Azithromycin versus doxycycline for the treatment of urogenital Chlamydia trachomatis infection—a retrospective study
,,,,,,,,
Dermatology Hospital,Southern Medical University,Guangzhou 510091,China
[Abstract]Objective:The objective of this retrospective study was to compare the efficacy of azithro-
[Keywords]Chlamydia trachomatis;azithromycin;doxycycline
One-gram single dose of azithromycin or 7-day doxycycline is recommended as the 1st line antimicrobial agents for the treatment of urogenitalChlamydia trachomatisinfection by the World Health Organization(WHO)Guidelines in 2016,[1]and guidelines from other countries[2-3].In China,the recommended 1st line treatments for urogenitalChlamydiainfection are the same as the one recommended by WHO,while the evaluation of the efficacy of these two treatments in real medical practice is rare.We conducted a retrospective trial to assess the cure rate of 1 g single dose of azithromycin and 7-day doxycycline for patients with urogenitalChlamydiainfection.
1 METHODS
1.1 Study Design and Participants
We retrospectively reviewed patients'charts in the STI clinic of Dermatology Hospital,Southern Medical University(DHSM).All the patients who were positive for chlamydial from May 2017 to May 2019 were identified.Only patients were initially treated with either 1 g single dose of azithromycin or 7-day doxycycline,and received at least one test-ofcure after one week of the treatment and within 60 days of the final treatment.Because ceftriaxone does not affect the treatment ofChlamydia,patients treated with ceftriaxone for co-infection of gonorrhea were also included in the study.However,patients treated with other medications such as clarithromycin,other antibiotics and quinolones were excluded from the study.Thus,only patients who met the following criteria were included in the analyses:a)havingChlamydiatesting positive at the first visit,b)being initially treated with 1 g single dose of azithromycin,or 7-day doxycycline,and c)having at least one test-of-cure after one week and within 60 days of completion of the therapy(Figure 1).
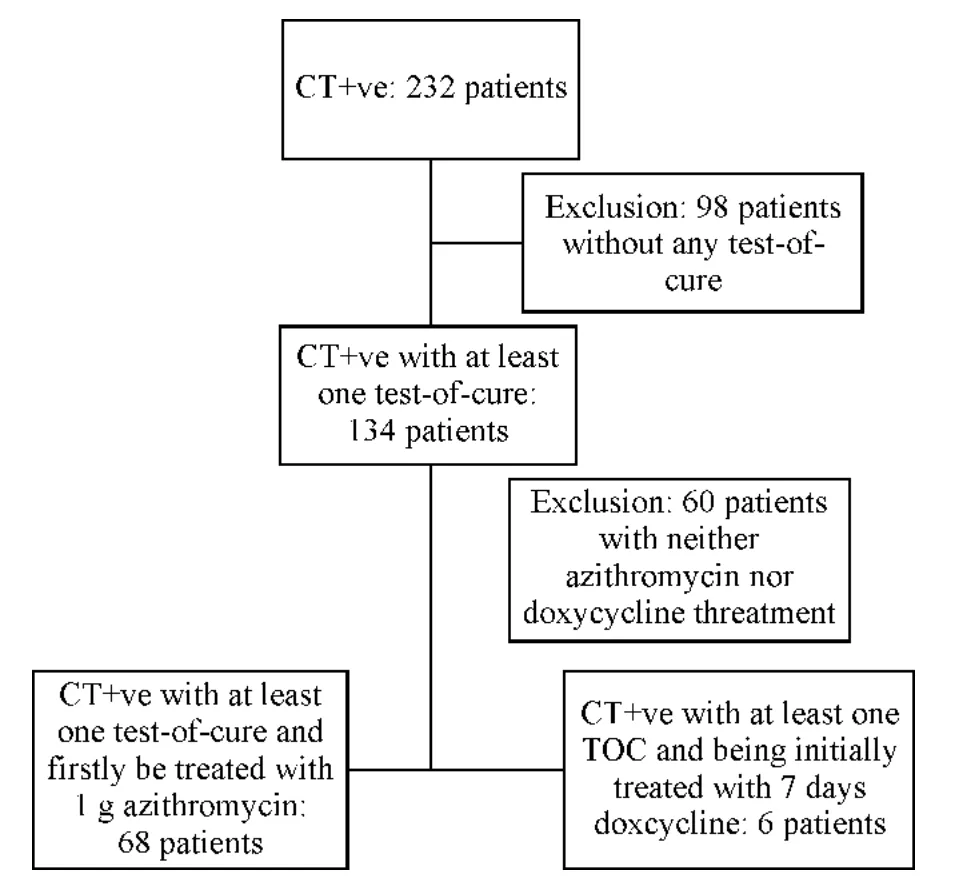
Figure 1 Flow chart of patients'enrollment.
