Current concepts in microsurgical soft tissue reconstruction of lower extremity trauma in a singlevessel extremity
Jenny L. Yu, Philip D. Tolley, Cameron Kneib, Erin A. Miller, Christopher S. Crowe
Division of Plastic Surgery, University of Washington, Seattle, WA 98104, United States.
Abstract Advancements in microsurgical techniques have allowed for the salvage of extremities that would otherwise be amputated. Despite these improvements, mangling injuries of the lower extremity with concomitant open fracture continue to present a challenge for reconstructive surgeons. These injuries are further complicated by circumstances in which a single vessel is perfusing the foot after trauma (Gustilo 3C injuries). Although the foot may remain perfused in this instance, free flap coverage of open fractures with concomitant vascular injury requires careful planning to maintain a viable foot. The surgeon must adapt surgical plans based on both preoperative and intraoperative findings. In this review, we discuss current concepts and reconstructive techniques for performing free flap reconstruction in single-vessel lower extremities.
Keywords: Lower extremity, reconstruction, microsurgery
INTRODUCTION
Soft tissue reconstruction of lower extremity trauma comprises a wide range of management strategies that depend on the condition and presence of critical structures within the wound. Gustilo III injuries requiring free flap coverage represent the highest tier of the reconstructive ladder, and deserve careful consideration by the reconstructive surgeon[1]. Several factors must be noted when planning free tissue transfer for large open fractures, many of which relate to the injured limb itself: the mechanism of injury and corresponding zone of injury (i.e., lowvs.high energy trauma), which tissues are missing, degree of contamination, presence of hardware, and need for additional fixation. A number of patient-related factors must also be evaluated prior to surgery: medical comorbidities, social support, participation in rehabilitation and postoperative care, and patient goals of care. The decision of amputationvs.limb salvage must be tailored to each individual case and should be a multidisciplinary discussion with the patient. Risk factors for amputation, such as compartment syndrome resulting in myonecrosis, prolonged warm ischemia time, and severe open injury to the hindfoot, should be identified and discussed[2]. An amputation may represent the most functional treatment plan and should be considered by the patient and treatment team during the discussion of limb salvage[3]. There have been numerous attempts at developing injury scoring systems to aid in predicting the need for amputation, such as the Mangled Extremity Severity Score, the Predictive Salvage Index, and the Limb Salvage Index, but there is no scoring system that has been validated in large prospective studies[2]. If amputation is to be avoided, limb salvage comprises both durable osseous support and a stable soft tissue envelope for return to weight-bearing and ambulation.
Traumatized lower extremities with a single perfusing vessel across the ankle mortise (i.e., Gustilo IIIB in patients with peripheral vascular disease and Gustilo IIIC injuries) present an even more challenging reconstructive dilemma. Although these extremities are not necessarily dysvascular, microsurgical treatment requires a greater degree of planning. A collaborative “orthoplastic” approach is even more important in these cases[4]; while soft tissue reconstruction is often the final step of treatment addressed for patients with significant extremity trauma, early collaboration with the plastic surgery team for debridement, timing for surgery, and surgical planning have been shown to improve outcomes[5-7].
Historically, large open fractures devoid of soft tissue and periosteum were previously not amenable to salvage; however, advances in microsurgical technique, flap selection and design, and improved understanding of lower extremity anatomy and perfusion in recent decades have resulted in the successful reconstruction of these mangled limbs. Contemporary rates of successful lower extremity free flap coverage are reported as 91% to 97%. This rate falls to as low as 80% in extremities in the presence of arterial injury and thus is the focus of this discussion[8-14]. With careful planning and a methodical approach to reconstruction, salvage of complex lower extremity wounds with single vessel perfusion can be achieved.
