The evolution of lower extremity reconstruction
Brogan G. A. Evans, David L. Colen
Division of Plastic and Reconstructive Surgery, Department of Surgery, Yale School of Medicine, New Haven, CT 06510, USA.
Abstract Reconstruction of the lower extremity is a complex task that has evolved greatly in both technique and indication over the past century. Early advances in treating traumatic lower extremity injuries focused on primary amputation to avoid the high mortality of infection. The introduction of antibiotics improved surgical debridement and local reconstructive options, enhancing the viability of lower extremities with simple and proximal defects. With the advent of microvascular surgery, free tissue transfer techniques provided a means to reconstruct more distal and complex problems. As these surgical techniques have continued to evolve, so too have indications for reconstruction, patient management and post-operative care-now with a greater emphasis on patient quality of life and limb function. The purpose of this article is to outline the evolution of lower extremity reconstruction, and how the standard of practice has changed over time.
Keywords: Lower extremity, lower extremity reconstruction, limb salvage, free flap, perforator flap, vascularized bone flap, orthoplastic surgery
INTRODUCTION
Traumatic injuries of the lower extremity are complex in nature. Mechanism of injury often predisposes these wounds to contamination, with high rates of infection when not appropriately debrided[1-7]. Prior to the industrial revolution, injuries of the lower extremity were largely sustained on the battlefield secondary ballistic or blunt force trauma[8,9]. High mortality rates from infection made primary amputation the standard of care in treating lower extremity injuries[9,10]. The invention and increasing accessibility of penicillin in the 1940s ushered in a new era, greatly decreasing mortality rates secondary to infection and demonstrating promise for potential limb salvage[10]. As a result, amputation no longer became an obligatory life-saving measure, thus shifting surgical goals from primary amputation to limb salvage.
Methods of lower extremity reconstruction have evolved greatly over the past century, beginning with the understanding of muscle, axial and perforator flaps, as well as skeletal stabilization, and most importantly, microvascular surgery. The ability to transfer healthy vascularized tissue from distant anatomic locations to reconstruct severe trauma made limb salvage a realistic and pragmatic option for the most complex defects[1,3,4,11-19]. At the forefront of microvascular reconstruction was Dr. Marko Godina, who made numerous contributions in reconstructive surgery stemming from his early work in limb salvage management. Since Godina, refinements in technique and indications have continued to shape our understanding of how, when, and why to reconstruct lower extremity injuries.
CLASSIFICATION AND DECISION MAKING
Prior to reconstructive planning of the lower extremity, the surgeon must be able to effectively evaluate an injury and determine its candidacy for salvage. In 1976, Gustilo and Anderson developed the most widely used classification system for open fracture-comparing the force of impact, the extent of soft-tissue injury, and degree of wound contamination in a retrospective review of 1025 patients[6]. Fractures were then categorized into three types, with higher rates of infection and complications noted with increasing severity[5,6].
Management of lower extremity open fractures was widely adopted based on Gustilo’s original classification system in 1976. While Type I and II injuries demonstrated predicable outcomes, Gustilo observed varying degrees of prognosis in Type III injuries. In 1984, Gustilo further subdivided Type III injuries based on adequate soft tissue coverage (IIIA), periosteal stripping (IIIB), and limb ischemia necessitating vascular repair (IIIC)[20].
The varying complexity of lower extremity trauma has driven authors to expand on Gustilo’s work and refine their implications[21]. Many adaptations have since been proposed to better categorize injuries with portending worse outcomes based on the extent of vascular injury and degree of soft tissue deficit, notably > 10 cm[1,22-27]. Concerted data has since demonstrated increasing rates of infection, non-union, and flap failure in injuries with worse vascular injuries, further emphasizing the importance of distal limb perfusion in functional limb salvage[24-26].
While numerous studies have proposed modifications to the Gustilo classification system, it remains the most widely used open fracture classification system today. Its framework allows surgeons to effectively communicate across multidisciplinary teams and reasonably predict patient outcomes in lower extremity reconstruction.
