Clinical study of warm needling moxibustion combined with entecavir in the treatment of compensated cirrhosis due to chronic hepatitis B
NIE Lu (聂璐), ZHANG Juanli (张娟丽)
Hubei Provincial Hospital of TCM, Wuhan 430060, China
Abstract
Keywords: Acupuncture-moxibustion Therapy; Warm Needling Therapy; Acupuncture Medication Combined; Entecavir;Hepatitis B, Chronic; Liver Cirrhosis; Liver Function Tests
Cirrhosis is a common outcome of the development of several chronic liver diseases, characterized by diffuse fibrosis, regenerative nodules, and pseudolobule formation[1]. In China, viral hepatitis is the primary cause of cirrhosis, with chronic hepatitis B (CHB) being the most prevalent[2]. Data show that the hepatitis B surface antigen positivity rate in the general population aged 1-59 years is 7.18%, while the 5-year incidence of cirrhosis in CHB patients is 12%-25%[3-4]. Liver cirrhosis can be divided into compensated and decompensated cirrhosis according to the progression of the disease.Among them, compensated cirrhosis has mild clinical symptoms and has the possibility of reversal with treatment. If cirrhosis progresses to decompensated cirrhosis without effective treatment, the disease is irreversible and can be complicated by hepatorenal syndrome, gastrointestinal hemorrhage, and hepatic encephalopathy, which can seriously affect the survival quality of patients and even threaten their lives[5-6].Therefore, antiviral, and anti-fibrotic treatment in patients with compensated cirrhosis can delay the progression of cirrhosis, improve the quality of life, and prolong the survival of patients. Research suggested that acupuncture treatment for CHB can regulate the body’s immunity, inhibit viral replication, and improve clinical symptoms[7]. Warm needling moxibustion can effectively improve the symptoms of non-infectious diarrhea and ascites in patients with cirrhosis[8-9], but there are few clinical studies on acupuncture for compensated cirrhosis due to CHB. Therefore, in this study, warm needling moxibustion combined with entecavir was adopted to treat compensated cirrhosis due to CHB to observe its efficacy and explore the possible mechanism of action.
1 Clinical Materials
1.1 Diagnostic criteria
1.1.1 Diagnostic criteria in Western medicine
Referred to the diagnostic criteria of compensated cirrhosis due to CHB in theGuideline of Prevention and Treatment for Chronic Hepatitis B:A 2015 Update[10].History of CHB and exclusion of other family causes of cirrhosis; abdominal ultrasound showed a shrunken liver with an uneven, serrated, or wavy surface, shallow liver margins, uneven, enhanced, and nodular echogenicity of the liver parenchyma, widened inner diameter of the portal and splenic veins, thin and twisted hepatic veins; gastroscopy showed varices of the esophagogastric fundic veins; normal or mildly elevated liver function parameters, and elevated hyaluronic acid (HA), laminin (LN), procollagen type Ⅲ(PCⅢ), and type Ⅳ collagen (Ⅳ-C); there were no serious complications such as rupture and bleeding of esophagogastric fundic varices, ascites, and hepatic encephalopathy.
1.1.2 Syndrome differentiation criteria in traditional Chinese medicine (TCM)
Referred to the TCM identification criteria for stasis blocking liver collaterals in theConsensus on the Treatment of Liver Cirrhosis with Integrative Chinese and Western Medicine[11]. The main symptoms are pain like stabbing in the hypochondrium, pain that does not move, accumulation of lumps under the hypochondrium, a dark purple tongue with or without dark spots, and purple or brown lips. Secondary symptoms include a dark or dull face, red spots and red wisps on the head, neck, chest, and abdomen, dark stool, and hesitant pulse beats.
1.2 Inclusion criteria
Met the aforementioned diagnostic criteria in Western medicine and the syndrome differentiation criteria in TCM; aged 20-60 years, male or female;voluntarily participated in this trial and signed the informed consent form.
1.3 Exclusion criteria
Patients with combined hepatitis A virus, hepatitis C virus, or other infections; with hepatic encephalopathy,hepatorenal syndrome, gastrointestinal hemorrhage, or other complications; with severe splenomegaly; with prolonged prothrombin time; women during pregnancy or lactation; with severe heart, brain, or kidney diseases;allergy to drugs in this test; with mental disorders or language or intellectual disabilities.