PREOPERATIVE EVALUATION
Open fractures can be distracting features of the trauma patient’s presentation. It is critical to remember that these injuries usually represent a high-energy mechanism associated with a number of concomitant injuries[15,16]. A comprehensive trauma evaluation, including the primary, secondary, and tertiary surveys with appropriate diagnostic imaging, should be completed in addition to addressing the injured limb. When appropriate, early transfer to a higher level of care should be considered[17]. On initial presentation, the appearance and hemostasis of the wound should be noted. Photographs of the wound should be taken for documentation in the medical record and for communication with the reconstructive team if they are not present at the initial evaluation.
A careful and thorough neurovascular exam is critical as concerns about limb perfusion should prompt a vascular surgery consult for emergent revascularization. Dorsalis pedis and posterior tibial pulses should be evaluated by palpation, and if not palpable, a handheld Doppler probe should be used to verify distal perfusion. A baseline motor and sensory nerve exam should be documented. An ankle-brachial index (ABI) should be performed for all open fractures of the lower extremity. Abnormal pulses, pulsatile bleeding, or an ABI < 0.9 indicate that additional vascular imaging, such as arteriography or computed tomographic angiography (CTA), should be obtained[2]. At our center, CTA is used to evaluate for vascular injury and for preoperative planning as the equipment is readily available and scans can be obtained quickly.
Vascular imaging for patients with lower extremity trauma is typically obtained in one of two contexts. The first is at the time of initial presentation if a vascular insult is suspected based on the physical exam. If perfusion is intact, debridement and preliminary orthopedic fixation are performed in a timely manner. The final reconstructive team may decide the utility of preoperative imaging for surgical planning if one has not already been obtained[18,19].
One limitation of CTA is the presence of hardware that may distort or obscure vessel path and patency. Nonetheless, CTA may provide valuable information when planning microvascular reconstruction [Figure 1]. When imaging or exam concerns limited perfusion to the leg, planning of free tissue transfer should consider the principles discussed below.

Figure 1. CT Arteriogram of a patient with an open right tibia-fibula fracture, and large soft tissue eschar and necrotic subcutaneous tissue. CTA identified a single-vessel extremity with thrombosed anterior tibial vessels. The patent posterior tibial vessels were ultimately used for flow-through free flap reconstruction. CTA: Computed tomographic angiography.
SURGICAL PRINCIPLES FOR SINGLE-VESSEL FREE FLAP RECONSTRUCTION
General considerations
Reconstruction of the lower leg follows the same principles as reconstruction in other anatomic zones. The “reconstructive ladder” and subsequent iterations advise balancing complexity and durability[20]. Especially in Gustillo III injuries, plans for future reoperations should be considered. If the orthopedic team needs repeat access to the fracture site or hardware, more robust tissue should be selected instead of the lowest rung on the reconstructive ladder. In the setting of the significantly traumatized leg with single-vessel distal perfusion, a surgical plan must also account for perfusion of the transferred tissue in addition to ensuring continued flow to the foot.
Recipient vessel selection
Selection of recipient vessels depends on several important factors: the location of the defect, the extent of the zone of injury, exposure of vessels within the wound bed, and known patency of the lower extremity vessels on CTA[9,21,22]. Single-vessel perfusion to the distal leg or foot precludes an end-to-end anastomosis using the remaining inflow vessel as this would result in ischemia distal to the flap.
We recommend dissecting a sufficient length of artery and vein(s) (> 4 cm) to allow for ample mobility of the vessels. All major branches should be preserved until the final anastomotic plan is determined. Once vessels are prepared, the recipient artery should be clamped to confirm that distal perfusion is adequate. This can be assessed by examining for a palpable pulse or Doppler signal. Sufficient perfusion may also be confirmed by retrograde flow from the distal end of a transected vessel; however, this requires division of the vessel, which is an irreversible step. As venous insufficiency is rarely problematic in these limbs, veins can be divided distally and reflected proximally to allow for subsequent end-to-end anastomosis.