MARKO GODINA’S METHOD
Marko Godina was a pioneer in reconstructive microsurgery, shaping the field with his many contributions despite his career being tragically cut short in 1986[2]. In his seminal work, Godina showed that with aggressive debridement and early soft tissue coverage (emergent free flap), reliable outcomes could be achieved. Additionally, Godina emphasized the correlation of successful limb salvage with surgeon experience and multidisciplinary care, noting an increase in his free flap success rate with increased experience and familiarity with his team[2,3,28,29].In his thesis, reviewing 826 free flaps, Godina found that only 1% of patients developed an infection when acutely debrided and reconstructed within 72 hours, with a flap failure rate of 0.75%. Conversely, when reconstruction was delayed beyond 72 hours, the flap failure rate was noted to be 8%-12%, with an infection rate of 9%-18%[2]. While optimal timing in extremity reconstruction has evolved throughout the years, Godina’s principle of early intervention survives as a principal tenet in extremity reconstruction.
Godina additionally emphasized the importance of preserving vascular patency to the distal extremity. While adequate perfusion can be met with a single vessel runoff in the lower extremity, Godina encouraged the use of end-to-side anastomoses in his reconstructions to ameliorate the risk of vascular insufficiency[28,30]. Although complications rates are equivalent between end-to-end and end-to-side anastomosis, Godina focused on preserving maximum perfusion when able[15,19].
While the plastic surgeon’s toolbox and flap selection have expanded largely since Godina’s time, Godina performed many of his free tissue transfers based on the subscapular axis[30]. The patient was placed in the lateral decubitus position, with posterior access utilized in dissecting out recipient vessels within the lower extremity. Godina advocated for beginning dissection of recipient vessels outside the zone of injury and dissecting distally to the first evidence of pathology. Although fallen out of favor for other modalities, Godina believed that all anastomoses should be done proximal to the zone of injury, and that an arterial autograft should be utilized to bridge gaps within the zone of injury[30-36].
Godina’s flap selection was limited by his time. Soft tissue coverage was typically achieved with free muscle flaps with skin grafting or, less frequently, myococutaneous flaps[3,37]. Moreover, muscle flaps were believed to be a highway for antibiotic therapy to bathe contaminated wounds, making them preferential in the reconstruction of traumatic injuries[12,16,17,38,39]. Today, fasciocutaneous and perforator flaps are exceptional flap options for reconstruction of the lower extremity and demonstrate less donor site morbidity when compared to muscle flaps[40-45]. Ultimately, as advancements in lower extremity reconstruction continue to emerge, it is evident that the “Godina Method” remains at the foundation of reconstructive microsurgery[46,47].
BUILDING ON GODINA’S FOUNDATION-INNOVATIONS IN LOWER EXTREMITY RECONSTRUCTION
While Godina advocated for early debridement and coverage of injuries within 72 hours, surgeons have continued to investigate optimal timing for extremity reconstruction. Time to coverage has since been refined, with multiple authors showing improved outcomes with early soft tissue coverage extending to 7-10 days[1,2,23,48,49]. Overall improvements in infection rates, bony union and flap success have demonstrated the utility in delaying reconstruction to an urgent setting (7-10 days), emphasizing the importance of serial debridement of non-viable tissue and preparing an adequate wound bed prior to functional limb salvage.
In 2000, Gopalet al.[4], introduced the “fix and flap” method in which lower extremity traumatic injuries were treated via a combined orthopedic and plastic surgery approach[50]. The authors suggested treating lower extremity injuries in a single stagged procedure in which early radical debridement, skeletal stabilization, and soft tissue coverage were performed. Results demonstrated favorable outcomes for surgeries performed within 72 hours of injury and comparable data compared to stagged reconstruction. Overall, timing in lower extremity reconstruction remains at the surgeon’s discretion. The literature appears concerted that early debridement in the acute setting is critical to decreasing complication rates, and reconstructive efforts should be ideally performed prior to 10-14 days[1,3-6,23,48,49,51].
WOUND TEMPORIZATION - DEVELOPMENTS IN SUBACUTE THERAPY UNTIL DEFINITIVE RECONSTRUCTION
Reconstructive efforts in unstable patients with traumatic lower extremity injuries are contraindicated until cleared by Advanced Trauma Life Support practice management. In these patients, wounds can be temporized with devices such as negative pressure wound therapy (NPWT), dermal matrices, and external delayed primary closure devices (e.g., DermaClose, Jacob’s Ladder, Shoe-String Method)[50,52-60].
