid Content In order to determine the content of D-lactic acid,the two strains were centrifuged at 3000 r/min for 20 min to take the supernatant,and then the bacterial suspension was diluted with PBS to a concentration of 1×106CFU/mL.After repeated freezing and thawing,the supernatant and the centrifuge were repeated until no precipitation was observed.The enzyme D-lactic acid (D-LA)enzyme-linked immunoassay (ELISA) kit was used to detect the content of D-lactic acid.By taking the concentration of the standard on the abscissa and the value OD on the ordinate,a standard curve was plotted,and the corresponding concentration (μmol/L)was found from the standard curve as a function of the OD value of the sample multiplied by the dilution factor.MRS liquid medium was used as a blank control.
1.2.5 Hemolytic Activity Hemolytic activity of L12 and L20 was determined on MRS blood agar plate as per the standard method[10]with a slight modification.Briefly,after diluting the experimental bacterial solution to 1×106CFU/mL with physiological saline,the diluted bacterial solution was spread evenly on the blood plate culture medium with an inoculation quantity of 100 μL.Once the bacterial solution was completely absorbed,it was placed upside down in an incubator at 37 ℃ for 18 to 24 hours to observe if hemolysis occurs.Staphylococcus aureus(AS11861-beta hemolysis) was used as positive control experimental strain.
1.3 In vivo Toxicology Studies
1.3.1 Animal Management and Ethical Aspects Sixteen female Kunming mice (aged 6~8 weeks),procured from the Experimental Animal Center of Bethune Medical College,Jilin University,were used for the study.All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Jilin Agricultural University,Chinese Government Technical Specifications for Inspection and Evaluation of Health Food 2003 Edition and approved by the Animal Ethics Committee at Jilin Agricultural University (approval no.SCXK 2015-0001).
1.3.2 Acute Oral Toxicity Study (30 Days Repeated-Dose Toxicity Study) The acute oral toxicity test was conducted according to the procedure and methods of the Food Safety Toxicological Assessment GB-15193.3-2014 (People Republic of China).A total of 18 female mice were randomly assigned to three groups (L12,L20 and control) of six mice each.The two animal groups were administered with 0.2 mL (109CFU/mL) of experimental bacterial solution(L12 and L20,respectively) via gavage each day for 30 days.Another group served as control was also administered normal saline at a dose of 0.2 mL for the same time duration.The time of gavage was the same every day.During this period,the behavior and activities of mice were observed and recorded daily.Weight was measured once a week and food intake was measured 2 to 3 times a week.After the 30-day of intragastric administration,on the 31st day,the spinal dislocation method was effected,the general dissection was performed,the internal organs were taken and weighed.Relative organ weight of spleen,heart,liver,and kidney was calculated as the organ weight (mg) divided by the actual body weight in grams (before the sacrifice).
Histopathological examination was also performed on the liver.After the mice were sacrificed,the livers were soaked in 40 g/L paraformaldehyde for 2 h,and then the mouse liver tissues treated with 40 g/L paraformaldehyde solution were dehydrated for 24 h in an automatic tissue processor.Each sample was cut to 5 μm-thickafter embedded in paraffin wax using a paraffin embedding system.The sections were fixed on a glass slide,treated with 10% formalin,heated at 58 °C for 4 h until dried.Thereafter,the sections were stained with hematoxylin and eosin(H&E),dehydrated with gradient alcohol for 5 minutes and subjected to microscopic examination.