End-to-end anastomoses using transected or injured vessels
The injured anterior or posterior tibial artery is one option for a recipient vessel. Antegrade or retrograde flow can potentially be utilized proximal or distal to the area of injury, respectively. The primary advantage of this recipient is that an end-to-end anastomosis can be performed without disturbing perfusion to the distal extremity. Should flow through the transected vessel prove inadequate, the uninjured vessel is still available as a backup. The primary limitation of using the injured vessel is that the recipient vessel is far more likely to be within the zone of injury and, therefore, may be thrombogenic, friable, and prone to vasospasm. Proximal dissection (or distal in the instance of retrograde flow) outside the zone of injury is critical but wide dissection to find adequate inflow may necessitate a longer flap pedicle.
End-to-side anastomoses using an uninjured vessel
If the injured vessel is unusable or if vessels have been chronically occluded, another option is to perform an end-to-side anastomosis on the remaining viable artery. This creates a surgical branch point in the vessel that redirects a portion of blood flow to the newly transferred flap while maintaining circulation through the distal extent of the vessel [Figure 2A]. This has been found to be a reliable anastomotic technique with no significant difference in rates of free flap failure or vessel patency when compared to end-to-end[23-25]. End-to-side anastomoses are, however, more technically challenging to perform than end-to-end. They require meticulous microsurgical skills, and if there is any indication of clotting at the anastomosis, there should be a low threshold to revise the anastomosis.
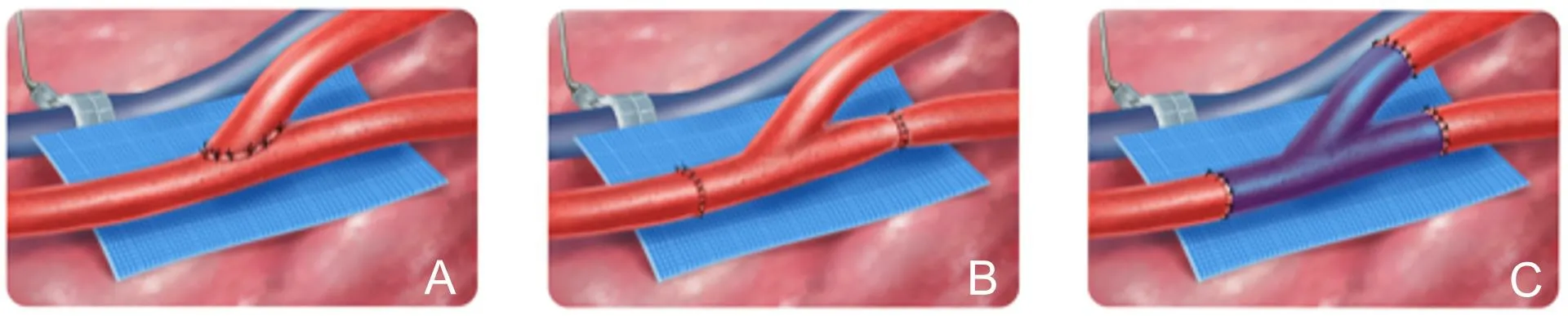
Figure 2. A: End-to-side anastomosis; B: flow-through anastomosis; C: branched interposition vein graft.
Several methods for performing an end-to-side have been described[26-28]. There are technical considerations that can improve the success of an end-to-side anastomosis. Sufficient proximal dissection of the vessel is important as being out of the zone of injury is even more critical in an end-to-side procedure. When setting up the orientation of the pedicle, the angle of the flap artery off of the recipient artery should be arranged to be a more gentle “V” rather than a 90-degree angle in order to promote linear flow. Blood flow may be stopped in the recipient artery using either a Satinsky clamp or Acland clamps per surgeon preference. Additional caution should be used in severely atherosclerotic vessels. A Satinsky clamp should be avoided as the calcium plaques on the vessel may fracture, and Acland clamps frequently do not provide enough pressure to occlude these very stiff vessels. We prefer to use vessel loops and a red rubber catheter as a Rummel tourniquet to atraumatically stop inflow during anastomosis in these cases.
