Effects of electroacupuncture pretreatment on motor function and cerebral blood flow in MCAO model rats
TAO Miaomiao (陶苗苗), DENG Yunyi (邓韵怡), CHENG Aifang (程爱芳), ZHANG Yingjie (张英杰), XU Mingshu (徐鸣曙)
1 Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine,Shanghai 201889, China
2 Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200030, China
3 Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China
Abstract
Keywords: Acupuncture Therapy; Electroacupuncture; Cerebral Infarction; Infarction, Middle Cerebral Artery;Cerebrovascular Circulation; Gait Analysis; Vascular Endothelial Growth Factor; Rats
Cerebral stroke is a cerebrovascular disease that is caused by a variety of factors leading to blood circulation disturbance in the brain, resulting in ischemia and hypoxic necrosis of brain tissue, thus damaging the structure and function of the nervous system. Clinical symptoms are mainly manifested as neurological deficits such as motor, sensory, learning,memory, language, and emotional dysfunction. There are two types of stroke: ischemic and hemorrhagic.Ischemic stroke accounts for 80% of strokes,characterized by high morbidity, mortality, and disability rates. Most patients develop disabilities after the onset of the disease, which causes both mental and economic pressure on the patient’s family and society[1-2].
Electroacupuncture (EA) is a treatment method combining acupuncture and electrical stimulation, with a widely recognized role in treating cerebrovascular diseases. EA pretreatment embodies the idea of“preventive treatment” in traditional Chinese medicine(TCM) preventive health care[3]. Studies have also confirmed that EA pretreatment has a certain promoting effect on the recovery of neurological function. EA pretreatment can reduce the brain damage caused by cerebral ischemia-reperfusion injuries,improve limb motor function in rats, restore cerebral blood flow in ischemic injury areas, promote the expression of various growth factors, inhibit neuronal apoptosis, and restore neuronal function[4-6].
In this study, to explore the effect and mechanism of EA pretreatment in relieving cerebral ischemiareperfusion injuries, the effects of EA pretreatment on Catwalk gait parameters, modified Bederson neurological deficit score, cerebral blood flow, cerebral infarction volume, and cerebral vascular endothelial growth factor (VEGF) were observed in rats with middle cerebral artery occlusion (MCAO). It is expected to provide certain experimental evidence for preventive EA pretreatment and gait rehabilitation after stroke.
1 Materials and Methods
1.1 Experimental animals and groups
A total of 24 healthy specific-pathogen-free male Sprague-Dawley rats [License No. SYXK (Shanghai) 2018-0040, body mass: (180±20) g, Shanghai Jihui Laboratory Animal Co., Ltd., China] were raised in the Animal Experiment Center of Yueyang Integrated Traditional Chinese and Western Medicine Hospital, Shanghai University of Traditional Chinese Medicine, with a rearing temperature of (24±2) ℃, humidity 45%-65%,dark/light 12 h/12 h, and free access to food and water.This experiment was approved by the Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine (Ethics No. YYLAC-2019-004). The experimental operations were in compliance with the animal ethics regulations. According to the principle of randomization, 24 rats were randomly divided into a normal group (without any treatment), a model group (MCAO modeling), and an EA group (EA pretreatment before MCAO modeling), with eight rats in each group.
1.2 Main instruments and reagents
1.2.1 Reagents
Isoflurane (Lot No. 217180501, Reward Life Technology Co., Ltd., China); 2,3,5-triphenyltetrazolium chloride (TTC, Lot No. T8170, Beijing Solarbio Science &Technology Co., Ltd., China); 0.9% sodium chloride injection (Lot No. 19993124, Zhejiang Jimin Pharmaceutical Co., Ltd., China); VEGF antibody (Lot No.ab1316, Abcam, USA).