1.3.3 Malondialdehyde Serum Concentration Malondialdehyde (MDA) serum concentration was measured by using the edible malondialdehyde micro assay kit (ABIN1884700).After 30 days intragastric administration of experimental bacterial solution,the eyeballs of the mice were bled and centrifuged at 10000 r/min for 3 min.According to the malondialdehyde micro assay kit,each reagent were added to the test tubes,then the test tubes were fixed with plastic wrap and water bathed at 95 ℃ for 40 minutes.Thereafter,the test tubes were cooled at 532 nm,1 cm light path,and adjusted to 0 with distilled water.Finally,the absorbance of each tube was measured.The MDA content was obtained by the following formula:

1.3.4 Total Liver Glutathione Concentration In order to analyze liver glutathione (GSH) concentration,0.1 g of the liver from each mouse was added with 0.9 mL of saline to make a tissue homogenate,which was centrifuged at 2500 r/min at 4 ℃ for 10 minutes and the supernatant was taken.Total concentration of glutathione was measured using Coomassie Leucocyanin assay kit and reduced glutathione assay kit following manufacturer’s instructions.
1.4 Statistical Data Analysis
Drug sensitivity (diameters),D-lactic acid concentration,bile salt hydrolase activity and organ relative weight were analyzed using SPSS Statistics 25.0 (SPSS,Inc.,Chicago,IL,USA).Differences in mean C,L12,and L20 treatments were tested for statistical significance by analysis of variance(ANOVA).One-way ANOVA was used to test for differences in mean C,L12,and L20 during the mouse growth.In case of significant differences among the means,DUNCAN significant differences atP=0.05 test was used.Figures were created in Origin Pro 8.Means and standard errors (S.E) from the statistical analysis were brought into Origin Pro 8,and diagrams were created using the line+symbol and column graph tools.All the results obtained in this study were expressed as mean±standard deviation,and the differences are considered significant ifP<0.05.
2 Results and Discussion
2.1 Antibiotic Susceptibility
LAB strains have important requirements in regards to resistance to different antimicrobials because any antibiotic resistant genes present in probiotic strains may transfer to other bacteria and increase the potential for virulence[11].TheLactobacillusstrains used in this study showed varied susceptibility to the tested antibiotics,with most of the antibiotics being sensitive.These results are similar to those reported by Maheshwari et al.[12].As shown in Table 1,both L12 and L20 strains were sensitive to ampicillin,penicillin,erythromycin,gentamicin,streptomycin,and rifampin.Comparing to L12,L20 strain showed greater sensitivity to cefotaxime (inhibitors of cell wall synthesis) and kanamycin,being resistant only tovancomycin,ciprofloxacin and norfloxacin.On the other hand,L12 strain was sensitive to ciprofloxacin and norfloxacin.
For some probiotic strains,resistance to certain antibiotics can be considered a positive characteristic[13]when this strain is co-administered with an antibiotic to which it is resistant,this characteristic will guarantee its survival and transfer of beneficial effects to the recipient in the Gastrointestinal Tract[14].At least one of the strains evaluated showed resistance to vancomycin or kanamycin,these results were similar to those observed by Guo et al[15]and Amraii et al[16].The main concern with safety in the use of probiotic strains to health is related to the possible transfer of antibiotic-resistant genes to pathogenic microorganisms present in the intestinal habitat,which can represent a serious risk for the treatment of infected patients[17].On the other hand,the resistance of the genusLactobacillusto vancomycin and some other antibiotics are intrinsic,these genes are chromosomally encoded,and will not be transferred to pathogenic microorganisms[18].Therefore,it is worth mentioning that,given these results,the strains used in this study do not present health risks and can be used in probiotic health products and foods.