1.2 Study Procedures
All data were obtained as part of routine medical care.Clinicians used standardized form to record information about socio-demographic information,sexual behavior,STI history,symptoms,concurrent STIs,and findings of physical examination.Some patients received their treatment at the time of the initial evaluation if the impressive diagnosis was urethritis or cervicitis or patients reported recent sexual contact to a partner diagnosed with this condition.Some asymptomatic patients received their treatment because their chlamydia testing was positive.All clinical data were audited and entered into the patient database on a weekly basis.
During the study period,azithromycin-P(P stand for a drug brand)was the drug of choice,azithromycin-X(X stand for a drug brand)was temporally used due to shortage of azithromycin-P supply.
Throughout the study period,the male urine samples and female cervical swab samples were collected to test chlamydia using the Cobas 4800 system(Roche Moleculer Systems,Inc.New Jersey,USA).
1.3 Outcomes and Populations Used for Analyses
Symptomatic male patients were defined as those having subjective symptoms such as pain on urination,urethral itching,and/or pus discharge from the external urethral meatus.Symptomatic female patients were defined as white blood cell(WBC)count≥30/per high power field(HPF)in microscopic examination of the cervical smear.Subjective symptoms and clinical examinations were not included for defining a symptomatic female case because it was not as sensitive as for male urethritis.Asymptomatic patients were defined as having a positive result of chlamydia,but not meeting the criteria for symptomatic case.These asymptomatic patients visited us for evaluating urogenital chlamydial infection as part of STD screening after risk sex,or because their sexual partners had been diagnosed with chlamydia infection.
Microbiological cure was used as the only index to evaluate the clinical outcome and was defined as having a negative result of the chlamydia testing between 7 to 60 days after the final treatment.
1.4 Statistical Analysis
Descriptive analyses were conducted on cure rates of different treatment regimens and different brand of drugs.The Chi-square test was used to compare categorical variables between symptomatic and asymptomatic groups.All data were analyzed using SAS 9.4(SAS int.Cary,NC,USA).
1.5 Ethical Approval
Ethical approval for this study was obtained from the Medical Ethical Committee of the Dermatology Hospital of Southern Medical University(GDDHLS-20171203).Informed consent was waived because the committee believed that the research presents no potential risk to identify the harm resulting from a breach of confidentiality.In the data analysis,the patient's names were deleted to further protect the patient's identity from disclosure.
2 Results
A total of 232 patients were diagnosed with urogenital chlamydia infection in this trial.Out of the 232 patients,134 patients completed at least one test-of-cure in the 7-60 days after completing the treatment.Of the 134 patients,68 patients initially received a 1 g single dose azithromycin and 19 patients received doxycycline treatment(6 initially doxycycline treated patients,13 azithromycin failure patients)(Figure 1).The mean age of the 74 patients was 33.5 years old for males(22-61 years old),and 26 years old for females(21-31 years old).