1.2.2 Instruments
CatWalk XT gait analysis system (Noldus Information Technology, Netherlands); SAS-44611 stereotaxic apparatus (ASI, USA); JA5003 electronic balance(Shanghai Liangping Instrument Co., Ltd., China); laser Doppler flowmeter (Moor Instruments Ltd., UK);universal small animal anesthesia machine (Reward Life Technology Co., Ltd., China); portable coagulator(Kanghua Medical Instrument Co., Ltd., China); suture for MCAO of 0.36 mm in diameter and 30 mm in length(Beijing Cinontech Co., Ltd., China); infrared physiotherapy lamp (Chongqing Guoren Medical Equipment Co., Ltd., China); Hwato brand acupuncture needles of 0.25 mm in diameter and 13 mm in length(Suzhou Medical Appliance Factory, China); KWD-808I pulse electrotherapy apparatus (Wujin Great Wall Medical Instrument Co., Ltd., China); -80 ℃ refrigerator(Thermo, USA); electric thermostatic water bath(Shanghai Yiheng Technology Co., Ltd., China).
1.3 Model preparation and identification
Rats in both the model group and the EA group underwent MCAO modeling. Rats in the EA group received EA intervention for 7 d before modeling. The modeling was performed with the suture-occluded method by LONGA Z E,et al[7-8]. After being fasted for 8 h while allowed free access to water, rats were anesthetized with 4% isoflurane, maintained in the anesthetized state with 2.5% isoflurane, and fixed on the rat plate in a supine position. The middle of the rat neck was routinely disinfected with 75% alcohol. The neck skin was cut with ophthalmic scissors, followed by blunt separation of the subcutaneous tissue and the muscles with forceps until the right anterior cervical triangle was clearly seen and the common carotid artery (CCA), the cervical external carotid artery (ECA),and the internal carotid artery (ICA) were exposed. The surface mucosae of the ECA were removed. The communicating branch and the ECA branch were coagulated using electrocoagulation. The CCA and the ICA were clamped with arterial clips. Two nylon threads were passed through the ECA, respectively. The distal end was tied tightly, and the proximal end was tied with a loose knot. A small wedge-shaped opening was cut using fine scissors between the ligation at the distal end of the ECA and the loose knot at the proximal end. The suture was inserted through the opening and pushed into the “Y”-shaped trident. Completely cut the wedgeshaped opening of the ECA, and released the ICA artery.Adjusted the direction of the suture appropriately and continued to insert the distal end of the ICA to the mark on the suture. Released the CCA artery clip, tied the loose knot, and sutured the skin when the bleeding was stopped. After 1.5 h, the suture for occlusion was withdrawn slowly and cut off when resistance appeared.
1.4 Intervention methods
Normal group: The same grasping and high-place setting were performed as in the EA group.
Model group: The same grasping, high-place setting,and MCAO model preparation were performed as in the EA group.
EA group: Before MCAO modeling, EA was performed at bilateral Fengchi (GB20)[9]. After the acupuncture needle was inserted into the points, an EA instrument was connected to the bilateral needle handles,respectively, and a continuous wave of 2 Hz and 3-5 mA were set to make the two rat auricles slightly shake. The intervention lasted for 30 min each time. The treatment was performed during the same time period every day for seven consecutive days. The rats were returned to their cages at the end of the intervention. The experimental process is shown in Figure 1.
1.5 Observation items
1.5.1 Gait training and collection
After training, gait data were collected at T1 (before EA intervention), T2 (after EA intervention), and T6(24 h after ischemia-reperfusion).
Rat gait training method: The rats were placed in the end cassette of the CatWalk gait analysis system one by one to adapt for 40-60 min with food inducements in the cassette. Each rat entered the runway on its own after being placed at the entrance of the runway in the CatWalk gait analysis system. One complete test was defined as passing the runway smoothly without any obvious pause or turn-backs within 8 s. Each rat repeated the test three times. Food rewards were provided after the test. The surface of the runway was cleaned using 75% alcohol spray to remove the odor.The traces on the runway were wiped off with a soft damp cloth after each rat was tested and trained. Each training time was fixed. The gait training and acquisition were carried out in a dimly lit and quiet environment.
1.5.2 Modified neurological deficit score
Modified Bederson neurological deficit score was obtained from the surviving rats at T2 (after EA intervention), T4 (immediately after ischemiareperfusion), T5 (3 h after ischemia-reperfusion), and T6(24 h after ischemia-reperfusion), with the highest score of 4 points. Behavioral disturbances in the rats were positively correlated with the scores. The scoring rules are as follows[10].