2.2 BSH Activities of Lactobacilli Strains
Table 2 shows the bile hydrolase activity of the twoexperimental strains (L12 and L20).The test results showed that the enzymatic hydrolysis of the bile salts in the L12 and L20 strains were 227.32 U/L and 245.92 U/L respectively,with a bile hydrolase activity of L20 greater than that of L12.This means that both experimental strains have the ability to produce bile salt hydrolysis enzyme.According to Bustos et al.[19],the activity of bile salt hydrolase has the effect of reducing blood cholesterol.Therefore,L12 and L20 have an ability of lowering blood cholesterol
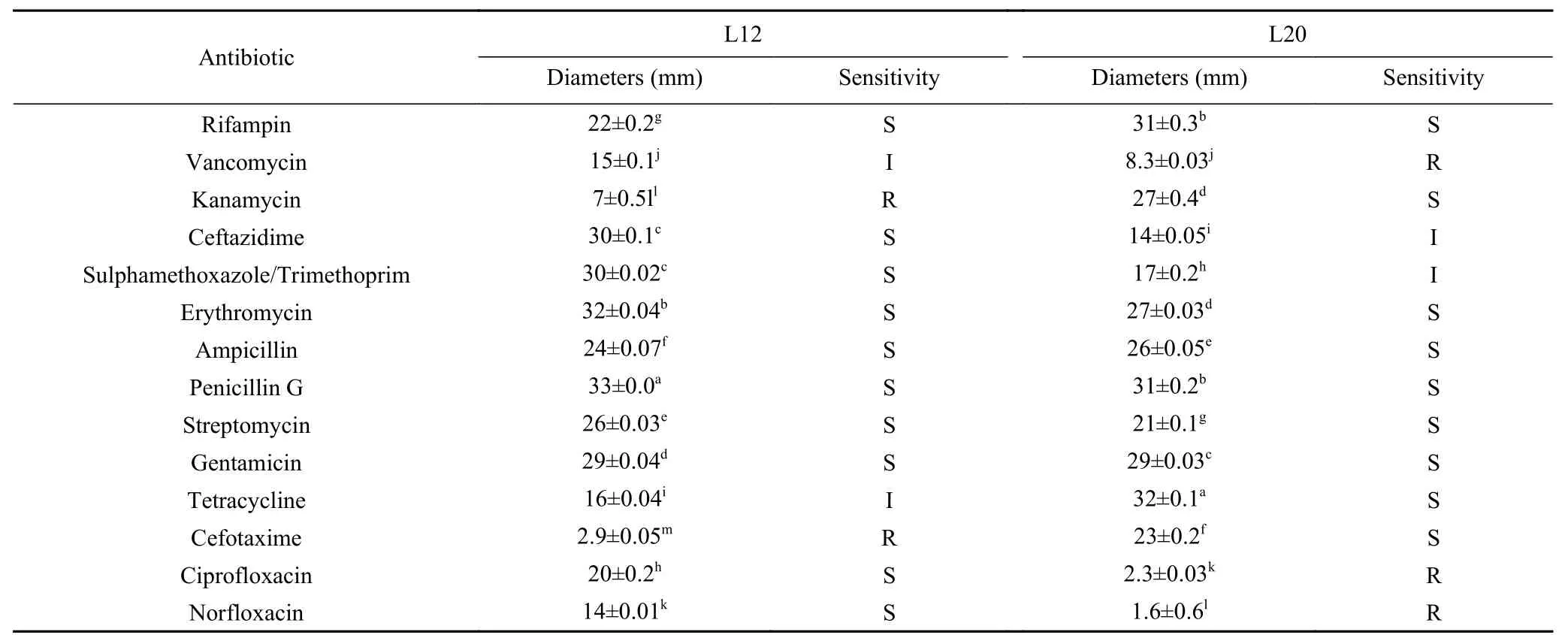
Table 1 Antibiotic susceptibility of Lactobacillus strains L12 and L20
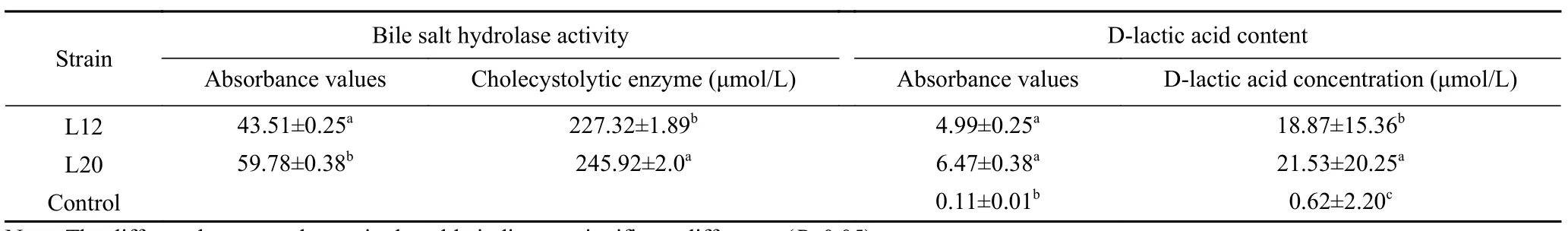
Table 2 Test results of D-lactic acid content and bile salt hydrolase activity of strains
2.3 D-lactic Acid Content
D-lactic acid is a harmful enantiomer of lactic acid[20].Most lactic acid bacteria secrete D-lactic acid during fermentation in the intestinal tract.D-lactic acid can cause lactic acidosis in people with short bowel syndrome when its production and absorption in the intestinal tract exceed the capacity of hepatic metabolism[13].Generally,in healthy adults’ serum Dlactate concentration ranges from 11 to 70 nmol/L[21].In this study,the D-lactic acid content of the two experimental strains L12 and L20 was within the allowed range,respectively 18.87 μmol/L and 21.53 μmol/L (Table 2),both of them were beyond the concentration standard of lactic acid in human serum,so these two strains are safe.
2.4 Hemolytic Activity
Hemolysis is usually related to the ingestion of hemolytic bacteria.Huseby et al.