Eco-Friendly pH Indicator Based on Natural Anthocyanins from Lycium ruthenicum
ZHANG Hongjie(张宏杰), LIAN Yasi(连雅思), YU Jiayan(余佳燕), LU Run(卢 润), ZHANG Jie(张 洁), QIU Yiping(邱夷平), 3, FENG Lili(冯丽丽)*
1 College of Textiles and Apparel, Quanzhou Normal University, Quanzhou 362000, China2 Shishi Institute for Ecological & Smart Textile, Quanzhou Normal University, Quanzhou 362000, China3 College of Textiles, Donghua University, Shanghai 201620, China
Abstract: An eco-friendly and visual pH indicator was developed based on cotton fabrics dyed with natural Lycium ruthenicum extract (LRE), whose color changed from red to purple, blue and green at pH of 2-10. Three methods for dyeing cotton fabrics with LRE were attempted, namely pre-mordanting, simultaneous mordanting, and post-mordanting methods with different dyeing temperatures, dyeing times and dyebath pH values. The cotton fabrics exhibited the highest K/S value when they were dyed under a dyebath of pH 6 at 20 ℃ for 90 min in the case of the simultaneous mordanting method. Meanwhile, the dyed cotton fabrics also showed reversible pH-dependent color changes. The developed flexible pH indicator based on renewable natural materials is suitable for multiple applications in environmental monitoring and smart textiles.
Key words: Lycium ruthenicum; anthocyanins; pH indicator; environmental monitoring; smart textiles
Introduction
The development of smart materials based on natural functional dyes has received considerable attention in recent years. One of the most promising natural dyes is anthocyanins[1-2], which are a class of natural flavonoids widely existed in flowers, fruits, and vegetables[3-4]. They have many special functions, such as anti-oxidant, anti-microbial, anti-inflammatory, and anti-tumor activities[5-6]. Anthocyanins are water-soluble and responsible for a wide variety of colors in plants. Due to structural transformation of anthocyanins under different pH conditions, their colors show a high sensitivity to pH changes, and the color changes are reversible in a certain range of pH values. The pH sensitivity and eco-friendliness of anthocyanins extracted from renewable source make them possible to be designed as promising natural visual indicators for real-time monitoring the surrounding pH values[7-8].
Most pH indicators based on anthocyanins are in the form of films, which are utilized as smart packages to monitor food freshness[9-10]. Anthocyanins extracted fromBauhiniablakeanaDunnhave been immobilized in chitosan, and the visual color changes of the resultant pH indicators from red to green have been observed at different pH values, demonstrating the possibility to use them as food freshness labels[11]. Similarly, a novel wide range pH-sensing colorimetric film has been developed by incorporating κ-carrageenan with anthocyanin extract[11]. The film showed colorimetric response in the pH range of 2-10 and the color changes were reversible. The developed pH sensing film showed great potential for monitoring the freshness of milk and aquatic products[12]. To further enhance pH-sensing capabilities of smart packages, the cellulose nanofiber films were labeled with anthocyanins from purple sweet potatoes, which displayed a color response over a wide pH range with excellent antimicrobial activity. Therefore, it could be utilized as an intelligent food packaging to monitor food quality and prolong its shelf life during the storage[13]. Additionally, in order to overcome the environmental problems arising from utilization of petroleum-based plastic packaging, sustainable and biodegradable polymers including starch, agar, and chitosan have been employed as the matrix for intelligent packaging[14-15].
The integration of chemical sensing materials into fibrous materials has recently gained much interest to develop wearable sensor devices, because they can detect acidic or alkaline values generated by the human body for disease diagnosis and health monitoring. However, little has been reported about the pH sensors based on natural anthocyanins in fibrous textiles. In this study, two natural materials are integrated to develop a flexible pH indicator.Lyciumruthenicumextract (LRE) containing natural anthocyanins is firstly prepared and subsequently immobilized onto cotton fabrics by means of dyeing with alum as a mordant. UV-Vis absorption spectroscopy is employed to investigate color changes of LRE at different pH values. The pH-sensing capability and reversibility of the dyed cotton fabrics are tested to evaluate their potentiality as pH indicators.
1 Experiments
1.1 Materials
Cotton fabrics (48/2 tex × 48/2 tex) with area density of 112 g/m2, andLyciumruthenicumwere obtained from local retailers. Alum, acetic acid, and sodium hydroxide were purchased from Xilong Science Co., Ltd., Shantou, China.
1.2 Methods
1.2.1 Extractionofanthocyanins
Lyciumruthenicum(100 g) was added to distilled water (1 L), and stirred for 90 min at room temperature. After that, the extracted solution was centrifuged at 7 000 r/min, and 4 ℃ for 20 min, and the supernatant obtained after centrifugation, namely LRE, was used for the subsequent experiments.