1.4 Criteria for dropout
Serious complications; excessive transaminases during the treatment; withdrawal from the trial.
1.5 Criteria for elimination
Failed to administer medication as prescribed;incomplete information affected the judgment of efficacy.
1.6 Statistical analysis
The SPSS version 22.0 software was used for analysis.Count data were analyzed by Chi-square test;measurement data conforming to assumptions of normality and homogeneity of variance were expressed as mean ± standard deviation (x±s) and analyzed usingt-test; measurement data not conforming to assumptions of normality or homogeneity of variance were tested using the nonparametric test.P<0.05 was considered statistically significant.
1.7 General data
Ninety patients with compensated cirrhosis due to CHB admitted to our hospital between January 2018 and December 2019 were selected. The random number table method combined with the order of patient visits was used to divide them into a control group and an observation group, with 45 cases in each group. During the treatment period, one case in the control group dropped out for work-related reasons and four cases were excluded due to self-administration of other drugs; two cases in the observation group dropped out due to needle sickness, two cases dropped out for work-related reasons, and one case was excluded due to self-administration of other drugs. A total of 80 cases finally completed this trial, including 40 cases in the control group and 40 cases in the observation group. There were no statistical differences between the two groups in terms of gender, age, and disease duration (P>0.05), suggesting the comparability(Table 1).
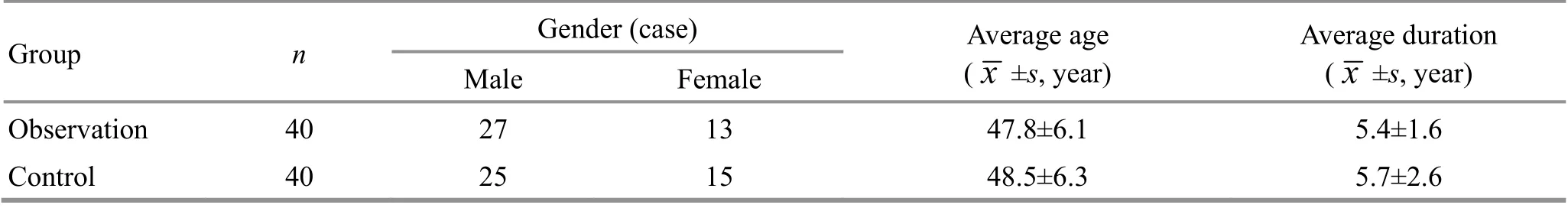
Group n Gender (case) Average age( ±s, year)Male Female x Average duration( ±s, year)x Observation 40 27 13 47.8±6.1 5.4±1.6 Control 40 25 15 48.5±6.3 5.7±2.6
2 Treatment Methods
2.1 Control group
Patients in the control group were treated with oral entecavir tablets (State Food and Drug Administration Approval No. H20100019, Chia Tai Tianqing Pharmaceutical Group Co., Ltd., China) at 0.5 mg once a day for 6 months.
2.2 Observation group
Patients in the observation group were treated with warm needling moxibustion in addition to the intervention in the control group.
Points: Bilateral Geshu (BL17), Ganshu (BL18),Danshu (BL19), Yanglingquan (GB34), and Sanyinjiao(SP6).
Methods:Patients took a prone position, and the skin of the selected points was exposed. After routine disinfection, Geshu (BL17), Ganshu (BL18), and Danshu(BL19) were punctured with Hwato brand disposable sterile acupuncture needles of 0.25 mm in diameter and 40 mm in length. The needles were inserted obliquely by 0.5-0.8 Cun toward the vertebral body;Yanglingquan (GB34) and Sanyinjiao (SP6) were punctured perpendicularly for 1.0-1.2 Cun with needles of 0.25 mm in diameter and 40 mm in length. During this period, a moxa stick of 2 cm in length was inserted onto the needle handles at Geshu (BL17), Ganshu(BL18), and Danshu (BL19) and lighted. A piece of cardboard was placed between the moxa stick and the skin to prevent burns. The treatment was carried out once a day, 5 times a week for 6 months.