[22]shows that many common bacteria can produce one or more hemolysin and cause hemolysis.Therefore,lactic acid bacteria may also be potential hemolytic bacteria.In this study,the hemolytic activity of the two experimental strains L12 and L20 showed γ-type hemolysis,that is,non-hemolysis (no α-hemolysis and β-hemolysis),although α-hemolysis among lactobacilli from foods and dairy products is not uncommon[23].During the blood agar plate culture process,the two test strains L12 and L20 did not show grass-green hemolysis ring and well-defined and completely transparent hemolysis ring.The hemolysis of the twoLactobacillus plantarumshowed that L12 and L20 were safe.WhenStaphylococcus aureus(AS11861) in the control group was cultured on a blood plate,a 2~4 mm,well-defined,completely transparent hemolytic ring was formed around the colony,which wasβhemolysis (Fig.1).
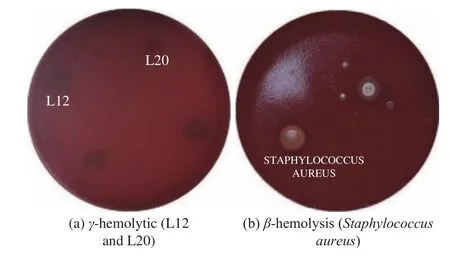
Fig.1 Hemolytic activity in treated and control groups
These results indicate that these two strains are not hemolytic bacteria and do not affect red blood cells,so they can be used in food because they are not harmful to health.Similar results have been presented by Halder et al.[24]who evaluated the native probiotic isolates ofLactobacillusfrom the curd and observed that alllactobacilli(L.AnimalisLMEM6,L.PlantarumLMEM7,L.acidophilusLMEM8 andL.RhamnosusLMEM9) were nonhemolytic.Likewise,it also found that the strains ofLactobacillusisolated from Sardinian dairy products did not exhibit any hemolytic activity (γ-hemolysis,α-hemolysis and/orβhemolysis) after 48 hours of incubation in blood agar plates supplemented with defibrinated sheep blood(5%).
2.5 Mortality and Clinical Signs
In the acute oral toxicity study,the 30 days of intragastric administration of experimental bacterial solution did not cause death or produce any clinical sign of toxicity.The mental state and activities of the mice were normal,no death or treatment-related adverse clinical reactions was observed in any of the animals throughout the experimental period (Table 3).Previous studies on LAB have shown that certainLactobacillusspecies such asL.Plantarum,L.acidophilusandL.reuteri,produce no oral toxicity in animals[25].