1.2.2 Mordantinganddyeingprocedure
The mordanting and dyeing of cotton fabrics were carried out in an infrared dyeing machine (Mebon company, China). Three different mordanting methods, namely pre-mordanting, simultaneous mordanting, and post-mordanting methods, were used to improve dyeability of cotton fabrics with LRE. In the simultaneous mordanting method, mordanting and dyeing were carried out in the same bath using a material-to-liquor ratio of 1∶50 and alum (10 g/L) was used as a mordant. Pre-mordanting and post-mordanting methods were two-bath processes with a material-to-liquor ratio of 1∶50 for both mordanting and dyeing processes. The sample was first mordanted and then dyed in the pre-mordanting method, while it was just opposite in the post-mordanting method. In the case of pre-mordanting and post-mordanting methods, the mordanting was performed at 20 ℃ for 30 min, and the dyeing process was carried out at 20 ℃ for 30 min. The dyed cotton fabrics were washed with running water and then dried at room temperature.
1.2.3 Characterization
The spectral features of LRE at different pH values were measured using a Lambda 365 UV-Vis spectrophotometer (PerkinElmer, USA) in the visible light range of 400-700 nm. The color strength (K/S) and CIELAB of the dyed cotton fabrics were tested using a Datacolor 800 spectrophotometer (Datacolor company, USA) under illuminant D65. TheK/Svalue was the average value from five consecutive measurements at different positions. In CIELAB systems,a*represents the position on the red-green axis (positive value indicates red, and negative value indicates green),b*represents the position on the yellow-blue axis (positive value indicates yellow, and negative value indicates blue),C*represents saturation,L*represents luminosity, andh*represents metric hue angle.
To test pH-sensitive property, the dyed cotton fabrics were titrated with aqueous solutions in the pH range of 2-10, which was prepared by adding appropriate amounts of acetic acid (0.1 mol/L) or sodium hydroxide solutions (0.1 mol/L) in pure water. The color variations of the dyed cottons were monitored for 3 min.
2 Results and Discussion
2.1 Characterization of LRE
Figure 1(a) shows color variation of LRE at different pH values (2-10). It was bright red under highly acidic environments. The color of LRE changed from violet to blue as pH values varied from 4 to 8, and green color was observed when pH value increased to 9. The spectral features of LRE at different pH values were measured using UV-Vis absorption spectroscopy in the visible light range of 400-700 nm. As shown in Fig. 1(b), it displayed a strong single absorption band at around 528 nm in acidic solutions at pH of 2-3. Absorption band became broader when the pH values further increased from 4 to 7, and a shoulder band around 604 nm was observed at pH of 8. When pH value changed from 9 to 10, a narrowed absorption band appeared at 607 nm along with an increasing intensity.
The color variation can be attributed to molecular structure transformations of anthocyanins with pH changes[16-17]. As shown in Fig.1(c), red flavylium cation predominates in the structures of anthocyanins solution at pH of 2-3[18-19]. As pH is between 4 and 5, formation of colorless carbinol pseudobase occurs due to a rapid proton loss of flavylium cation. When pH changes from 6 to 7, the carbinol pseudobase is hydrated to produce quinoidal species[20]. Co-extist of equilibrium forms, including flavylium cation, carbinol pseudobase and quinoidal base, leads to an integrated violet color. The equilibrium shifts towards a deep blue ionized quinoidal base at pH of 7-8. Further increasing pH value can open the central pyran ring of anthocyanins, thus forming yellow chalcone[21]. Therefore, the color of LRE solution changes to green when the pH value increases to 9.
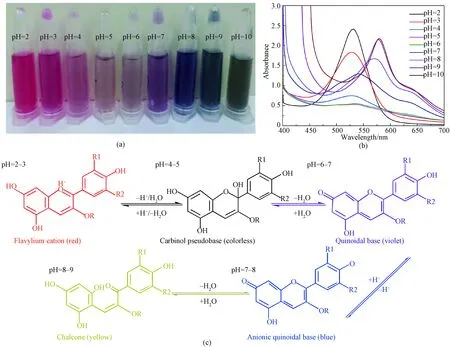
Fig. 1 Characterization of LRE at different pH values: (a) optical image; (b) UV-Vis absorption spectra; (c) molecular structure transformation (R, R1, and R2 represent substituent groups)
2.2 Dyeing cotton fabric with LRE
Dyeing cotton fabrics with nature dyes is often difficult since cotton possesses only hydroxyl groups in its molecular structure, which results in weak bonding between the fiber and the nature dye. Metallic mordants are mostly favored to improve the affinity between cotton fiber and natural dyes[22]. Therefore, alum was used as a mordant, while three mordant methods including pre-mordanting, simultaneous mordanting, and post-mordanting methods were employed to improve dyeability of natural LRE to cotton fabrics.
As shown in Table 1, the dyed cotton fabrics without mordanting exhibited rather low color strength (K/S=0.12). The application of alum exerted obvious effect on dyeing performance of cotton fabrics with LRE. Among three mordant methods, cotton fabrics obtained the highest color strength in the case of the simultaneous mordanting method. It was observed thatK/Svalue of the dyed cotton fabrics increased to 0.48, four times as high as its counterpart without mordanting.