Care should be taken to create the appropriate size arteriotomy - an arteriotomy that is too large will cause the flap vessel to take on an oval shape which predisposes to backwalling during anastomosis. In smaller recipient arteries, a stay stitch may be used to help the assistant retract the side of the arteriotomy and provide adequate visualization for the surgeon. Sutures should be placed closer together at the “heel” of the anastomosis, as these are the points that are most likely to leak, and a rescue stitch in this location is quite difficult to visualize. We find these sutures are best taken in two steps to ensure the appropriate angle. After restoring flow through the vessel, the pedicle is frequently vasospastic and may require additional adventitial stripping. Allowing the flap to re-perfuse for several minutes before restoring perfusion to the foot may help diminish this vasospasm.
Flow-through flap
Flow-through free flaps are a valuable technique for lower extremity reconstruction in the setting of vascular injury. A flow-through flap is characterized by anastomosis at two sites along the recipient artery using a segment of flap artery as an interpositional graft [Figure 2B] to reconstruct the recipient artery. The blood flow enters the flap through a native branch at an anatomic angle. Historically, flow-through flaps were performed for simultaneous reconstruction of soft tissue and vascular defects. Soutaret al.[29]initially described a flow-through radial forearm free flap for head and neck reconstruction, which preserved blood flow through the facial artery. This technique was subsequently expanded to include the reconstruction of extremities with segmental vessel loss[30].
This technique is well-suited for free flap coverage of single-vessel extremities. In general, two end-to-end anastomoses tend to be less technically demanding compared to a single end-to-side anastomosis. Additional benefits are that a segment of injured or unhealthy vessels can be replaced with healthy vessels, and dissection does not need to be carried as far out of the zone of injury. The flow-through flap can also be used as a second attempt for the reconstruction of a previously unsuccessful end-to-side anastomosis with excision and interpositional reconstruction of the perfusing vessel.
Performing a flow-through flap requires appropriate anatomy from the donor - the perfusion to the flap needs to come off a branch of a larger segment of expendable artery. Three commonly used flow-through flaps used in lower extremity reconstruction include the latissimus dorsi muscle, anterolateral thigh fasciocutaneous, and radial forearm fasciocutaneous flaps.
The latissimus dorsi flap has several options as a flow-through flap. The largest caliber flow-through vessel comes from the continuation of the circumflex scapular artery from the subscapular artery; the thoracodorsal branch comes off this intervening segment. The subscapular is used as the proximal anastomosis and the circumflex as the distal when transplanted to the leg. During pedicle dissection, the serratus branch is kept intact as a backup flow-through option. If aberrant anatomy is encountered, the serratus branch and proximal thoracodorsal can be used as the flow-through segment, with the distal thoracodorsal preserved for flap perfusion. A flow-through latissimus dorsi flap for reconstruction in a traumatized lower extremity with single-vessel runoff is demonstrated [Figures 3 and 4].
For the flow-through ALT flap, the descending branch of the lateral femoral circumflex artery can be taken distal to the site of perforator takeoff and used as the distal flow-through segment. This creates a long length to reconstruct a vessel with a large zone of injury. The length of the total flow-through segment is dependent on the position of the most distal perforator being utilized. A shorter flow-through segment can be created with the ALT flap by taking an additional length of the rectus branch, as this vessel is frequently quite large in caliber and can serve as the distal anastomotic site.
For the radial forearm fasciocutaneous flap, the radial artery acts as the flow-through vessel, and the proximal and distal ends are anastomosed to the recipient vessel. The perforators branching from the radial artery provide blood supply to the fasciocutaneous flap.