Given that the rat tail was lightly lifted about 50 cm off the ground, if the rat’s forelimbs symmetrically extended toward the ground, scored 0 point; if the shoulder or elbow joints of the contralateral forelimb flexed or internally rotated, scored 1 point. The rat was placed on a smooth surface and pushed to the other side by the shoulders. If equal and strong resistance was found on both sides, scored 0 point; if a decreased resistance was found from the surgical side toward the opposite side, scored 1 point. Moreover, the two forelimbs of the rat were placed on a metal mesh, if equal and strong muscle tension was found on both sides, scored 0 point; if a decreased muscle tension of the contralateral forelimb was detected, scored 1 point.Lastly, raised the rat tail about 50 cm off the ground, if there were no spinning or other behaviors, scored 0 point; if the rat kept rotating to the opposite side of the operation, scored 1 point.
The sum of the above scores was taken as the modified Bederson neurological deficit score of the rat,the higher the score the more severe the behavioral disorder.
1.5.3 Cerebral blood flow monitoring by laser Doppler flowmeter
Time points of laser Doppler monitoring the blood flow in the cerebral cortex nourished by the middle cerebral artery in rats were T2 (after EA intervention),T3 (immediately after ischemia), and T4 (immediately after ischemia-reperfusion). The detection process is as follows.
Fasting started 8 h before the surgery, while free access to water was allowed. After anesthesia with 4%isoflurane, the rat was fixed on the brain stereotaxic apparatus in a prone position when its tail had no response to gentle pinching. The rat was maintained under anesthesia with 2.5% isoflurane throughout the operation. The scalp was opened to remove the fascia tissue, and the rat skull was exposed. The right middle cerebral artery blood supply area of the rat was located according to the rat brain stereotaxic atlas [anteroposterior (AP)[11]: -3.0 mm; mediolateral(ML): -5.0 mm].
The skull surface was marked and moistened with saline, and then the skull was thinned with a flat head drill at the mark site. The blood flow was monitored with a stereotaxic holder optical fiber, which was perpendicular to the thinned skull point to ensure accurate positioning. Rinsed the surface of the cranial window with diluted levofloxacin hydrochloride injection. After the skull surface was dried, placed the thin plastic tube vertically at the marked hole, fixed the tube with glass ionomer cement, and sealed the surface of the cranial window. The optical fiber was clamped by the stereotaxic device holder and inserted into the thin tube. The position was adjusted so that it was perpendicular to the skull surface. The recording started when the cerebral blood flow was stable, lasting for 1 min.
1.5.4 TTC staining of brain tissue
At T6 (24 h after ischemia-reperfusion), after all thein vivodata were collected, four rats were randomly selected from each group, and their brains were collected under deep isoflurane anesthesia after cervical dislocation. The intact brain tissue was rinsed with physiological saline and placed on the outer surface of the petri dish in a -80 ℃ refrigerator. Five coronal sections approximately 2 mm in thickness were prepared from anterior to posterior of the frozen brain tissue. The tissue sections were put into 2% TTC solution, incubated in a 37 ℃ water bath for 30 min in the shades, and turned every 10 min to make the staining uniform. Took out the tissue sections to fix with 4% paraformaldehyde solution for 24 h, arranged them in order, dried up, and photographed. The percentage of cerebral infarction volume in the fourth brain slice was analyzed with ImageJ software. The percentage of cerebral infarction volume = Infarction volume of the fourth slice ÷ Full volume of the fourth slice × 100%.