NPWT was introduced in 1996 as a method for delayed wound closure, in which an open-cell polymer foam is placed within a wound bed and subjected to negative pressure to promote wound contracture and granulation tissue formation[61]. Since its introduction, evidence has shown that NPWT can effectively temporize and shrinks wounds, as well as assist in converting full-thickness wounds with exposed bone or tendon into a granulated wound bed for skin grafting[18,53,62,63]. However, in the contaminated field or areas of severe soft tissue defects, indications are limited. While NPWT has been shown to improve overall wound hygiene, it does not definitively decrease bioburden or infection rates, and is not a substitute for early operative debridement when able[22,64,65]. Newer versions of NPWT include the instillation of irrigation to continually cleanse contaminated wounds[56-58,66-68]. Instillation solutions vary widely, with studies demonstrating comparable efficacy amongst solutions, suggesting a utility in the process of irrigation rather than the solution itself[67]. Overall, the adjuvant of an instilling NPWT can help change a static wound to a variable environment, which may ultimately help cleanse contaminated wound beds.
In addition to NPWT, the utilization of acellular dermal regenerative templates, such as Integra, has provided surgeons with an additional tool to temporize and close wounds secondarily. These dermal matrices are composed of a bilaminate sheet of cross-linked bovine tendon collagen and shark glycosaminoglycans, which serve as a collagen scaffolding for the growth of a neodermis[50,55,69]. Wounds of the lower extremity that would previously be treated with free flap reconstruction can now potentially be closed with Integra application and skin grafting following 3-4 weeks of neodermis development. While dermal matrices can be a useful tool in soft tissue reconstruction, their overall efficacy remains poor in contaminated wound beds[69].
ORTHOPEDIC ADVANCEMENTS IN SKELETAL STABILIZATION AND BONEY DEFECTS
Traumatic lower extremity wounds are inherently contaminated. Open fractures should be managed with the initiation of intravenous antibiotics and washout within 6 hours. Severe open fractures such as Gustilo IIIB or IIIC injuries, may result in large bony defects or a grossly contaminated wound in which immediate internal fixation with hardware is contraindicated. In these injuries, antibiotic-impregnated cement is commonly used as temporization[70-73]. While first described in the 1970s, antibiotic-impregnated cement continues to be routinely used to eliminate dead space and elute antibiotics at high local concentrations to decrease bacterial burden in contaminated wound beds[71,74]. Prior to definitive skeletal fixation, or flap coverage, the beads are removed.
Skeletal defects of the lower extremity offer a unique challenge. Autologous bone grafting can provide structural cortical bone and osteogenic potential to fill smaller defects. For larger defects, modalities such as limb shortening and distraction osteogenesis are effective but morbid and inherently complex[75]. By convention, bony defects greater than 6cm are largely reconstructed with vascularized bone graft. While this convention has been largely adopted, Allsoppet al.[76], determined that this indication is not evidence-based. Today, a variety of techniques have gained traction in reconstructing complex boney defects of the lower extremity.
Distraction osteogenesis has proven to be a reliable method for repairing segmental bony defects measuring up to 10 cm[75]. In the Ilizarov technique, first described in the 1950s, distraction is achieved via placing an external fixation device with carefully planned corticotomies to preserve blood supply[77]. Following an initial latency phase of 3-10 days, gradual distraction of the proximal and distal segments is achieved at an average gain in length of 1cm every 30 days. Upon completion of distraction, the newly formed bone within the distraction gap is allowed to bridge and corticalize via a consolidation phase. Numerous frames and external fixation devices have since been described to improve techniques in distraction osteogenesis. The Taylor spatial frame is a modern modification to the traditional Ilizarov technique, in which a multiplanar external hexapod frame is used to improve versatility in correcting rotation, angulation and translation of bony deformities[75,78].