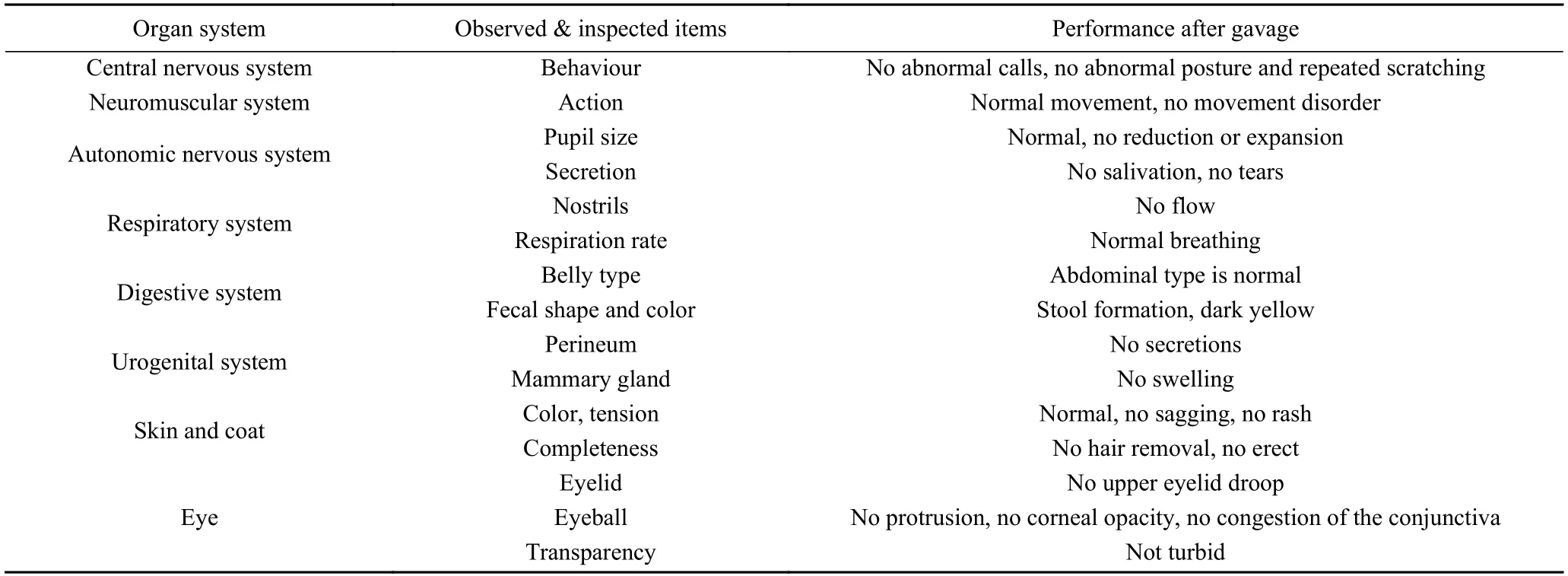
Table 3 Treatment-related sign of toxicity during the 30-days study period
2.6 Body Weight and Food Consumption
With regard to body weight gain,results show that,the body weight of mice increased by an average of 4~5 g in the first seven days (Fig.2a) and by 1.5~2 g per week during the next three weeks (Fig.2b).The average weight gain in 30 days of gavage was between 8.5 g and 11 g,with a more rapid increase in the first two weeks.When comparing the initial and final weights of the experimental mice after one month of intragastric administration of bacterial strains,there were no statistically significant differences in body weights between L12 and control groups (P>0.05).In contrast,there were statistical differences between the control and L20 groups (P<0.05),demonstrating that there is some interaction between the L20 strain and a decrease inbody weight.These results agree with the preliminary results of Dayong et al.[9],who discovered that L20 has the functionality of lowering lipids and cholesterol.No significant differences in food intake were observed between the treatment groups and the control group(data not shown).