1.5.5 Immunohistochemistry (IHC) of brain tissue
Tissue in the target area was paraffin-embedded for future use. The tissue wax blocks were serially sectioned at 3 μm, attached to a glass slide, and baked at 60 ℃ for 1 h to make the tissue closely adhere to the glass slide. The slices were dehydrated in 100%, 90%,80%, and 70% graded alcohol successively, each for 10 min, and rinsed with tap water for 30 min. The slices were put into a container with citric hydrochloric acid buffer. Placed the container in a pressure cooker at 100 ℃ for 6 min, cooled to room temperature, and taken out. Added 3% H2O2solution dropwise, incubated in the shades for 5 min, rinsed with distilled water for 5 min, and washed with buffer 3 times, 5 min/time.Blocked with 5% normal goat serum, placed at room temperature for 30 min, and shook off the excess liquid.Added the primary antibody (1:100) dropwise,incubated at 4 ℃ overnight, placed in a 37 ℃ incubator for 30 min, and washed with buffer 3 times, 5 min/time.Added 50 μL biotin-labeled secondary antibody dropwise, incubated at 37 ℃ for 30 min, washed with buffer 3 times, 5 min/time. Added horseradish peroxidase-labeled streptavidin working solution dropwise, incubated at 37 ℃ for 30 min, washed with buffer 3 times, 5 min/time. In a centrifuge tube containing 1 mL distilled water, added one drop of reagents A, B, and C provided by the kit one by one in the shades, mixed it well, and added it to the slices. The color development reaction was terminated after 5 min followed by hematoxylin counterstaining for 3 min. The slices were dehydrated in 70%, 80%, 90%, and 100%gradient alcohol in turn, followed by transparency with xylene for 5-8 min, and then air-dried naturally. Neutral gum was added dropwise and mounted with a coverslip.
1.6 Statistical methods
All data were statistically analyzed by the SPSS version 24.0 statistical software. Measurement data conforming to normal distribution and homogeneity of variance were presented as mean ± standard deviation(±s), and the one-way analysis of variance was used for comparisons among groups. The data not conforming to normal distribution were presented as the median (lower quartile, upper quartile) [M (QL, QU)],and a nonparametric test was used. The difference was statistically significant atP<0.05.
2 Results
2.1 Modified neurological deficit score
At T2 (after EA intervention), T4 (immediately after ischemia-reperfusion), T5 (3 h after ischemiareperfusion), and T6 (24 h after ischemia-reperfusion),the modified Bederson neurological deficit score was evaluated. Each time-point for the normal group without MCAO modelling and the two time-points before and after EA intervention for the other two groups were scored 0 point. Comparing the difference in the neurological deficit score between different time points can better reflect the difference between the modeling and the EA intervention.
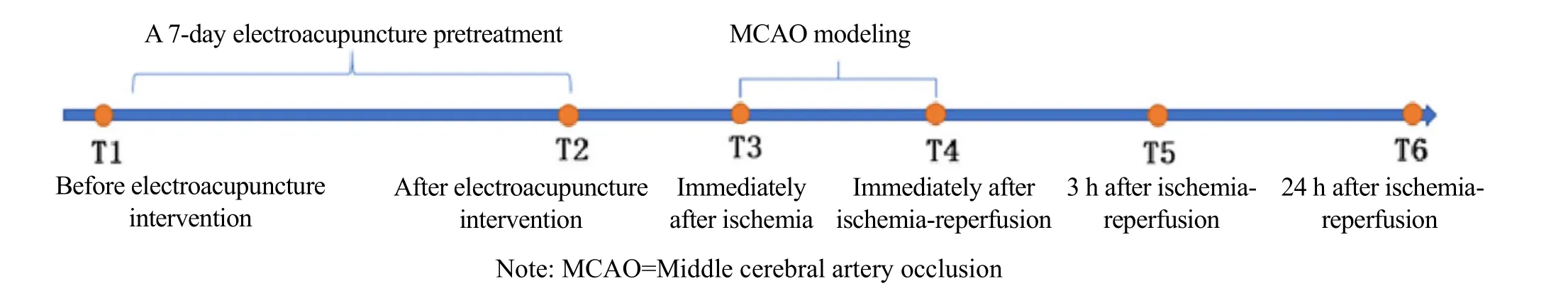
At each time point, compared with the normal group,the neurological deficit score in the model group and EA group significantly increased (P<0.05). In the EA group,the figure of the neurological deficit score at T6 minus the score at T4 was larger than that of the score at T5 minus the score at T4, and was greater than that in the model group (P<0.05). It is suggested that EA pretreatment can improve the symptoms of nerve defects in rats (Table 1).
