Formulating Novel Halogen-Free Synergistic Flame Retardant Epoxy Resins for Vacuum Assisted Resin Infusion Composites
WANG Ming(王 明), WEI Yi(魏 毅)*, QIU Yiping(邱夷平)
1 College of Textiles, Donghua University, Shanghai 201620, China2 Center for Civil Aviation Composites, Donghua University, Shanghai 201620, China
Abstract: The most common process to manufacture advanced composites is the costly autoclave. One of the out-of-autoclave alternatives is the low-cost vacuum assisted resin infusion (VARI) which produces quality parts with less pollution. Epoxy resin is a widely used composite matrix resin, but its high flammability limits its use as interior composite parts for vehicles. The usual flame retardant for epoxy involves halogen, which is effective but has high smoke toxicity. As a result, halogen-free flame retardant epoxy resin systems become dominant. In this paper, phosphorus flame retardant was combined with benzoxazine (BOZ) to produce synergistic effect and achieve satisfactory flame retardance, as well as mechanical improvement for the epoxy resin. Differential scanning calorimetry (DSC), dynamic mechanical analysis (DMA), thermal gravitational analysis (TGA), the cone calorimeter (CC), and limiting oxygen index (LOI) were used to characterize the resins. The results showed significant improvement on the flame retardance of the synergistically modified resins. Specifically, the carbon residue increased by 113.6%, and the char thickness increased by 6 to 7 times, compared to those of the flammable benchmark resin. The LOI reached 33 and passed the UL94 V-0 vertical burn rating. The modified resins also exhibited adequate stability and viscosity suitable for VARI processes.
Key words: synergistic flame retardant; epoxy; benzoxazine(BOZ); vacuum assisted resin infusion(VARI); composite
Introduction
Epoxy resin is a class of thermosetting polymers extensively used in various industrial fields such as coatings, molding compounds, adhesives, and composite materials[1-3]. However, it is a very combustible material with a high heat release rate and fast flame propagation during combustion. Dense smoke and toxic gas are produced during the burning process, which limits its use as interior parts for vehicles[4-5]. Traditionally, the flame retardant modification mainly use halogen-containing flame retardant of epoxy resins, but its adoption is halted because of the generation of toxic acids and dioxins during burning[6-7]. As a result, halogen-free flame retardant epoxy resin has gradually become mainstream.
Flame retardants for epoxy resins can be divided into reactive flame retardants and additive flame retardants. Reactive flame retardants incorporate flame retarding elements into the resins or curatives, which are usually pricy and involve complicated synthesis processes. Additive flame retardants are formulated into the resin and are added during mixing which is relatively simple and cheap[8]. In this research, additive flame retardants are adopted. Considering that the vacuum assisted resin infusion (VARI) process requires low resin viscosity, usually less than 800 mPa·s[9], and the conventional high-efficiency flame retardants are usually in powder form with a particle size at micron levels, which will be filtered by the filaments during infusion, this research selects liquid flame retardants.
The most common additive liquid flame retardants in the market are phosphate esters. Although they are effective flame retardants, significant plasticizing effect is introduced at the same time, which lowers the service temperature of the cured resin and reduces the composite mechanical properties. An alternative is to limit the level of the liquid flame retardants in formula, and to make the cured epoxy resin more flame retardant at the same time. Co-cure epoxy resin with benzoxazine (BOZ) emerges is an interesting option. As a special type of phenolic resin inherently flame retardant, BOZ is a six-membered heterocyclic compound containing oxygen atoms and nitrogen atoms. It can crosslink with epoxy and maintain excellent mechanical and flame retardant properties with low curing shrinkage and no condensed water. The nitrogen contained in BOZ resin can also form synergistic flame retardant effect with the additive phosphorus flame retardants[10-13]. BOZ resin has low viscosity which is favorable to maintaining the low infusion viscosity for VARI.