Autologous nonvascularized bone grafting is largely considered the gold standard in repairing small bony defects of the lower extremity. Cancellous bone can be accessibly harvested from iliac crest, femur, or tibia, and grafted into the defect for repair[75]. Additionally, smaller corticocancellous bone flaps, such as the medial femoral condyle flap, have also demonstrated efficacy in repairing small defects in post-traumatic non-unions[79-82][Figure 1]. In the reconstruction of large bony defects, significant structural and weightbearing support is needed for functional limb salvage[75,83]. Historically, these defects were reconstructed with large cadaveric allografts, as vascularized bone flaps were associated with prolonged immobilization and early fracture. While allograft reconstruction has proven successful in limb salvage, it is associated with a higher incidence of infection and non-union when compared to vascularized bone grafts[75,84-88]. The first vascularized bone grafting was described in 1905 when a pedicled fibular graft was utilized to fill a large tibial defect[85]. In 1975, the first microsurgical vascularized bone graft was performed, using a fibula to fill a large tibial defect in the contralateral leg[88]. While the fibula remains one of the most commonly used vascularized bone flaps for repairing large bony defects, multiple adaptations have contributed to its success in lower extremity reconstruction. One of these adaptations is the Capanna Technique, which was first described in 1993. The Capanna technique combines the advantages of allograft structural support and vascularized bone graft osteogenesis. In this technique, the free fibula acts as an intramedullary rod within an allograft conduit, and is used to reconstruct large boney defects to provide early structural integrity and decreased rates of non-union and infection[75,83,89,90].

Figure 1. Vascularized bone flaps for lower extremity reconstruction of bony defects. (A) free fibula osteocutaneous flap. (B) in situ medial femoral condyle flap isolated on descending geniculate vessels for the reconstruction of a small bony defect.
In addition to reconstruction with bone grafting, several autologous and allograft products are available to help augment fracture healing[75]. Platelet-rich plasma, bone marrow mesenchymal stem cells, and adiposederived stem cells, are examples of autologous therapies that can be used to promote wound healing in areas of traumatic injury. These therapies work similarly to polarize M2 macrophages and upregulate key growth factors such as transforming growth factor β (TGFβ), vascular endothelial growth factor, and fibroblast growth factor within the wound bed[91]. The newly polarized M2 macrophages and increased levels of growth factors work synergistically to augment wound healing by reducing inflammation, inducing collagen synthesis, and promoting angiogenesis. Similar to autologous options, allograft material such as bone morphogenic protein (BMP), demineralized bone matrix, and ViviGen are additional adjuvant therapies that can be used to promote fracture healing. BMP contains functional growth factors to promote bone regeneration via osteoinduction, whereas demineralized bone matrix acts as a scaffold to promote bone formation via osteoconduction[90]. Vivigen is a unique cellular allograft made up of three components to promote bone formation: Lineage committed bone cells to induce osteogenesis, a natural bone scaffolding to promote osteoconduction, and growth factors to promote osteoinduction. Overall, advancements in these adjuvant therapies may help further decrease complication and non-union rates following lower extremity trauma fixation.
THE EVOLUTION OF OPERATIVE PLANNING AND FLAP SELECTION
Early reconstructive options in lower extremity trauma relied heavily on physical exam and duplex Doppler ultrasound for surgical planning. Formal arteriography was utilized to evaluate vascular patency in lower extremities injuries concerning ischemia, however, not routinely used for surgical planning[20,92]. Upon surgical exploration, the traumatic nature of these injuries often distorts soft tissue planes and anatomic landmarks, making the determination of adequate recipient vessels difficult within the zone of injury. Today, computed tomography (CT) angiography is routinely used as a minimally invasive way to evaluate distal limb perfusion and target recipient vessels[92]. In early reconstructive efforts, it was believed that flap anastomoses should be performed with a recipient vessel proximal to the zone of injury[3,28,32,92]. Kolkeret al.[31], determined that there was no difference in complication rates for flaps based on vessels proximal, or distal to the zone of injury. With the knowledge that distal vessels can be adequate targets in lower extremity reconstruction, CT angiography allows the surgeon to effectively look at distal patency in vessels that pass through the zone of injury.
Flap selection in lower extremity reconstruction had changed considerably since 1854, when Hamilton first described the use of a cross-leg flap for the treatment of chronic lower extremity wounds[8]. In the late 1890s and early 1900s, pedicled muscle flaps and vascularized bone flaps began to emerge as a useful tool in reconstructing soft tissue and boney defects[85,93,94]. In the 1960s-1970s, microsurgical options in extremity reconstruction emerged, with the first successful lower extremity free tissue transfer performed by Daniel and Taylor in 1971[95].
With the emergence of microsurgical techniques, free muscle transfers with skin grafting and myocutaneous free flaps soon became the gold standard for lower extremity reconstruction[2,3,12-14,31,44,46,51]. Severe soft tissue deficits of the lower extremity that were once considered too distal, or too large for pedicled flap reconstruction, could now be covered with free tissue transfer.