There are several disadvantages to the use of flow-through flaps. There is a risk that potential thrombosis could cause distal perfusion/limb viability issues when operating on the only vessel providing blood flow to the distal extremity. There is a theoretical risk of steal syndrome. With two sites of vessel anastomoses, there are two sites of potential thrombosis, which could lead to either flap or foot ischemia. Frequently, one site of anastomosis has a vessel mismatch and requires precise suture placement for correction. Awareness of these pitfalls prompts the surgeon to use a meticulous technique, and at our institution, we have found flowthrough flaps to be very reliable.
Vein grafts
If there are no viable recipient vessel sites within a reasonable distance of the defect, which can be the case with a large zone of injury, vein grafts are required to bring inflow from healthy proximal vessels. This is most often done using an arteriovenous (AV) loop, which can be performed as a single or two-stage procedure[31,32]. The two-stage procedure has a higher graft occlusion rate and lower limb salvage rate, and thus, the single-stage AV loop is recommended[31-33]. AV loops are reliable for providing inflow when none is present distally - Momeniet al.[34]showed no difference in reconstructive outcomes between traumatic lower extremity wounds reconstructed with a single-stage AV loop and those without an AV loop in a pairmatched study.
In creating an AV loop in the lower extremity, the greater saphenous vein is most commonly used. If enough length is able to be obtained distally, the saphenous vein may be used in situ: the distal end is mobilized and reflected proximally to anastomose with the femoral artery, necessitating only one anastomosis. When a greater length is needed, or the more distal vein is in the zone of injury, the contralateral saphenous may be harvested and used as a free vein graft to connect to the femoral artery and femoral vein in the adductor hiatus. The anastomosis to the femoral artery may be achieved in an end-toside fashion or end-to-end with a branch of the femoral artery. A temporary fistula is created through the graft with continuous circulation. After flap harvest, the AV loop is divided at its apex, and the arterial and venous segments are anastomosed to the respective flap pedicle vessels. This minimizes the number of anastomoses performed during flap ischemia. It also allows for inflow and outflow through the loop to be evaluated prior to free flap anastomosis. In the two-stage procedure, the AV loop is first created and allowed to mature, and the flap is transferred during a second stage.
Branched vessel grafts can also be used analogously to the pedicle of a flow-through flap - facilitating microsurgical anastomoses for a free flap as well as reconstructing a portion of artery [Figure 2C]. We have found this technique only to be necessary when a flow-through flap or end-to-side anastomosis is unsuccessful or not viable. A length of vein graft with a branch is harvested and used as an interposition vein graft to reconstruct the injured vessel. The vein branch is then anastomosed to the flap pedicle in an end-to-end fashion. There is frequently a valve at the vein graft branch point that requires a valvulotomy prior to interposition[35]. An expendable branching arterial graft (e.g., DIEA, descending branch of LCFA) can also be harvested and used similarly without the need for valvulotomy.
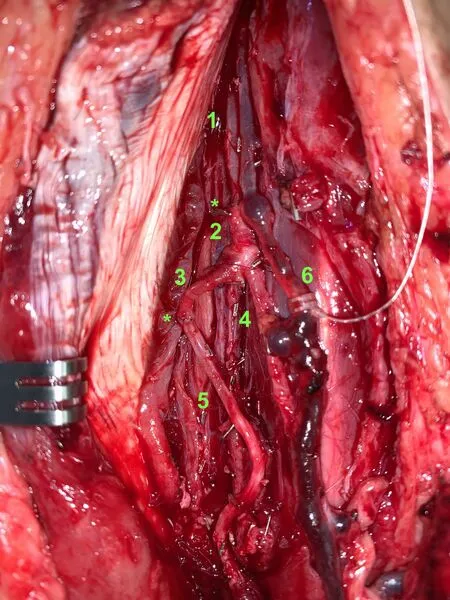
Figure 4. Flow-through anastomosis of latissimus dorsi flap to posterior tibial vessels as recipients. 1: Proximal Posterior tibial artery; 2: subscapular artery; 3: circumflex scapular artery; 4: thoracodorsal artery (pedicle to latissimus dorsi flap); 5: distal posterior tibial artery; 6: venous anastomosis. ★Denotes arterial end-to-end anastomoses.