2.2 CatWalk gait analysis
2.2.1 Stance duration
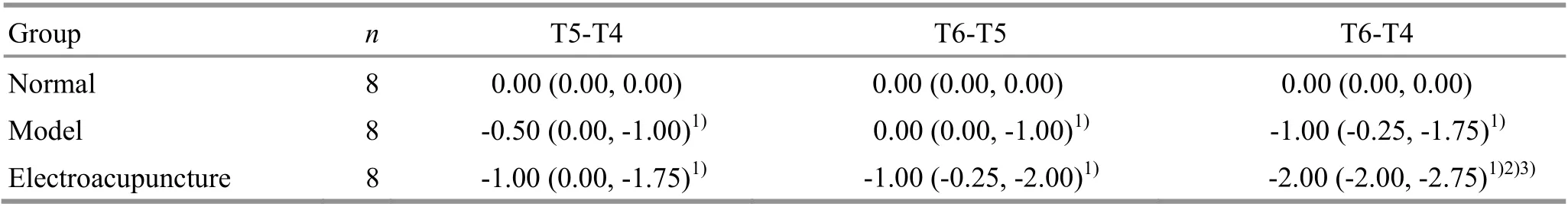
At T1 (before EA intervention), there were no statistical differences in the stance duration of rat left or right forelimbs among the groups (P>0.05), indicating that the groups were comparable. At T2 (after EA intervention), the stance durations of the left and right forelimbs in the model group and the EA group were not significantly different from those in the normal group (P>0.05). At T6 (24 h after ischemia-reperfusion),compared with the normal group, the stance durations of the rat left and right forelimbs in the model group and the EA group were significantly increased (P<0.05);compared with the model group, the stance durations of the left and right forelimbs in the EA group were decreased (P<0.05), (Table 2).
Group n T5-T4 T6-T5 T6-T4 Normal 8 0.00 (0.00, 0.00) 0.00 (0.00, 0.00) 0.00 (0.00, 0.00)Model 8 -0.50 (0.00, -1.00)1) 0.00 (0.00, -1.00)1) -1.00 (-0.25, -1.75)1)Electroacupuncture 8 -1.00 (0.00, -1.75)1) -1.00 (-0.25, -2.00)1) -2.00 (-2.00, -2.75)1)2)3)
Group n T1 T2 T6 LF RF LF RF LF RF Normal 8 0.13±0.01 0.13±0.01 0.12±0.01 0.12±0.02 0.13±0.02 0.14±0.02 Model 8 0.11±0.01 0.12±0.01 0.13±0.02 0.12±0.01 0.20±0.041) 0.20±0.041)Electroacupuncture 8 0.12±0.02 0.12±0.02 0.13±0.01 0.13±0.01 0.16±0.031)2) 0.15±0.031)2)
2.2.2 Gait cycle
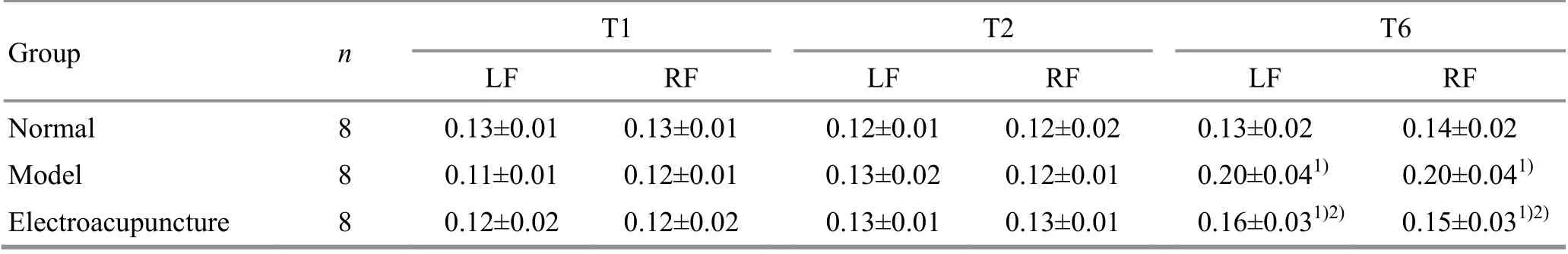
At T1 (before EA intervention), there were no statistical differences in the gait cycles of the rat left or right forelimbs among the groups (P>0.05), indicating that the groups were comparable. At T2 (after EA intervention), the left and right forelimb gait cycles in the model group and the EA group were not significantly different from those in the normal group(P>0.05). At T6 (24 h after ischemia-reperfusion),compared with the normal group, the left and the right forelimb gait cycles of the rats in the model group and the EA group were significantly increased (P<0.05);compared with the model group, the gait cycles of the left and right forelimbs in the EA group were significantly reduced (P<0.05), (Table 3).