In this paper, an epoxy resin capable of room temperature VARI with good flame retardant properties is prepared. And the effects of dimethyl methylphosphonate (DMMP) (25% phosphorus content), methylene di-aniline (MDA)-BOZ, bisphenol F-BOZ and bisphenol A-BOZ as flame retardant modification for epoxy resins are evaluated based on their flame retardant and mechanical behaviors.
1 Materials and Experiments
1.1 Materials
The molecular structure of BOZ is shown in Fig. 1. The materials used are listed in Table 1.

Fig. 1 BOZ molecular structure: (a) bisphenol F-BOZ; (b) bisphenol A-BOZ; (c) MDA-BOZ

Table 1 Experimental materials
1.2 Preparation of flame retardant resin
NPEF-170 and DMMP were mixed under agitation at room temperature until the mixture became transparent. HTDA and DMP-30 were then added to the mixture to form a uniform solution. Similarly, the BLANK sample were prepared but without the addition of DMMP. For the BOZ flame retardant resins, NPEF-170 and BOZ were first blended under agitation at 90 ℃ for 30 min to form a transparent solution. Then the solution was cooled to room temperature, and DMMP, HTDA, and DMP-30 were added and mixed until the solution became transparent. The formulae of all samples are listed in Table 2.

Table 2 Resin formulae in weight
1.3 Characterization
Resin viscosity was measured by the Brookfield DV2T viscometer and #51 spindle at 10 r/min and 21 ℃. Differential scanning calorimetry (DSC) was performed on a NETZECH 214 of TA Co., Ltd.,USA, at a heating rate of 10 ℃/min under a nitrogen atmosphere to analyze the resin curing behavior. The rheological properties of resins were investigated on the TA Instrument DHR rheometer, USA. Dynamic mechanical analysis (DMA) was performed on a TA Instrument DMA Q800 Co., Ltd., USA, equipped at a heating rate of 5 ℃/min with a cooling system. Thermal gravimetric analysis (TGA) was carried out by a TGA 4000 thermogravimetric analyzer from Perkin Elmer Instruments at a heating rate of 10 ℃/min in the temperature range from 35 ℃ to 800 ℃ under nitrogen flow of 50 mL/min.
The limiting oxygen index (LOI) value was measured according to standard GB/T2406. The dimension of all samples was 110 mm × 6.5 mm × 3 mm. The UL94 vertical burning level was tested according to standard GB2408. The dimension of samples was 125 mm × 13 mm × 3 mm. The 6810-A001 cone calorimeter (USA) was used to measure the flammability samples according to standard ISO 5660 by Suzhou Standard Combustion Testing Technology Service Co., Ltd.,China. The samples with the dimension of 100 mm×100 mm×3 mm were exposed to a radiant cone under a heat flux of 35 kW/m2. The tensile properties of flame retardant epoxy resins were tested on a Wance ETM104B-EX electronic universal testing machine(USA), following standard ASTM D638.
2 Results and Discussion
2.1 Resin viscosity
In general, the lower the viscosity of the resin used, the easier it wets the fiber filaments. Therefore, the material screening based on the viscosity of the resin system is first conducted.
Comparing the BLANK sample with the 25% DMMP sample in Fig. 2, it could be seen that DMMP significantly reduced the overall resin viscosity, due to its very low viscosity. It helped the resin that formulated with BOZ to maintain reasonable viscosity required for VARI.