3 Observation of Therapeutic Efficacy
3.1 Observation items
3.1.1 Liver function indicators
The serum alanine transaminase (ALT), aspartate transaminase (AST), and albumin (ALB) levels of the patients in the two groups were measured before and after treatment using a fully automatic biochemical analyzer.
3.1.2 Ultrasound indicators
The internal diameters of the portal vein and the splenic vein, and the thickness of the spleen were measured by ultrasound examination in both groups before and after treatment.
3.1.3 Liver hardness value
Liver hardness value was measured in both groups before and after treatment using the liver transient elastic fiber Fibrotouch.
3.1.4 Four indicators of liver fibrosis
Fasting venous blood was collected from patients before and after treatment, and HA, LN, PCⅢ, andⅣ-C levels were measured by radioimmunoassay.
3.1.5 Serum interleukin (IL)-21 and platelet-derived growth factor (PDGF)
Fasting venous blood was collected from patients before and after treatment, and serum IL-21 and PDGF levels were measured by enzyme-linked immunoassay.
3.2 Results
3.2.1 Comparison of the serum ALT, ASR, and ALB levels
Before treatment, there were no statistical differences in the serum ALT, AST, or ALB levels between the two groups (P>0.05). After treatment, the serum ALT and AST levels decreased (P<0.05), and the serum ALB level increased (P<0.05) in the two groups; the differences in the serum ALT, AST, and ALB levels between the two groups were statistically significant(P<0.05). For details, see Table 2.
3.2.2 Comparison of the portal vein internal diameter,splenic vein internal diameter, and spleen thickness
Before treatment, there were no statistical differences in the internal diameter of the portal vein,the internal diameter of the splenic vein, or spleen thickness between the two groups (P>0.05). After treatment, the portal vein internal diameter, splenic vein internal diameter, and spleen thickness decreased in both groups (P<0.05), and the portal vein internal diameter, splenic vein internal diameter, and spleen thickness in the observation group were smaller than those in the control group (P<0.05). For details, see Table 3.
3.2.3 Comparison of the liver hardness value
Before treatment, there was no statistical difference in the liver hardness value between the two groups(P>0.05). After treatment, the liver hardness value decreased in both groups (P<0.05), and was lower in the observation group than in the control group(P<0.05). For details, see Table 4.

Group n ALT (U/L) AST (U/L) ALB (g/L)Before treatment After treatment Before treatment After treatment Before treatment After treatment Observation 40 96.49±19.25 53.62±14.341)2) 80.67±16.85 43.15±9.981)2) 30.27±8.26 42.12±11.881)2)Control 40 92.09±19.82 76.35±16.881) 86.44±15.52 65.17±15.071) 31.35±7.46 35.56±8.961)

Group n Portal vein internal diameter Splenic vein internal diameter Spleen thickness Before treatment After treatment Before treatment After treatment Before treatment After treatment Observation 40 14.46±1.66 12.64±1.311)2) 9.74±1.09 8.22±1.201)2) 45.82±8.16 34.18±7.231)2)Control 40 14.52±1.33 13.66±1.251) 10.01±1.18 9.02±1.081) 47.61±9.11 38.89±8.071)
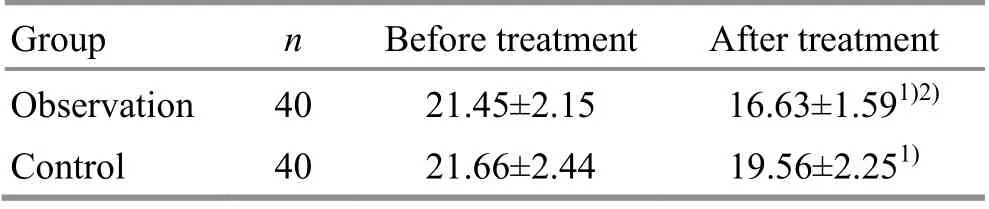
Group n Before treatment After treatment Observation 40 21.45±2.15 16.63±1.591)2)Control 40 21.66±2.44 19.56±2.251)
3.2.4 Comparison of the serum HA, LN, PCⅢ, andⅣ-C levels
Before treatment, there were no statistical differences in the serum HA, LN, PCⅢ, or Ⅳ-C levels between the two groups (P>0.05). After treatment, the serum HA, LN, PCⅢ, and Ⅳ-C levels decreased in both groups (P<0.05), and were lower in the observation group than in the control group (P<0.05). Please see Table 5 and Table 6.data are detailed in Table 7.