2.2.3 Gait cadence
At T1 (before EA intervention), there were no statistical differences in the gait cadence of the rats among the groups (P>0.05), indicating that the groups were comparable. At T2 (after EA intervention), the gait cadence in the model group and the EA group was statistically equal to that in the normal group (P>0.05).At T6 (24 h after ischemia-reperfusion), compared with the normal group, the gait cadence of the rats in the model group and the EA group significantly decreased(P<0.05); compared with the model group, the gait cadence in the EA group increased (P<0.05), (Table 4).
2.3 Cerebral blood flow
At T2 (after EA intervention), there were no significant differences in the cerebral blood flow among the groups (P>0.05), indicating that the groups were comparable. At T3 (immediately after ischemia),compared with the normal group, the cerebral blood flow of the rats in the model group and the EA group was significantly decreased (P<0.05). At T4 (immediately after ischemia-reperfusion), compared with the normal group, the cerebral blood flow in the model group was significantly reduced (P<0.05); compared with the model group, the cerebral blood flow in the EA group was significantly increased (P<0.05), (Table 5).
Group n T1 T2 T6 LF RF LF RF LF RF Normal 8 0.25±0.02 0.25±0.02 0.24±0.02 0.24±0.03 0.25±0.03 0.26±0.04 Model 8 0.24±0.01 0.24±0.02 0.24±0.02 0.24±0.02 0.34±0.031) 0.35±0.031)Electroacupuncture 8 0.24±0.02 0.25±0.02 0.25±0.01 0.25±0.02 0.29±0.041)2) 0.30±0.051)2)

Group n T1 T2 T6 Normal 8 15.81±1.06 16.60±2.09 15.48±2.03 Model 8 16.55±1.12 16.32±1.46 11.50±1.401)Electroacupuncture 8 16.16±1.47 15.41±0.88 13.28±1.841)2)

Group n T2 T3 T4 Normal 8 517.22±171.99 509.94±173.21 519.20±171.20 Model 8 529.20±204.47 148.90±72.601) 254.45±98.431)Electroacupuncture 8 533.87±146.64 152.69±52.281) 405.95±161.712)
2.4 TTC staining of brain tissue
After TTC staining, the ischemic sites are shown in white and non-ischemic sites are shown in red. At T6(24 h after ischemia-reperfusion), compared with the normal group, different degrees of infarction sites occurred in the model group and the EA group, and the cerebral infarction volume ratio in the model group and the EA group was higher (P<0.05); compared with the model group, the cerebral infarction volume ratio of the rats in the EA group was decreased (P<0.05), (Figure 2 and Figure 3).
2.5 IHC of VEGF in the brain tissue
Positive expression in the cytoplasm and cell membrane was stained reddish-brown with IHC examination and observed under a microscope(Figure 4). The positive VEGF expression level in the model group was significantly higher than that in the normal group (P<0.05). The positive VEGF expression level in the EA group was significantly higher than that in the model group (P<0.05), (Figure 5). It is suggested that MCAO modeling increases the VEGF expression in the marginal area of rat cerebral infarction, and EA pretreatment significantly increases VEGF expression.
3 Discussion
TCM believes that wind, fire (heat), phlegm (fluid retention), Qi and blood stasis are the main causes of stroke. Liver Yang induces inner wind to cause disordered Qi and blood flow, straightly damaging the brain, and the clear orifices are closed. Studies have shown that acupuncture improves cerebral blood supply, relieves cerebral ischemia, induces cerebral ischemic tolerance, reduces cerebral ischemic damage,and protects the cerebral nervous system[12]. With the concept of “acupuncture pretreatment”[13], many studies have proven that acupuncture pretreatment can resist ischemia-reperfusion injury.