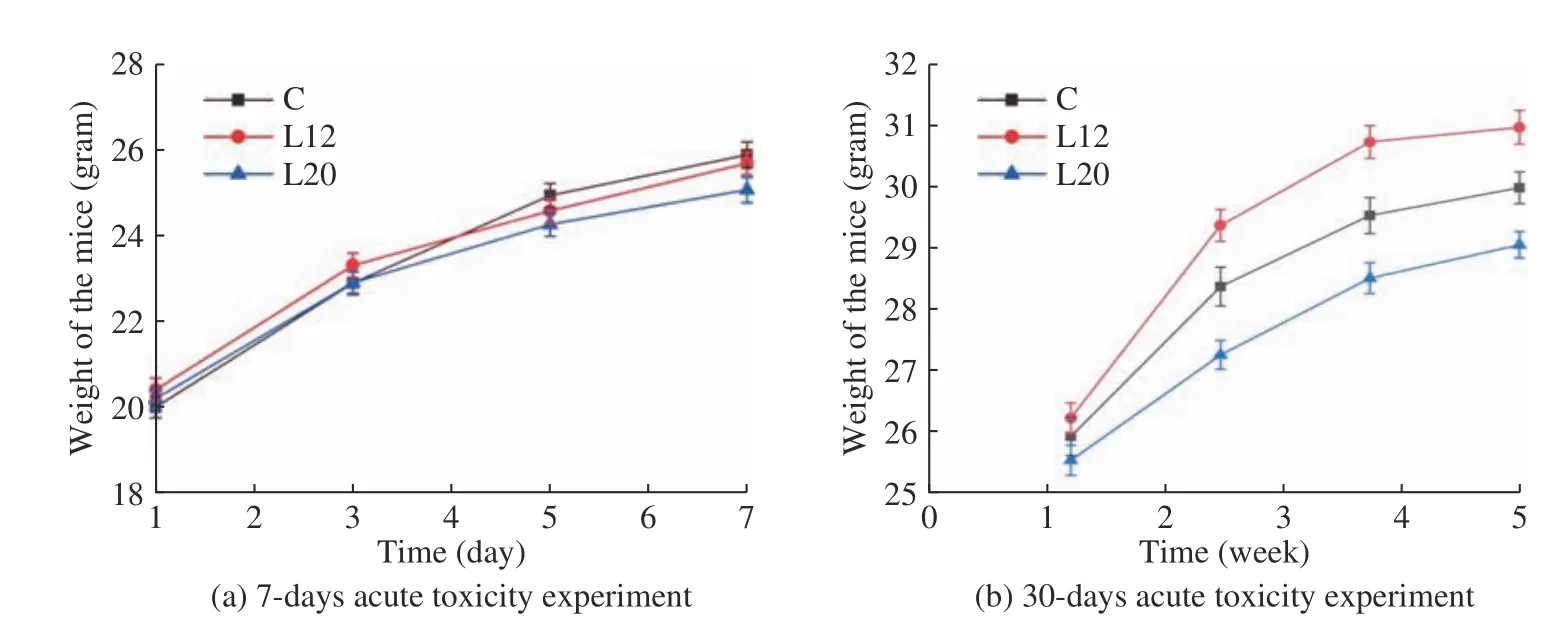
Fig.2 Weight of conventional mice
2.7 Relative Organ Weights and Histopathological Examination
Changes in organ weights are another indicator of chemical or toxicological effects in experimental animals[26].In this study,the relative organ weights and morphology of the three experimental groups did not exhibit any significant differences or microscopic changes.No statistically significant difference(P>0.05) was observed in relative body weight between the treated groups (L12 and L20) and control(Table 4).No abnormalities,tenderness,or swelling were observed in the internal organs (i.e.,heart,liver,spleen,and kidneys).No obvious infiltration of inflammatory cells in the liver tissue were observed in all the groups of mice (Fig.3).Compared with the control group,the liver lobules of mice in treated groups were intact with no abnormal pathological changes.These results demonstrate that the intragastric administration of bacterial strains did not have harmful alterations in the histology of the analyzed organs of the mice.Furthermore,the results suggests an unchanged renal function of the kidney,metabolic activity of the liver,and defensive function of spleen against microorganisms.Variable results have been observed in similar studies conducted by Shokryazdan et al.[27],and Ding et al.[28].However,these results did not affect the conclusions drawn from the safety assessment.