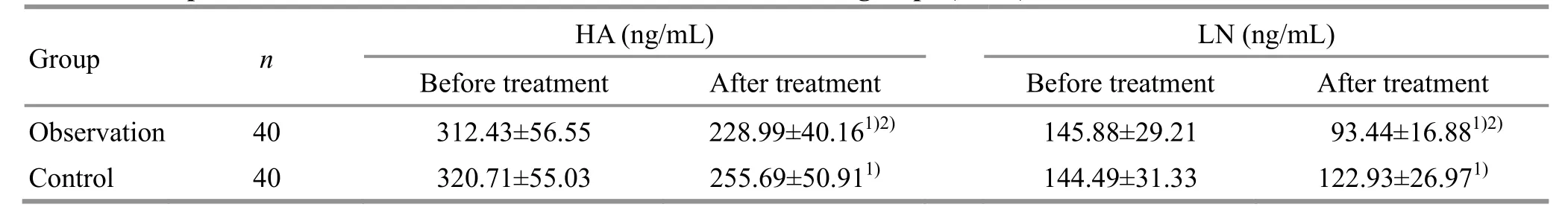
Before treatment After treatment Before treatment After treatment Group n HA (ng/mL) LN (ng/mL)Observation 40 312.43±56.55 228.99±40.161)2) 145.88±29.21 93.44±16.881)2)Control 40 320.71±55.03 255.69±50.911) 144.49±31.33 122.93±26.971)
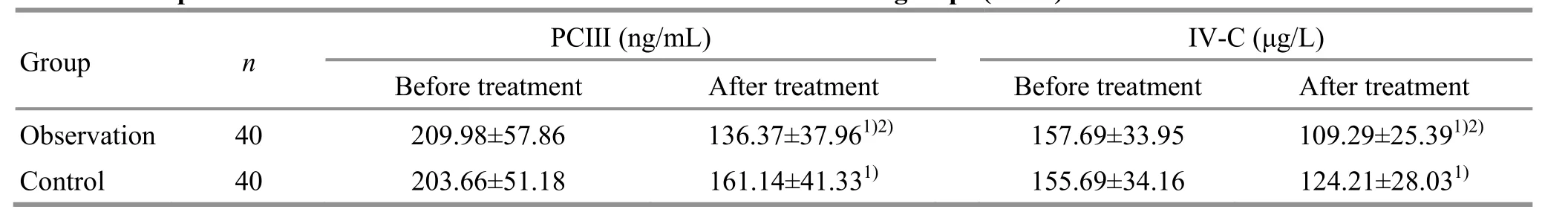
Before treatment After treatment Before treatment After treatment Group n PCⅢ (ng/mL) Ⅳ-C (μg/L)Observation 40 209.98±57.86 136.37±37.961)2) 157.69±33.95 109.29±25.391)2)Control 40 203.66±51.18 161.14±41.331) 155.69±34.16 124.21±28.031)
3.2.6 Adverse reaction records
During the treatment, no serious adverse reactions occurred in either group. One case of headache and two cases of gastrointestinal reactions occurred in the control group; two cases of dizziness occurred in the observation group. The symptoms were mild. There was no statistically significant difference in the incidence of adverse reactions between the groups (P>0.05).