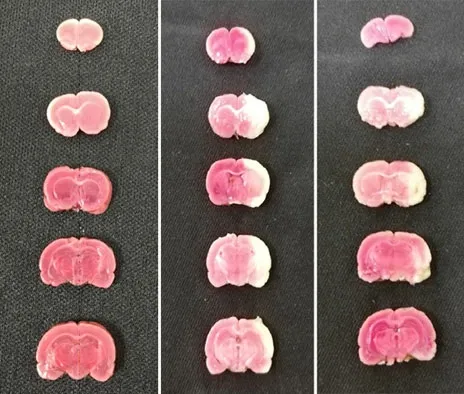
Figure 2. Rat brain tissue stained with 2,3,5-triphenyltetrazolium chloride
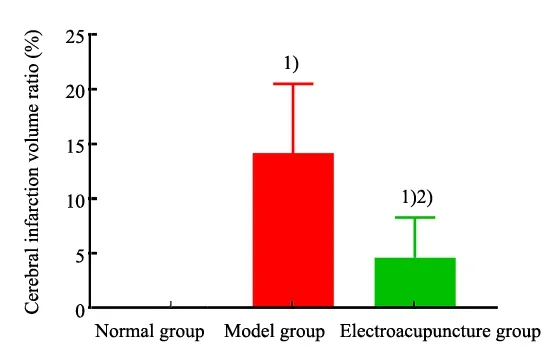
Figure 3. Comparison of the cerebral infarction volume ratio

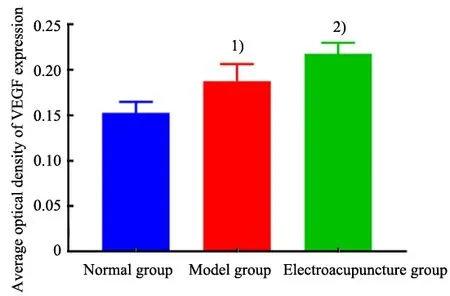
Figure 5. Comparison of the average optical density of vascular endothelial growth factor expression in rat brain tissue among groups
3.1 Effects of EA pretreatment on motor function in MCAO rats
In animal experiments, the modified Bederson neurological deficit score is of great significance for evaluating the degree of motor function impairment and the prognosis of MCAO model rats. To reflect the changes in the rat neurological deficit scores in different groups, the differences in the scores between different time points were compared in this study. Our findings showed that the rats had different neurological deficit symptoms after modeling, and EA pretreatment improved the neurological deficit symptoms of the model rats. However, the early stage of the modeling did not really reflect the improvement effect of EA pretreatment on the rat nerve defect symptoms due to the influence of anesthesia during operation.
The CatWalk gait analysis system objectively records and quantitatively and comprehensively analyzes the gait characteristics of experimental animals during walking. Our study found that the model rats had rigid limbs, unfavorable joint flexion and extension,decreased movement balance and coordination, slower movement speed, longer movement time, and longer limb standing time. In the EA group, the stance durations of the left and right forelimbs were significantly shortened after EA pretreatment,suggesting that EA pretreatment improves limb motor dysfunction in rats with cerebral ischemia. The gait cycle refers to the interval between the two times that the rat’s paw touched the glass plate. Combined with the standing time and the swing time of rat limbs, the gait cycle can reflect the rat gait coordination and is mainly manifested in the rat’s forelimbs[14]. The gait cadence represents the step numbers taken by rats in one second. Cerebral ischemia-reperfusion injury can cause limb motor dysfunction, uncoordinated movements,prolonged standing time, slower limb swing, decreased step numbers/unit time, poor coordination, and prolonged gait cycles in rats. Rats mainly rely on the forelimb support to exert force during normal walking.In this study, the forelimb gait parameters of MCAO rats changed significantly. After EA pretreatment, the forelimb standing time, gait cycle, and gait cadence were significantly improved. However, the effect of EA pretreatment on the hind limbs of MCAO rats needs to be further clarified by extending the observation time.