Supermicrosurgery
As microsurgical techniques and equipment have become more refined, supermicrosurgical flaps have emerged as a tool for lower extremity reconstruction[36]. Supermicrosurgery is defined as the anastomosis of vessels with lumen sizes less than 0.8mm[37]. This technique allows the use of flaps based on perforators and perforator-to-perforator anastomoses. A single perforator can be sufficient to supply a large area of skin, as seen when performing perforator flap dissections. Flow from a perforator recipient vessel is inadequate to perfuse a large muscle or fasciocutaneous flap that requires more blood flow.
The literature reports success with this technique in lower extremity reconstruction[38,39]. Hong describes 42 patients with lower extremity defects who were reconstructed with a perforator as the recipient vessel with only 1 flap failure. Of those 42 patients, 13 had single-vessel perfusion to the foot. The reported benefits of this technique are a decrease in time for dissection of both the flap and the recipient site (as only the perforators are dissected), preserved perforator vessels even in the presence of trauma or atherosclerosis, and no risk of injuring major vessels of the extremity[38]. This technique requires a high level of technical skill and should only be attempted by experienced microsurgeons. The size limitations of these perforator flaps must also be kept in mind when considering supermicrosurgery as a reconstructive option.
Salvage procedures
When there are no recipient vessels or local flaps available, recipient vessels or donor tissue from the contralateral extremity remain a viable option. Cross-leg flaps are not ideal as they have a high morbidity rate and require prolonged immobilization as well as multiple operations[40,41]. If the alternative is amputation and the patient is highly motivated to pursue this path, these flaps can be a final attempt at limb salvage. The three types of cross-leg reconstruction are the pedicled cross-leg flap, the free cross-leg flap, and the free cable bridge flap. For the pedicled cross-leg flap, the flap is raised on the contralateral extremity and inset to the defect with the pedicle remaining attached to the contralateral leg. For the free cross-leg flap, a free flap is an inset to the defect, and the flap pedicle is anastomosed to a recipient vessel on the contralateral extremity. For the free cable bridge flap, Manriqueet al.[40]describe a multi-stage approach where a radial forearm free flap is anastomosed to the contralateral extremity during the first operation. During the second operation, another free flap is then attached to the radial forearm free flap and provides coverage for the soft tissue defect. The radial forearm free flap in this situation acts as an interposition graft to extend the reach for the second free flap. For all of the cross-leg flaps, the pedicle was divided after 3 to 4 weeks. An external fixator is placed to prevent avulsion of the flap.
CONCLUSION
There are multiple approaches and techniques that can be utilized in the reconstructive approach for traumatic lower extremity wounds with limited recipient vessels. Each case must be approached individually, and careful and considerate planning is critical for success. Lower extremity reconstruction can be very challenging from both decision-making and technical perspective, but the rewards of salvaging the limb are innumerable and should be attempted when possible.
DECLARATIONS
Authors' contributions
Performed literature review and primary manuscript writing: Yu JL, Tolley PD, Kneib C Performed review and editing of the manuscript: Yu JL, Miller EA, Crowe CS
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study has been approved by our Institution Review Board, study number: STUDY00013819. There are no specific ethical issues associated with the study.
Consent for publications
The copyright of the figures belongs to the authors.
Copyright
© The Author(s) 2022.
 Plastic and Aesthetic Research2022年5期
Plastic and Aesthetic Research2022年5期
- Plastic and Aesthetic Research的其它文章
- The evolution of lower extremity reconstruction
- Ehlers-Danlos syndrome: prevalence and outcomes in gender affirming surgery - a single institution experience
- Soleus muscle flap for reconstruction of lower extremity trauma. Workhorse or glue factory?
- Peripheral nerve allograft: how innovation has changed surgical practice
- AUTHOR INSTRUCTIONS