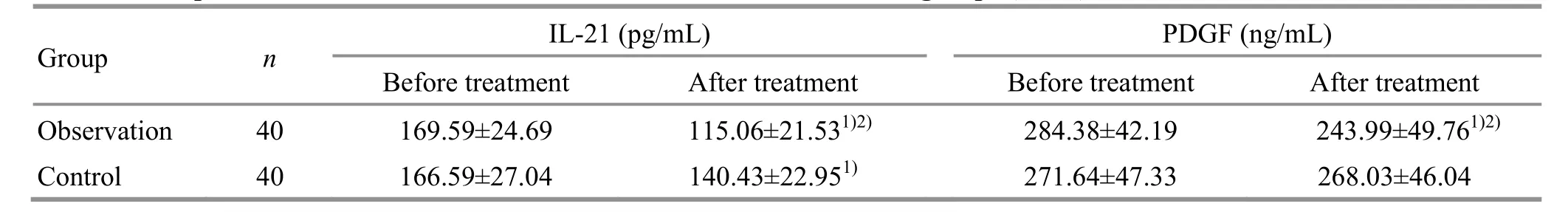
Group n IL-21 (pg/mL) PDGF (ng/mL)Before treatment After treatment Before treatment After treatment Observation 40 169.59±24.69 115.06±21.531)2) 284.38±42.19 243.99±49.761)2)Control 40 166.59±27.04 140.43±22.951) 271.64±47.33 268.03±46.04
3.2.5 Comparison of the serum IL-21 and PDGF levels
Before treatment, there were no statistical differences in the serum IL-21 or PDGF levels between the two groups (P>0.05). After treatment, the serum IL-21 level decreased in the control group (P<0.05), and the serum PDGF level did not change significantly(P>0.05); the serum IL-21 and PDGF levels decreased significantly in the observation group (P<0.05) and were lower than those in the control group (P<0.05). The
4 Discussion
Hepatitis B virus (HBV) replicates and multiplies in the liver for a long time, which can lead to chronic inflammation of the liver, hepatocyte injury, activation of hepatic stellate cells, promotion of extracellular matrix release, and inhibition of its degradation,resulting in excessive extracellular matrix deposition and formation of hepatocyte regeneration nodules;chronic inflammation persists, with alternating damage and repair, resulting in structural changes of liver lobules, pseudolobule formation, liver deformation,hardening, and progression to cirrhosis[12]. The compensated cirrhosis due to CHB is less severe than the decompensated cirrhosis, the liver structure is less damaged, and the liver function can still meet the needs of the body. Timely and effective antiviral and antifibrotic treatment for compensated cirrhosis can maximize the inhibition of viral replication, reduce the degree of liver inflammation, inhibit the activation of liver stellate cells, reduce the degree of liver fibrosis,and prevent the progression to decompensated cirrhosis[13-14].
Entecavir is a guanine nucleoside analogue that acts as an antiviral replicator by inhibiting HBV polymerase activity. It has a high resistance genetic barrier and stronger antiviral potency than lamivudine and adefovir and is recommended as a first-line antiviral drug[15]. A clinical study showed that after entecavir treatment in 57 patients with CHB, 96% had improved liver histological performance, including 88% of patients(10 with cirrhosis) who had significantly improved liver fibrosis scores[16]. This suggests that long-term application of entecavir can control further liver damage by HBV and reverse coarse fibrosis and cirrhosis.
According to the clinical symptoms such as pain in the hypochondrium, hypochondriac mass, yellowish skin, and abdominal bloating like a drum, CHB cirrhosis can be classified as “accumulation”, “liver accumulation”,“dropsy”, or “jaundice” in TCM[17]. Blood stasis is involved in the whole process of the occurrence and progression of liver cirrhosis and is the pathological basis of the disease. The pestilential pathogens stay in the blood and disrupt the flow of Qi and blood,resulting in stasis of blood and obstruction of collaterals;the physiological function of the liver is affected by the pestilential pathogens, and its ascending and dispersing functions are disordered, resulting in poor blood flow and stasis in the liver collaterals; liver disease transmits to the spleen, and the earth is stagnated and wood is depressed; spleen deficiency prevents the transformation of Qi and blood, and the liver collaterals are not nourished and filled, resulting in stasis due to deficiency; the pestilential pathogens burn and deplete body fluids, and then Yin and blood are insufficient,resulting in stasis[18]. SUN Y J[19]analyzed and compiled information in 337 patients with compensated hepatitis B cirrhosis hospitalized in the Department of Liver,Spleen, and Stomach Diseases, the Affiliated Hospital of Changchun University of Traditional Chinese Medicine between 2014 and 2018. The study showed that 48.2%of the patients were found to have evidence of stasis blocking the liver ligament. This suggests that the syndrome of stasis blocking liver collaterals is the most common syndrome of this disease, and treatment should be based on the main principle of dispersing blood stasis and unblocking collaterals.