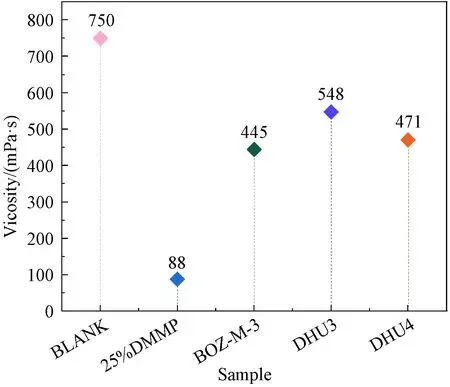
Fig. 2 Viscosity of epoxy resin formulations
2.2 Resin stability during infusion
In addition to low infusion viscosity, the VARI process also requires the resin to be stable during the infusion process, which can last from minutes for small parts to hours for large parts. Usually, the processing stability at given temperature can be characterized by the time for the resin viscosity to be doubled. Selecting suitable infusion temperature is important because it is advantageous to infuse at the lowest possible viscosity without risking resin gelling or exotherm. Above viscosity measurement indicated that the resins in this study had a viscosity low enough to be infused at room temperature, but for lower viscosity and a wider temperature window, 40 ℃ infusion temperature was selected for the resin stability. The viscosity increasing with time at 40 ℃ was recorded and shown in Fig. 3.

Fig. 3 Isothermal rheological curve of resins
As can be seen from Fig. 3, the viscosity of BLANK increased rapidly with time, reaching 800 mPa·s in 40 min which was more than 4 times higher than its initial viscosity. The modified resins exhibited much better stability, only doubling their viscosity in 80 min, and therefore were more suitable for the VARI process.
2.3 Analysis of resin curing behavior
As shown in Fig. 4(a) and Table 3, the epoxy group started to react with the amino group in HTDA at about 82 ℃, producing a large enthalpy of 473 J/g. Contrarily, the 25% DMMP resin had a significantly lower heat of reactions due to the dilution effect from the unreactive DMMP flame retardant. Different from the previous two resin systems, it was found that the epoxy resin systems containing BOZ exhibited two exothermic peaks, which represented two curing stages or chemistries. To determine the nature of these exotherms, DSC of NP-170 epoxy resin with MDA-BOZ at a weight ratio of 7.5∶2.5(7.5/2.5 BOZ-EP), and the self-polymerization of 100% MDA-BOZ were performed, as shown in Fig. 4(b). The red curve in Fig. 4(b) shows that the initial temperature of the self-polymerization of 100% BOZ is 215 ℃, corresponding to the second exotherm in Fig. 4(a). Therefore, the first exothermic peak in Fig. 4(a) should primarily be the cure of epoxy with amine. Based on the above DSC data, the curing condition for the BLANK resin and 25% DMMP resins was selected as 80 ℃ for 4 h, while for the resin systems containing BOZ, additional 1 h post cure at 190 ℃ was added to achieve complete cure.

Fig. 4 DSC curves of resins: (a) epoxy resin; (b) BOZ self-polymerization and cure with epoxy resin
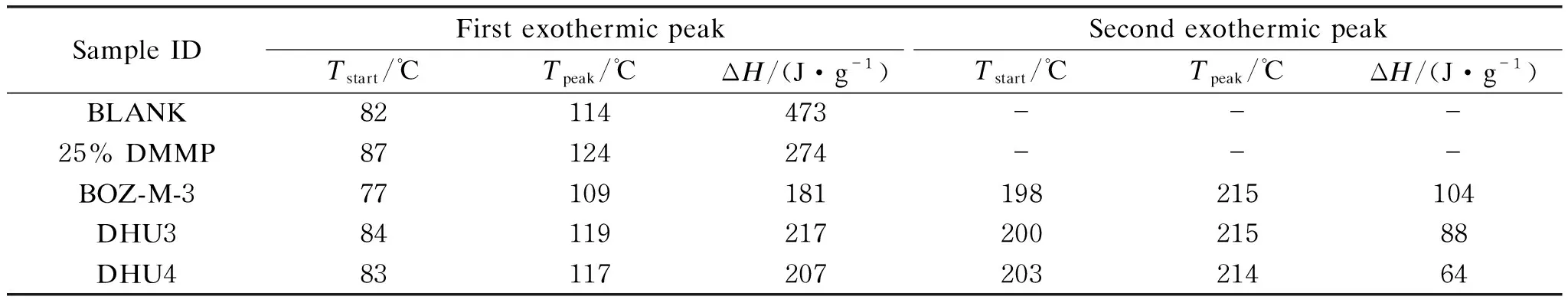
Table 3 DSC analysis of modified epoxy resin
2.4 DMA
It can be seen from Fig. 5 and Table 4 that the glass transition temperatureTgof the flame retardant resin is lower than that of the unmodified resin, especially that of the 25% DMMP resin which was reduced by 76 ℃, indicating the significant plasticizing effect by DMMP. As a result, the 25% DMMP cured resin was not suitable for structural composites. TheTgof BOZ-M-3, DHU3, and DHU4 resin was 92- 95 ℃, which were lower than the BLANK resin but were still significantly higher than that of the cured resin only containing DMMP, because the increase inTgcaused by the presence of rigid rings in BOZ offset the reduction ofTgcaused by the plasticization of DMMP. Resins having such level ofTgshould have adequate service temperature for interior composites.