In the late 1980s, the advent of the perforasome theory and perforator flaps further expanded flap options for lower extremity reconstruction. New fasciocutaneous and muscle-sparing myocutaneous flaps proved not only reliable in covering soft tissue deficits, but also demonstrated decreased donor site morbidity and improved cosmesis[12,40,41,43][Figure 2].

Figure 2. Perforator flap for lower extremity reconstruction. (A) Pre-operative markings of an anterior lateral thigh (ALT) flap based on three perforating vessels from the descending lateral femoral circumflex pedicle. (B) ALT fasciocutaneous flap with dissected perforating vessels. (C) Large skin paddles can be successfully elevated based on perforators for the reconstruction of soft tissue defects in the lower extremity. (D) ALT flap inset with preferential end-to-side anastomosis.
As a result of the perforasome theory, vascular mapping of perforators has developed numerous additional options for free tissue transfer and local tissue rearrangements in the lower extremity[43,46,96,97]. Perforatorbased local flaps have gained favor in reconstructing small soft tissue defects of the lower extremity. Numerous perforators exist within the lower limb for flap harvest, with a study by Morriset al.[98], demonstrating 93 perforators in 21 distinct territories for use. Increasing understanding of vascular perforators in the lower extremity has allowed local perforator flaps, such as the propeller and keystone flap, to not only replace like with like, but also reconstruct soft tissue defects that would previously require free tissue transfer[99,100][Figure 3].
Advances in microsurgery have placed a greater emphasis on the importance of decreasing donor site morbidity and reducing patient harm. In addition to utilizing perforasomes and fasciocutaneous flaps, peripheral nerve blocks, epidurals and local anesthesia have proven to be effective alternatives to general anesthesia for select patients[101-103]. Reconstruction of the lower extremity often necessitates multiple surgical procedures and long operative times for patients. Successful free tissue transfer and local flaps under nerve block may provide reconstructive options for patients who would otherwise be unable to tolerate general anesthesia. Additionally, without the need for endotracheal intubation, and airway protection, utilization of nerve blocks may allow for patients to maintain adequate nutrition, which is often interrupted with serial debridement and reconstructive efforts in traumatic injuries [Figure 4].
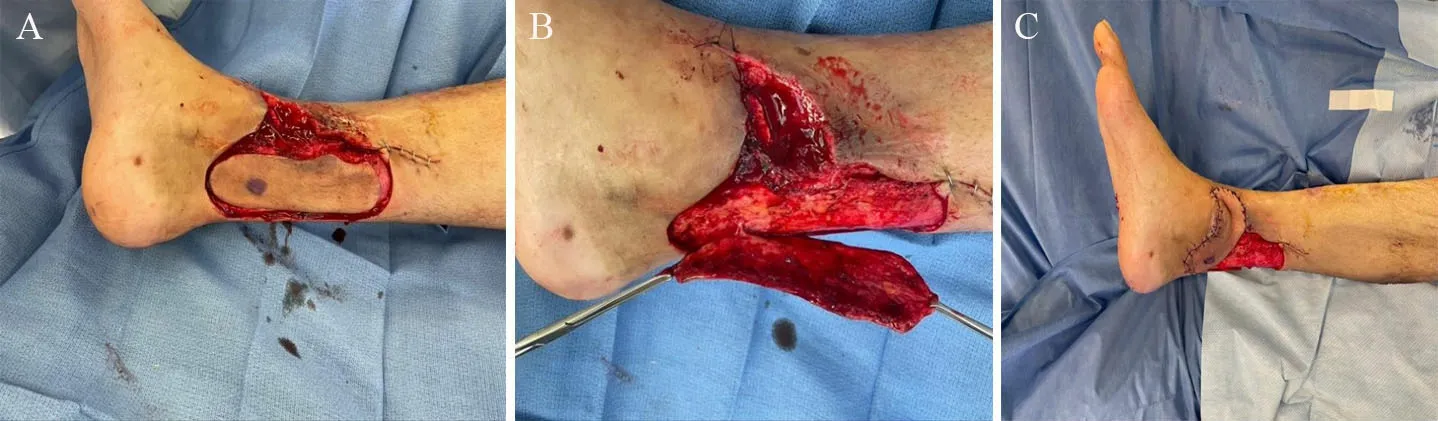
Figure 3. Propeller flap for local flap reconstruction of a lower extremity soft tissue defect. (A) Isolated propeller flap based on a perforator from the posterior tibial vessels for the reconstruction of an anteromedial soft tissue defect. (B) Raised propellor flap with isolated perforating vessels. (C) 90-degree rotation of flap with a successful inset for coverage of anteromedial defect. The donor site can be covered with a split-thickness skin graft or dermal matrix at the time of inset.