Table 1 Color characteristics of cotton fabrics dyed with LRE using different mordanting methods
2.3 Influence of dyeing temperature and dyeing time
The influence of dyeing temperature on the dyeability of cotton fabrics with LRE was studied in the range from 20 ℃ to 80 ℃ and the results are illustrated in Fig. 2. The data indicated thatK/Svalue of the dyed fabrics decreased with the increase of dyeing temperature, and a dyeing temperature of 20 ℃ was found to be the optimal condition for dyeing cotton fabrics with LRE, which might be attributed to the inferior thermostability of LRE.
The effect of dyeing time on the dyeability of cotton fabrics with LRE was performed for different durations of time (30-120 min). As shown in Fig. 2, an increase ofK/Svalues from 0.45 to 0.65 was observed as dyeing time increased from 30 min to 90 min. However, further prolonging dyeing time to 120 min did not increaseK/Svalue of the dyed cotton fabrics, indicating that the dyeing equilibrium was probably reached within 90 min.

Fig. 2 Effect of dyeing temperature and dyeing time on K/S values of dyed cotton fabrics
2.4 Influence of dyebath pH
The effect of dyebath pH on the dyeability of cotton fabrics with LRE was observed at a constant dyeing temperature of 20 ℃ and a dyeing time of 90 min.K/Svalues from Table 2 indicated that the color strength increased as dyebath pH varied from 3 to 6, while a decreasing tendency was observed with higher dyebath pH, which might be due to the instability of LRE under highly alkaline environment.

Table 2 Color characteristics of cotton fabrics dyed at different pH values
2.5 Cotton fabrics dyed with LRE for pH sensing
Since the color of anthocyanins is pH sensitive, cotton fabrics dyed with LRE containing anthocyanins may display different colors when they are exposed to aqueous solutions at different pH values. As shown in Fig. 3, the dyed cotton fabrics showed red color when it was titrated with aqueous solutions at pH of 2. The color of the dyed cotton fabrics changed from red to purple-red, purple, violet, blue, and green when the pH values varied from 2 to 10, suggesting that cotton fabrics dyed with LRE could be used as a flexible pH indicator for visualization of pH measurement.
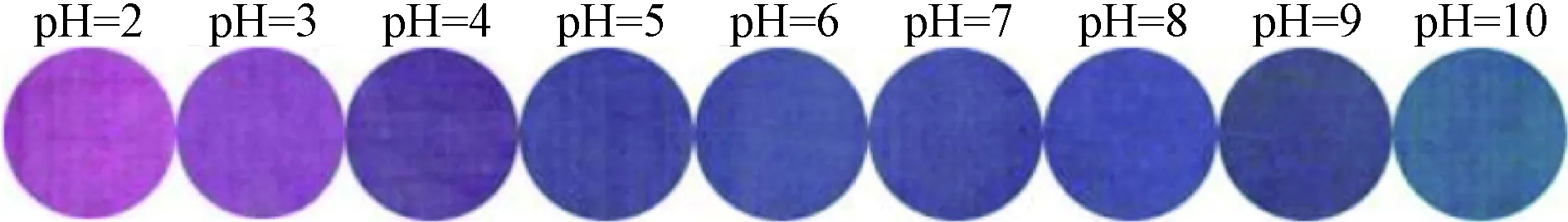
Fig. 3 Color changes of dyed cotton fabrics exposed to aqueous solutions at different pH values
The reversibility of the dyed cotton fabrics was evaluated through exposing them to acidic solutions alternated with alkaline solutions. The red-green-red color transition process was repeatable within diverse exposure cycles (shown in Fig. 4). However, the existence of color differences at the fifth cycle indicates that more efforts are needed to realize the reuse of a pH indicator based on natural LRE and cotton fabrics.

Fig. 4 Optical images of dyed cotton fabrics after repeated exposure to acidic and alkaline solutions
3 Conclusions
In this study, visual pH indicators were prepared through the combination of LRE and cotton fabrics. Optical images and UV-Vis absorption spectroscopy revealed that colors of LRE were sensitive to acidity or alkalinity, making the production of visual pH indicators possible. LRE was then used for dyeing cotton fabrics, and alum was employed as a mordant. The cotton fabrics exhibited the highestK/Svalue when they were dyed under a dyebath pH of 6 at 20 ℃ for 90 min in the case of the simultaneous mordanting method. Meanwhile, the dyed cotton fabrics showed pH-dependent color changes. The resultant pH indicators based on natural pH-sensitive dyes can be utilized for different sensing applications especially in wearable devices and health monitoring.
 Journal of Donghua University(English Edition)2022年2期
Journal of Donghua University(English Edition)2022年2期
- Journal of Donghua University(English Edition)的其它文章
- Electrospinning of Bead-on-String Sodium Alginate Nanofibrous Membrane
- Polysaccharides Based Random and Unidirectional Aerogels for Thermal and Mechanical Stability
- Formulating Novel Halogen-Free Synergistic Flame Retardant Epoxy Resins for Vacuum Assisted Resin Infusion Composites
- Electrochemical Reduction Determination of N-Nitrosodiphenylamine in Food Based on Graphene Electrode Material
- Hydrothermal Synthesis of Ordered ZnO Nanorod Arrays by Nanosphere Lithography Method
- Temperature-Dependent Growth of Ordered ZnO Nanorod Arrays