Fig. 5 Resin modulus as function of temperature: (a) storage modulus; (b) loss modulus

Table 4 Tg of epoxy resins
2.5 TGA
TGA is mainly used to study the thermal stability of materials. As can be seen from Fig. 6, the BLANK sample has one thermal decomposition stage, while the modified epoxy resins containing DMMP and BOZ show two stages. Since the boiling point of DMMP is 181 ℃, the TGA curve of the 25% DMMP resin reflected the gradual loss of DMMP above its boiling point, followed by the decomposition of epoxy. For the curves of resins containing both DMMP and BOZ, the DMMP seemed to be locked by the resins so as not to exhibit its loss. This might be a strong evidence that compositional synergies exited between the phosphate ester and BOZ, in addition to the synergies of flame retardance shown later in this research. Therefore, the first stage was considered the decomposition of epoxy resin, and the second was considered to be the decomposition of BOZ.
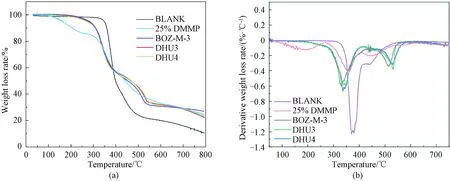
Fig. 6 Thermal stability of cured resins: (a) TGA; (b) DTGA

Fig. 7 THR curves of cured epoxy resins
The derivative TGA(DTGA) curves show that the temperature for the largest weight loss rates of BLANK resin was high (about 375 ℃), due to the excellent thermal stability of epoxy-BOZ resin. Contrarily, the first peak for the resin containing DMMP was as low as 0.4%/℃, which was due to the loss of DDMP above its boiling point. The maximum mass loss rates of BOZ-M-3, DHU3, and DHU4 shifted to lower decomposition temperatures, evidently due to the presence of DMMP.
The temperature at which 5% weight loss occurs, designated asT5%, is listed in Table 5. It can be seen thatT5%of the modified resins containing DMMP and BOZ is lower than that of the BLANK resin, caused by the plasticization of DMMP. The BOZ degradation at the temperature higher than 300 ℃ was due to the fracture of Mannich bridge, releasing aniline and different kinds of phenols, and finally pyrolysis and carbonization[14].

Table 5 Decomposition temperature T5% and carbon residue rate of cured resin
The carbon residue shown in Table 5 demonstrated effective flame retardance imparted by DMMP and benzene, particularly their synergy to char and form a physical barrier to the diffusion of oxygen and heat[12, 15].
2.6 Flame retardant properties of cured resin
2.6.1 VerticalcombustionandLOIanalysis
Vertical combustion ratings and LOI of cured resin are listed in Table 6. Table 6 showed that the flame retardant epoxy resin passed UL94 V-0. It was more significant that LOI of the resins containing both DMMP and BOZ reached 32, demonstrating again the effectiveness of flame retardant synergy. Common mechanism is that DMMP decomposes to produce PO· free radicals, promoting char and producing water, while BOZ releases inflammable gases, diluting flammables in gaseous phase, and resulting in synergistic flame retardant effect between phosphorus and nitrogen[15-16].