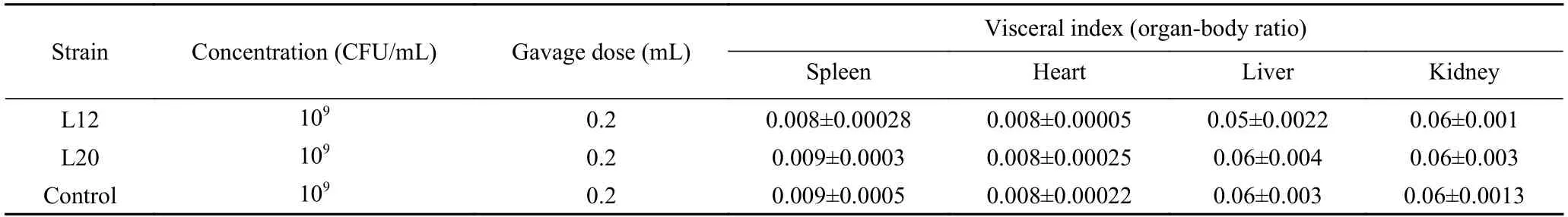
Table 4 Organ relative weights of control and probiotic-treated mice
2.8 Liver GSH Content and MDA Serum Concentration
GSH and MDA,as important indexes ofin vivoantioxidant evaluation,are very important in the safety evaluation of L12 and L20.The concentration of GSH in the liver was determined to analyze if there were changes in antioxidant defense due to probiotic treatment,and the serum MDA concentration was used to determine the extent of lipid peroxidation caused by the probiotic treatment.Results in Fig.4aand 4b show that the serum MDA concentration and liver GSH concentration of treated groups (i.e.,L12 and L20) were significantly different from the control group (P<0.05).Comparing the two trains,the L12 treatment increased MDA by 4.2% to that of L20(Fig.4a) while the L20 treatment contained the highest concentration of glutathione in the liver than L12(Fig.4b).These findings suggest that the Lactobacillus L20 strain has a stronger antioxidant capacity which may help lower cholesterol.A previous study have shown that increased serum MDA concentration,decreased antioxidant content,and changes in trace elements are highly linked to obesity[29].MDA is a product of lipid peroxidation,which can indirectly reflect the degree of cell damage.