3.2 Effects of EA pretreatment on cerebral blood flow in MCAO rats
Laser Doppler flowmetry is an instrument to continuously monitor the microcirculatory blood flow with a monochromatic cohesive laser[15-17]. By intuitively observing the changes of the cerebral blood flow, it is possible to determine whether the blockage degree is appropriate during rat MCAO model preparation, so as to avoid model differences due to animal species, body mass, suture diameter, or insertion depth, thus to ensure the stability of the ischemia degree in the animal model, which will improve the scientificity and strictness of the study[18-19]. This study showed that the rat cerebral blood flow in the model group and the EA group was decreased by 40% after embolization,indicating that the model was successfully prepared and relatively stable. Normal brain tissue appeared red,while ischemic infarcted tissue appeared white with TTC staining, which is helpful to investigate the specific position, size, and shape of the ischemic penumbra.Therefore, TTC staining is one of the morphological evidences for the successful MCAO modeling[20]. The results of laser Doppler blood flow monitoring were consistent with those of the TTC staining, indicating that MCAO modeling reduces the blood flow in the middle cerebral artery blood supply area, and EA pretreatment partly rescues the cerebral blood flow in the ischemic area. SHI L,et al[21]also proved that the EA intervention significantly improved the regional cerebral blood flow in MCAO model rats and increased the cerebral blood flow supply around the infarcted area.
At present, it is believed that angiogenesis is a key step in the recovery of ischemic disease and damage repair, and the new blood vessel formation at the ischemic site is beneficial to the ischemic brain tissue repair after cerebral infarction. VEGF has neurotrophic effect and plays a direct protective effect on the nervous system. VEGF helps prolong the nerve cell survival until the new blood vessel takes shape. Studies have shown that VEGF promotes the division and proliferation of vascular endothelial cells and enhances the angiogenesis ability, thereby stimulating new blood vessels in the ischemic penumbra, establishing collateral circulation, and promoting the recovery of blood supply in the lesions[22]. Many studies have shown that acupuncture at Shuigou (GV26), Dazhui (GV14), Ganshu(BL18), Shenshu (BL23), and Baihui (GV20) effectively increases the expression levels of VEGF and its related factors in brain tissue, promote angiogenesis, and improve the local cerebral blood flow, thus resisting cerebral ischemia injury[23-25], which has been further confirmed in our current study. MCAO modeling upregulates rat VEGF-positive cells in the cerebral infarction areas, which may be caused by cerebral ischemia-induced stress responses. The stress response is manifested as increased capillary density in the ischemic areas and the surrounding tissue, microvessel formation, and collateral circulation establishment. It improves the local blood supply and the neurological damage level after cerebral ischemic infarction[26]. This study showed that EA promoted VEGF expression in the cerebral ischemia areas of MCAO rats, which may be related to the local blood supply recovery after cerebral infarction. In conclusion, EA pretreatment at Fengchi(GB20) can improve motor function and promote the recovery of cerebral blood flow and microvascular reconstruction in the ischemic infarction areas of the MCAO rats, which may be associated with the promoted angiogenesis and nerve function recovery owing to the increased VEGF expression. However, the indicators in the later recovery stage of MCAO model rats affected by the EA pretreatment were not investigated or recorded due to the short observation time after model establishment in this study. To further reveal the effective time-point of EA pretreatment on VEGF expression in the cerebral infarction areas of MCAO model rats, the observation time should be appropriately extended.
Conflict of Interest
There is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Research Project of Shanghai Science and Technology Committee (上海市科学技术委员会科研计划项目, No. 17ZR1427500);Research Project of Shanghai Municipal Commission of Health and Family Planning (上海市卫生和计划生育委员会科研课题, No. 201640128); Shanghai Talent Development Fund (上海市人才发展基金项目, No.2017104).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 2 November 2020/Accepted: 28 July 2021
 Journal of Acupuncture and Tuina Science2022年3期
Journal of Acupuncture and Tuina Science2022年3期
- Journal of Acupuncture and Tuina Science的其它文章
- Effect of moxibustion on N-methyl-D-aspartate receptor subtype 2B expression in hippocampus of rheumatoid arthritis model rats
- Effects of scalp acupuncture plus acupuncture exercise therapy on walking ability in children with spastic cerebral palsy
- Therapeutic efficacy and safety rating of Tui-Pushing chest-back manipulation for children with cough variant asthma
- Clinical observation on moxibustion at Baihui (GV20)plus Tuina for children with postnasal drip syndrome
- Clinical study of warm needling moxibustion combined with entecavir in the treatment of compensated cirrhosis due to chronic hepatitis B
- Clinical study of warm needling moxibustion plus intra-articular injection of sodium hyaluronate for hip involvement in ankylosing spondylitis