Of the 68 patients initially treated with azithromycin,the microbiological cure rate was 79.4%(54/68).The cure rate of symptomatic patients was lower than that of the asymptomatic ones(74.4% vs 88.8%,Table 1).During the study period,azithromycin-X was temporally used due to the short supply of azithromycin-P.Of the 6 male and 2 female patients who received azithromycin-X treatment,two males and one female failed to reach a microbiological cure.The cure rate of azithromycin-P and azithromycin-X were 85.0%(51/60)and 62.5%(5/8),respectively.Among the azithromycin treated group,the average days after completing treatment to test-of-cure in failure group was much shorter than that in the cure group[7.9 days(IQR:7-8.5 days)vs 20.5 days(IQR:7-27-day)](Table 2).
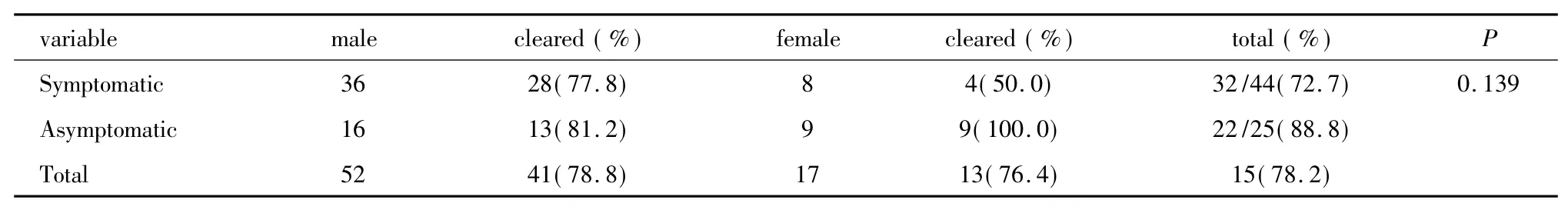
Table 1 Microbiological outcome in patients with urogenital chlamydia infection treated with azithromycin
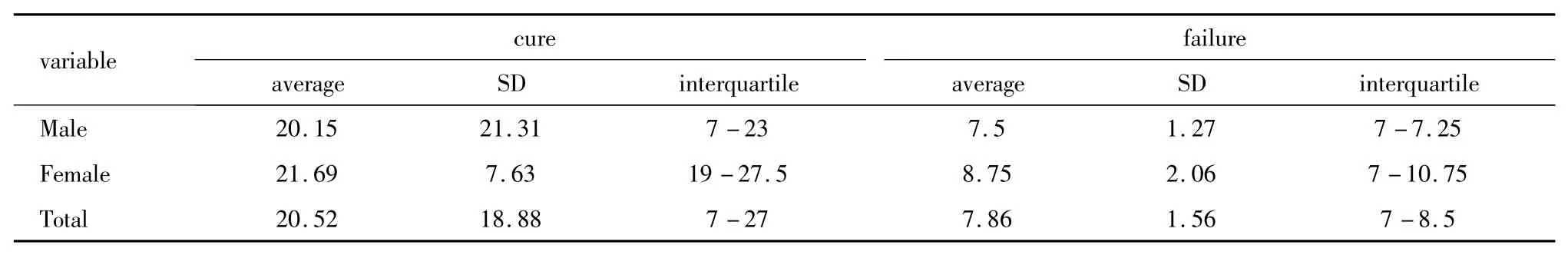
Table 2 Days to test-of-cure after completing treatment between cure and failure groups
In total,19 patients received doxycycline treatment(6 initially treated with doxycycline,13 azithromycin failure patients).The microbiological cure was achieved in all 19 patients,with a cure rate of 100% following 7-day treatment with doxycycline.Of the 19 doxycycline cured cases,8 patients received the test-of-cure within 1-2 weeks after completing the treatment,2 patients within 2-3 weeks,9 patients within 3 weeks to 2 months.