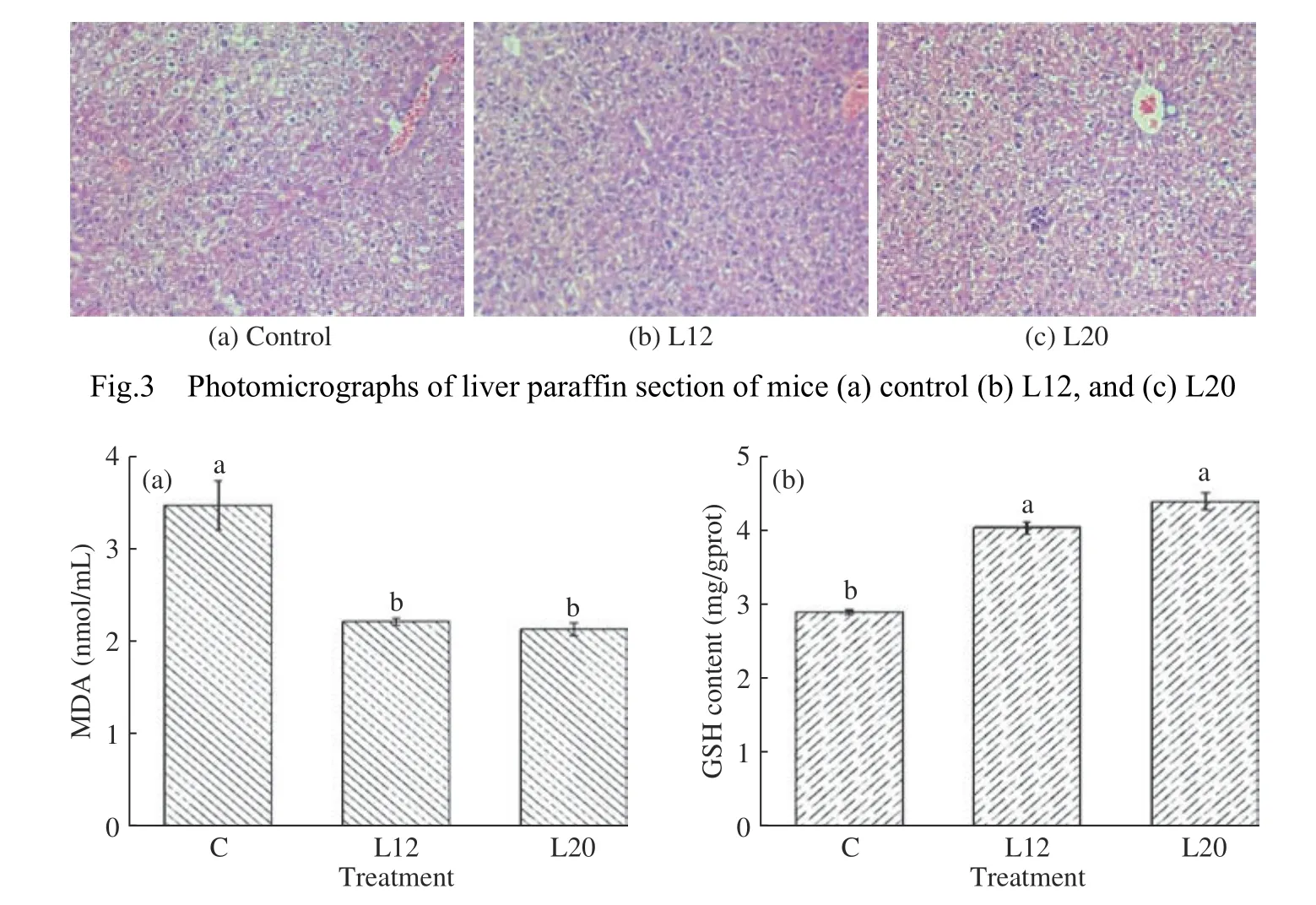
Fig.4 Serum MDA concentration (a) and total Liver GSH concentration (b) in control and probiotic-treated miceNote:Values are means with standard deviations shown by vertical bars (n=4).Bars with a different lowercase letter (s) in the same treatments indicate significant differences at P<0.05 among the treatments.
3 Conclusions
In the present study,twoLactobacillus(L.PlantarumL12 and L20) isolates from Northeast China spicy cabbage and hot sauce were found to have favorable characteristicsin vitroandin vivo.In general,the two strains obtained better probiotic properties,including susceptibility to antibiotic,low Dlactic acid content,ability to produce bile salt hydrolysis enzyme,and non-hemolysis.A 30-day intragastric administration of LAB strain did not influence the animals’ weekly weight gain in relation to the control group,neither induced abnormalities in the behavior,external appearance,or produce any clinical sign of toxicity.No change was observed in the organ weights(kidney,spleen,liver and heart) and histology of kidneys.It is suggested that these strains are nonpathogenic and safe for human consumption,hence can be used in food industries.