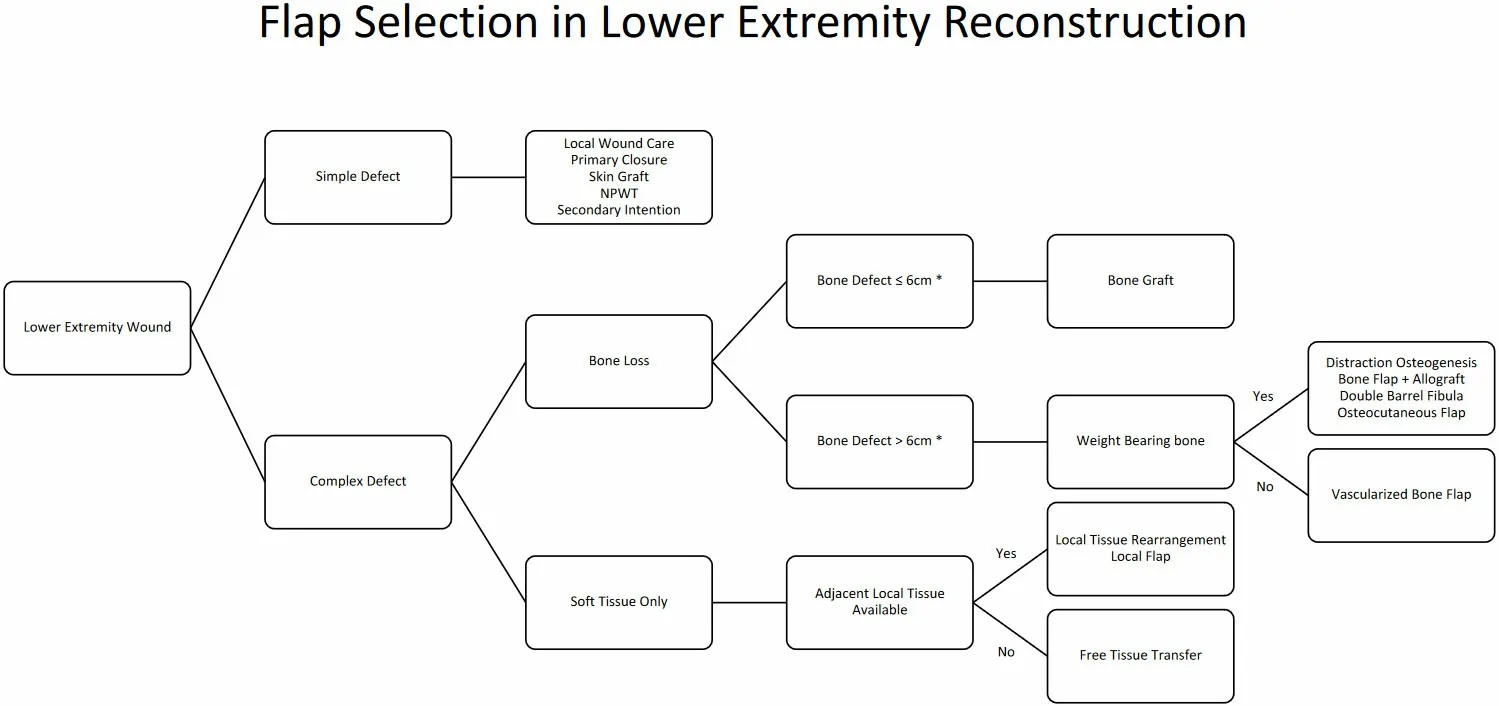
Figure 4. Flap selection algorithm for lower extremity reconstruction: Simple defects defined as wounds with healthy wound beds, sufficient tissue laxity, and absence of exposed hardware, tendon, bone, or neurovascular structures. Complex defects defined as open fractures, wounds with soft tissue deficit not amendable to primary closure, and wounds with gross contamination, exposed hardware, bone, tendon, or neurovascular structures.