Table 6 Vertical combustion ratings and LOI of cured resin
2.6.2 Conicalcalorimetric(CC)analysis
CC is a test to study the combustion behavior of materials in a condensed phase based on the principle of oxygen consumption. Such combustion environment is most close to the actual burning environment. Figure 7 indicates the high total heat release(THR) of the BLANK resin of 120 MJ/m2, while those of the flame retardant modified epoxy resins, BOZ-M-3, DHU3, and DHU4, were 48, 46, and 39 MJ/m2, respectively. The largest reduction was found to be 207% with BOZ-M-3 which contained the highest amount of nitrogen.
Figure 8 shows that the heat release rate(HRR) which is a key parameter to characterize the flame retardant effectiveness of resins. The smaller the HHR and the lower the THR, the more effective the flame retardant. It was confirmed that the flame retardant modified resins exhibited a much lower HRR, about 500 kW/m2, than the BLANK sample which was 1 400 kW/m2, equivalent to an average HRR reduction of 180%.

Fig. 8 HRR curves of cured epoxy resins
Figure 9 shows the carbon residue at end of the CC test. Normally, the thicker and denser the char, the higher the flame retardancy the resin. It can be seen from Fig. 9 that the char thickness of BLANK resin is less than 10 mm, while the char thickness of the flame retardant resins is much larger, namely 55 mm for BOZ-M-3, 68 mm for DHU3, and 70 mm for DHU4. The larger amount of char evidently came from the phosphoric carbonization of DMMP. However, the much thicker char layers of the BOZ containing resin exhibited again the synergistic flame retardance.