Warm needling moxibustion is effective in invigorating blood and Qi, dispersing stasis and unblocking meridians, warming Yang, and invigorating Qi, and is applicable to the syndrome of stasis blocking liver collaterals. Geshu (BL17) is the Influential Point for Blood in the Eight Influential Points, treating liver diseases and blood disorders, and is effective in improving blood circulation, dispersing stasis, regulating Qi, and unblocking vessels. Ganshu (BL18) and Danshu(BL19) are the Back-Shu Points of the liver and gallbladder, respectively and are important points in the treatment of liver and gallbladder diseases. They can regulate the liver and gallbladder, promote Qi, and disperse stasis. Yanglingquan (GB34) is the He-Sea Point of the Gallbladder Meridian and the lower He-Sea Point of the gallbladder. It can regulate the liver and gallbladder, dissipate and discharge Shaoyang Meridians,and treat liver and gallbladder diseases. Sanyinjiao (SP6)is the crossing point of the three Yin meridians of foot and can strengthen the spleen, regulate blood, calm the liver, and tonify the kidney. The above points are combined and complemented by warm needling moxibustion to promote blood circulation and disperse blood stasis, regulate the liver, unblock the collaterals,disperse nodules, and eliminate stagnation.
IL-21 is a cytokine secreted by activated CD4+T cells and NK cells. It has a powerful pro-inflammatory effect,up-regulating IL-6 and IL-1β expression and aggravating inflammatory damage in hepatocytes[20]. Studies showed that elevated serum IL-21 in CHB patients was positively correlated with total bilirubin and negatively correlated with ALB, suggesting that IL-21 can be used as an indicator to determine the degree of liver inflammatory activity, monitor the disease, and judge the prognosis[21]. In addition, IL-21 inhibits the apoptosis of hepatic stellate cells and up-regulates collagen production to accelerate the process of liver fibrosis[22]. PDGF is produced and secreted by vascular endothelial cells, Kupffer cells, etc. PDGF can activate hepatic stellate cells, shorten the cell division cycle of hepatic stellate cells, accelerate the division and proliferation of hepatic stellate cells, and accelerate the migration of newly synthesized cells to the relevant sites.It can also promote collagen production and deposition and induce hepatic stellate cells to secrete a variety of cytokines that aggravate liver damage and promote the generation and progression of liver fibrosis[23-24]. In this study, after treatment, the serum IL-21 level decreased in the control group (P<0.05) and the serum PDGF level did not change significantly (P>0.05), while the serum IL-21 and PDGF levels decreased significantly in the observation group (P<0.05) and were lower than those in the control group (P<0.05). This suggests that warm needling moxibustion combined with entecavir can reduce the serum IL-21 and PDGF levels, and the mechanism of action of warm needling moxibustion combined with entecavir in the treatment of compensated cirrhosis due to CHB may be related to this.
In summary, warm needling moxibustion combined with entecavir treatment can improve liver function,reduce the inner diameter of the portal vein and splenic vein and spleen thickness, reduce liver hardness, and improve liver fibrosis indicators in patients with CHB cirrhosis, which may be related to the reduction of the serum IL-21 and PDGF levels.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
There was no project fund support for this study.
Statement of Informed Consent
Informed consent was obtained from all individual participants.
Received: 16 November 2020/Accepted: 27 April 2021
 Journal of Acupuncture and Tuina Science2022年3期
Journal of Acupuncture and Tuina Science2022年3期
- Journal of Acupuncture and Tuina Science的其它文章
- Effects of electroacupuncture pretreatment on motor function and cerebral blood flow in MCAO model rats
- Effect of moxibustion on N-methyl-D-aspartate receptor subtype 2B expression in hippocampus of rheumatoid arthritis model rats
- Effects of scalp acupuncture plus acupuncture exercise therapy on walking ability in children with spastic cerebral palsy
- Therapeutic efficacy and safety rating of Tui-Pushing chest-back manipulation for children with cough variant asthma
- Clinical observation on moxibustion at Baihui (GV20)plus Tuina for children with postnasal drip syndrome
- Clinical study of warm needling moxibustion plus intra-articular injection of sodium hyaluronate for hip involvement in ankylosing spondylitis