NOT ALL LIMBS NEED SALVAGE-A FOCUS ON FUNCTIONAL OUTCOMES AND PATIENT QUALITY OF LIFE
Although innovations in microsurgery and skeletal stabilization have allowed surgeons to reconstruct injuries that would otherwise be amputated, outcomes are not always favorable. Limb salvage often requires several surgeries and prolonged physical therapy, often plagued by high cost, infection, and functional complications[104-108]. In an effort to evaluate patient outcomes following limb salvage, literature has demonstrated substantial physical, mental, and financial hardships that can follow these heroic attempts. Thus, while early management of lower extremity injuries emphasized reconstructive options, the paradigm has now shifted to focus on maximizing quality of life.
One of the most influential series investigating limb salvage versus primary amputation is the Lower Extremity Assessment Project (LEAP)[107]. As part of this multi-institutional series, a study published in 2009 demonstrated primary amputation after lower extremity trauma resulted in lower complication rates compared to limb salvage, with no statistically significant difference in self-reported health status and functional outcomes between both groups[106]. A portion of the limb salvage group (4%) went on to amputation as a result of complications (e.g., infection, osteomyelitis, non-union, etc.), compared to 5.4% of patients in the primary amputation group that required a revision amputation surgery.
In analyzing long-term outcomes for these patients, a 7-year follow-up demonstrated no statistically significant difference in return to work for primary amputation versus limb salvage patients. Moreover, patients who received soft tissue only reconstruction and primary below-knee amputations reported lower severity scores of their injuries than those who underwent both bone and soft tissue reconstruction for limb salvage[109].
While the LEAP series does not objectively outline which patient should receive limb salvage versus primary amputation, it does present comparable functional outcomes and subjective injury severity scores between both groups[104,108-110]. Overall, it is evident that severe lower extremity trauma is debilitating for patients regardless of attempted limb salvage or amputation. These injuries are often met with poor functional outcomes, complication rates, and chronic pain. Reconstructive options have greatly improved in functional limb salvage; however, it is apparent that greater emphasis is needed on post-operative care and patient rehabilitation.
In addition to analyzing the quality of life for primary amputation versus limb salvage, healthcare-associated costs should also be considered prior to reconstruction. In a study by MacKenzieet al.[111], it was estimated that the cost of the first two years following injury was comparable between primary amputation ($91,105) versus limb salvage ($81,996). In analyzing life-time cost, MacKenzieet al.[111]determined primary amputation costs to be substantially higher than limb salvage when factoring in costs for a new prosthesis, maintenance, and medical care ($509,275 versus $162,28, respectively). Chunget al.[112], demonstrated similar findings, with 40 years of life remaining cost estimated to be $350,465 for primary amputation and $133,704 for limb salvage.
CONCLUSION
Lower extremity reconstruction has evolved tremendously in a short few decades. Innovations in microvascular surgery, skeletal fixation, and patient management have contributed greatly to the ability to care for patients with traumatic injuries. While operative techniques continue to expand, a greater emphasis is needed on improving long-term outcomes in these patients. The authors believe that future efforts to improve physical rehabilitation, chronic pain, and minimize costs, are key factors in preserving limb function and patient quality of life following lower extremity trauma.
DECLARATIONS
Authors’ contributions
Primary author, performed review of manuscripts and organization of article flow: Evans BGA
Secondary author, provided administrative organization, editing and photographs for figures: Colen DL
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Both authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for Publication
Not applicable. All photos have been de-identified, with no identifying tattoos, or descriptions that may lead to patient identifications (e.g., date/time, location of surgery, mechanism of injury). Additionally, all photos submitted in this review were obtained via a photo consent, completed by Dr. Colen and the patient prior to surgery. This includes consent for use in articles/journals/lectures.
Copyright
© The Author(s) 2022.
 Plastic and Aesthetic Research2022年5期
Plastic and Aesthetic Research2022年5期
- Plastic and Aesthetic Research的其它文章
- Ehlers-Danlos syndrome: prevalence and outcomes in gender affirming surgery - a single institution experience
- Soleus muscle flap for reconstruction of lower extremity trauma. Workhorse or glue factory?
- Current concepts in microsurgical soft tissue reconstruction of lower extremity trauma in a singlevessel extremity
- Peripheral nerve allograft: how innovation has changed surgical practice
- AUTHOR INSTRUCTIONS