Ten symptomatic males patients and 1 symptomatic female patient co-infected with gonorrhea were treated with 1 g ceftriaxone plus 1 g azithromycin.Two male patients and 2 female patients co-infected with Ureaplasma urealyticum did not receive additional treatments with antibiotics.Another 2 male patients and 2 female patients were diagnosed with genital warts and treated with laser therapy.
3 Discussion
In this retrospective study,the microbiologic clearance rate was 79.4% in 68 cases of urogenital tract chlamydia infection following 1 g azithromycin therapy,while the microbiological clearance was 100% in 19 patients treated with doxycycline.Our results indicated that doxycycline had a higher microbial clearance rate than azithromycin in the treatment ofChlamydia trachomatisinfection of the urogenital tract.
Due to the limitations of our retrospective study,the efficacy of azithromycin might have been underestimated.First,at the time of data extraction,98(42%)of the initial 232 patients were not included due to loss of follow-up,and a significant number of these patients were treated with azithromycin.Patients failed to show up for the test-of-cure could be because either they were cured without symptom or they believed they needed no more test.But lost to follow-up might also be due to their unsatisfaction with the treatment outcome and seeking care in different clinics.Second,we used test results from one week to six months after completion of treatment to assess whether the patients were cured or not.Some failure cases defined in our study did not follow the recommended guideline to have tests three weeks after completion of treatment.A shorter time to perform test-of-cure after the treatment may increase the follow-up rates,and minimize the potential re-infection,but it may also produce false positive caused by DAN residue.Workowski and colleagues found that 3 out of 20 culture-negative cervical specimens had chlamydial DNA one week after the treatment.[4]In our study,the time to test-of-cure in failure group was shorter than in the cure group.Thus,the cure rate in our study might have been underestimated.However,test-of-cure of some subjects in both azithromycin and doxycycline groups was performed in a shorter time after the treatment.Thus,the effect of time to do test-of-cure on cure rates should be comparable between azithromycin and doxycycline group.Therefore,it is reasonable to conclude that the efficacy of the azithromycin is lower than the of doxycycline.
One-gram single dose azithromycin or 7-day doxycycline is recommended as the 1stline treatments for uncomplicated chlamydial infection in China,andChlamydiatreatment guidelines from WHO and other countries.[1-3]Though these guidelines recommend these two regimens equally,literatures show inconsistent efficacy of the two treatments.In 2011,a multicenter randomized controlled trial(RCT)showed thatChlamydiawas eradicated in only 41 out of 53(77%)male patients after treatment with 1 g single dose azithromycin,which was significantly lower than 7-day doxycycline(95%,P<0.01).[5]In 2015,Geisler and colleagues reported that treatment failure occurred in 5 participants in single dose azithromycin group,and none in 7-day doxycycline group although the cure rates did not differ significantly between the groups(97% vs 100%).[6]Similar results were observed by Kissinger and colleagues.[7]In their studies,failure of azithromycin treatment was between 6.2% and 12.8%.In our study,the azithromycin cure rate was lower than doxycycline group(79.4%~85.0% vs 100%).Our results indicate that a single dose azithromycin might not be as effective as a 7-day doxycycline for patients with urogenitalChlamydiainfection.
The limitations of our study include the aforementioned shorter time to test-of-cure in failure group,uncertainty of the treatment outcomes of patients who failed to return for a test-of-cure,small number of patients in doxycycline group and symptomatic group.In addition,no evaluation of drug side effects was performed.A larger sample size would be more desirable.
Our results indicate that a twice-daily 7-day regimen of doxycycline might be more effective for the treatment of urogenitalChlamydiainfection and we recommend it as the 1stline treatment for urogenitalChlamydiainfection in China.While azithromycin has a major advantage of being administered as a single dose,unlike doxycycline,it can be used in pregnant women,and more effective than multidose doxycycline for the treatment of another urogenital pathogen,mycoplasma genitalium infection.[8]Taken all these factors into consideration,single dose azithromycin should be recommended as the alternative treatment for uncomplicated urogenitalChlamydiainfection in China.