Fig. 9 Carbon residue from CC test
2.7 Tensile properties of cured resin
The preparation of casted test coupons includes pouring resin into the mold, vacuum degasification, curing, coupon polishing, and dimensional measurement, and the results are shown in Figs. 10 and 11.
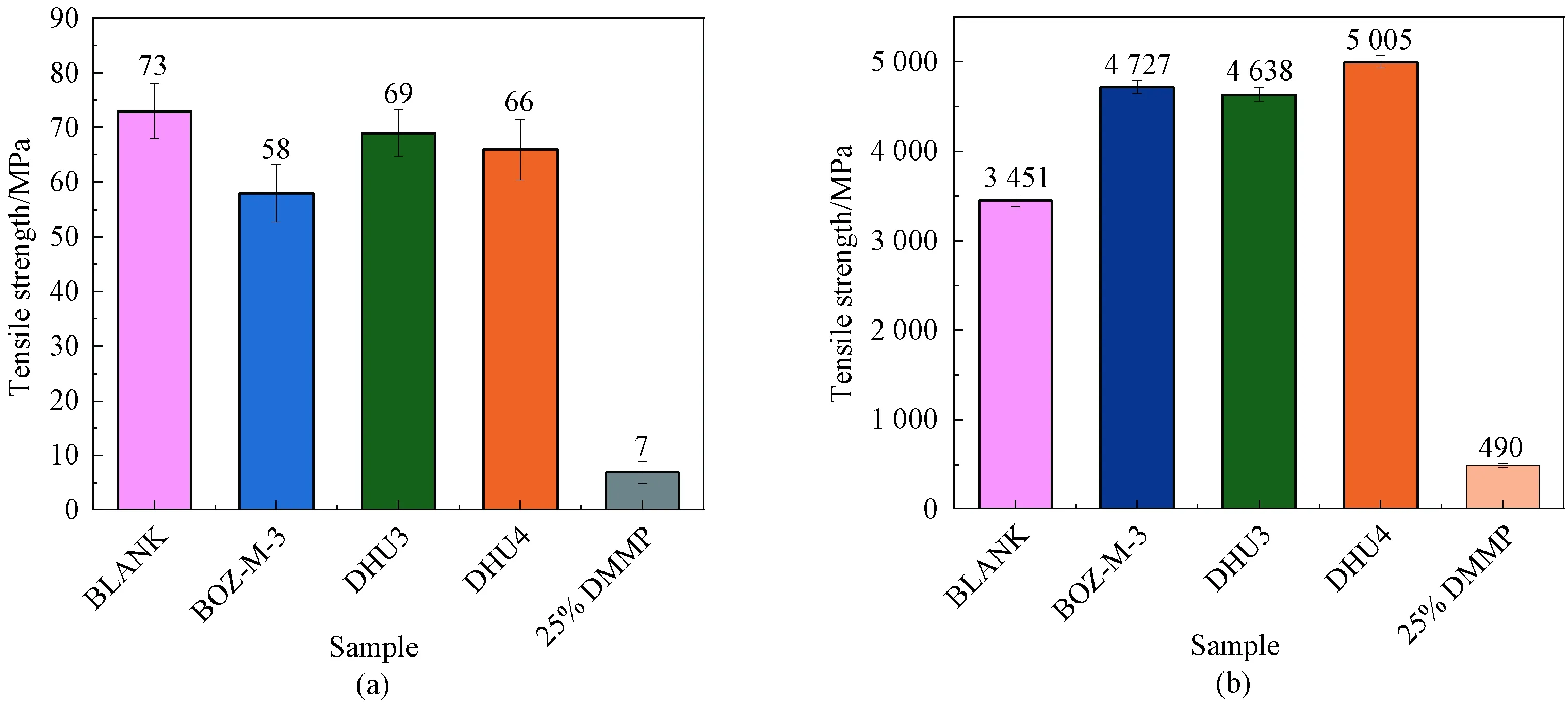
Fig. 10 Tensile properties of cured resins: (a) strength; (b) modulus
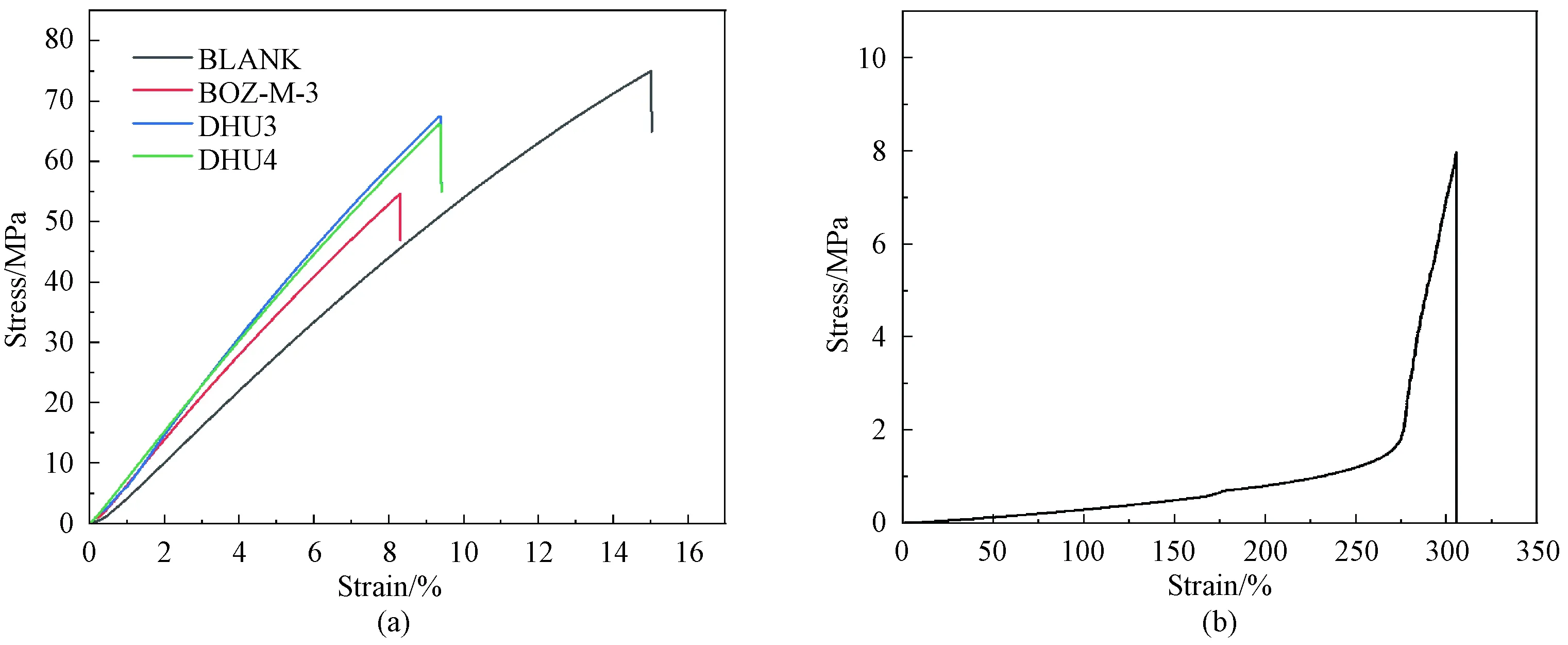
Fig. 11 Tensile stress-strain curves of cured resins: (a) various epoxy resins; (b) 25% DMMP epoxy resin
Figure 10(a) shows that the highest tensile strength is given by the BLANK resin, and the lowest was for 25% DMMP resin, from 73 MPa to 7 MPa, again showing the strong plasticizing effect by DMMP, especially by the extremely low modulus of the 25% DMMP resin[17-18](Fig. 10(b)). Adding DMMP increased the free volume which resulted in the reduction ofTgand modulus. However, the modified resins largely retained the tensile strength, and the slight reduction might be due to the additional defects or internal stress introduced by the incorporation of BOZ which cured differently to the epoxy and phase separated[19-20], as shown by the DSC results. In contrast to the tensile strengths, the tensile moduli of the resins containing BOZ significantly increased, showing that the stiffness improvement brought in by highTgand highly rigid BOZ offset the plasticization of DMMP.
3 Conclusions
Flame retardant epoxy resins formulated with an effective liquid phosphate ester flame retardant and a synergistic component BOZ were prepared for the application of VARI composites. The formulations produced resins of low viscosity which could be infused at slightly elevated temperature or even at room temperature.
It was found that using the phosphorous containing liquid flame retardant such as DMMP alone is far from adequate to produce satisfactory flame retardance and mechanical properties. This research demonstrated that the use of BOZ and DMMP for synergistic flame-retardant modified epoxy resin was an effective solution to the disadvantages. The synergistically formulated resins passed UL94 V-0 rating, produced low THR and HRR, and yielded high LOI. Test results also showed that the modified flame retardant resins largely retained, even improved the mechanical properties of the flammable benchmark resin, implying their potential applications as flame retardant interior parts manufacturable from the cost-effective VARI process.
 Journal of Donghua University(English Edition)2022年2期
Journal of Donghua University(English Edition)2022年2期
- Journal of Donghua University(English Edition)的其它文章
- Electrospinning of Bead-on-String Sodium Alginate Nanofibrous Membrane
- Polysaccharides Based Random and Unidirectional Aerogels for Thermal and Mechanical Stability
- Eco-Friendly pH Indicator Based on Natural Anthocyanins from Lycium ruthenicum
- Electrochemical Reduction Determination of N-Nitrosodiphenylamine in Food Based on Graphene Electrode Material
- Hydrothermal Synthesis of Ordered ZnO Nanorod Arrays by Nanosphere Lithography Method
- Temperature-Dependent Growth of Ordered ZnO Nanorod Arrays
