Liquid-phase synthesis of Li2S and Li3PS4 with lithium-based organic solutions
Jieru Xu(许洁茹) Qiuchen Wang(王秋辰) Wenlin Yan(闫汶琳)Liquan Chen(陈立泉) Hong Li(李泓) and Fan Wu(吴凡)
1Key Laboratory for Renewable Energy,Beijing Key Laboratory for New Energy Materials and Devices,
Beijing National Laboratory for Condensed Matter Physics,Institute of Physics,Chinese Academy of Sciences,Beijing 100190,China
2University of Chinese Academy of Sciences,Beijing 100049,China
3Tianmu Lake Institute of Advanced Energy Storage Technologies,Liyang 213300,China
4Yangtze River Delta Physics Research Center,Liyang 213300,China
5Nano Science and Technology Institute,University of Science and Technology of China,Suzhou 215123,China
Keywords: lithium sulfide,sulfide solid electrolyte,liquid phase synthesis,lithium-based organic solution
1. Introduction
All-solid-state batteries (ASSBs) are widely recognized as as one of the the most promising next-generation energy storage technologies to balance properties of high energy density, high power density, high safety and broadened working temperature range.[1]Among basic routes of ASSBs, sulfide solid electrolyte(SE)is widely recognized as the most promising one due to its high ionic conductivity[2-4]and favorable deformability.[5]Aiming to bridge the great gap between lab-scale fabrication and industrial large-scale production of sulfide-based ASSBs, the mass production of sulfide solid electrolytes is one of the most overwhelming issues. High-temperature solid state reaction[2-4]and mechanochemical method[6-8]are typical conventional solidphase synthesis methods of sulfide solid electrolytes with relatively high-energy cost in high-temperature heat treatment process[2-4,9-11]and high-energy ball milling process.[6-8]Recently, liquid-phase synthesis method has been widely reported,which can be an effective way for large-scale production of sulfide SEs with decreasing energy costs attributable to its mild reaction conditions. Taking the synthesis of Li3PS4as an example, which is one of the most studied sulfide solid electrolytes in the field of liquid phase synthesis since Lianget al.firstly synthesized nanoporousβ-Li3PS4by a wetchemical method in 2013.[12]In a typical synthesis, Li2S and P2S5with a stoichiometry of 3:1 were mixed in anhydrous tetrahydrofuran (THF), followed by overnight stirring at room temperature and subsequent centrifugation to obtain Li3PS4·3THF precipitation. After the removal of THF at 80°C and further heating at 140°C,crystallineβ-Li3PS4with ionic conductivity of 1.6×10-4S·cm-1at 25°C was synthesized. Lianget al. also employed acetonitrile (ACN) to synthesizeβ-Li3PS4nanoflakes,[13]and synthesized Li3PS4electrolytes with shape-controlled plate-like morphology via a special exchange process from THF to ACN solvent.[14]Except for the special morphology, the particle size of sulfide solid electrolytes can be mediated by controlling parameters (especially the solvent type) of liquid-phase synthesis. For instance,75Li2S·25P2S5solid electrolytes with small and uniform particle sizes (2.2±1.68 µm) were synthesized with the participation of anhydrous dibutyl ether.[15]The asprepared 75Li2S·25P2S5SEs exhibited ionic conductivity of about 3.1×10-4S·cm-1at room temperature,with small and uniform particle sizes, which can form intimate contact with active materials. However,even with the liquid-phase synthesis method,the costs of sulfide solid electrolytes are relatively high,considering the relatively high price of the crucial starting material, Li2S. Common synthesis methods of Li2S are summarized as follows. Carbothermic reduction of Li2SO4is a predominant commercial synthesis method. Specifically,Li2SO4is reduced with the oxidation of carbon during the reaction of molten Li2SO4and carbon at high temperature of 1000°C,with high energy cost and the release of CO2harmful gas.[16]An acid-base reaction at the temperature of 130-445°C is another synthesis route of Li2S,in which Li2CO3or LiOH are lithium precursors and H2S is used as the sulfur precursor. However, the formation of Li-S-O impurities needs additional purifying process via organic solvents.[17,18]Therefore,a new route for Li2S synthesis is eagerly expected,which is expected to have cheap precursors,mild reaction conditions and environmentally friendly and easily separated byproducts.Alkali metal(such as Li,Na and K)has been reported to react with certain aromatic hydrocarbons in ether solvent to form an alkali solution at room temperature since 1900s.[19]Such solutions, containing radical anions, can be used as reducing agents in chemical synthesis.[20,21]In this work,lithium reacts with biphenyl in 1,2-dimethoxyethane(DME)ether solvent to form a lithium solution at room temperature,which can react with sulfur at high reaction kinetics even at room temperature.This new synthesis route of Li2S starts with cheap precursors of lithium,sulfur,biphenyl and DME solvent,and the only remaining byproduct(DME solution of biphenyl)after the collection of Li2S product can be recycled and reused. Besides,the reaction can proceed effectively at room temperature with mild condition,reducing energy cost to a great extent. The assynthesized Li2S owns uniform and extremely small particle size, proved to be feasible in synthesizing sulfide solid electrolytes(such as the solid-state synthesis of Li6PS5Cl). Spontaneously, this lithium solution can be directly employed in the synthesis of Li3PS4solid electrolytes via liquid-phase synthesis method,in which the centrifugation and heat treatment processes of Li2S are not necessary,providing simplified production process. The as-synthesized Li3PS4exhibits typical Li+conductivity of 1.85×10-4S·cm-1at 30°C.
2. Experiment
2.1. Materials synthesis
2.1.1. Synthesis of Li2S
The synthesis process of Li2S is illustrated in Fig. 1(a).First, lithium-based organic liquid metal solution was prepared as the lithium source. Specifically, an aromatic hydrocarbon, biphenyl (Alfa, 99%), was fully dissolved in extra DME (Energy Chemical, 99%) solvent at room temperature via a simple string process. A transparent and colorless solution of biphenyl was formed with a concentration of 1 mol·L-1, referred as Bp1.0(DME)1.0. Then, lithium metal was cut into pieces(China Energy Lithium Co. Ltd,99.95%)and put into the Bp1.0(DME)1.0solution,forming a dark-green solution after a 12-h standing at room temperature. The molar ratio between Li and biphenyl was fixed at 1:1, referred as Li1.0Bp1.0(DME)1.0. Afterwards, S (50 mol% of lithium,Alfa, 99.5%) was immersed in the Li1.0Bp1.0(DME)1.0complex solution and allowed to react for 12 h during the string at room temperature. The suspension was then centrifugated and washed with DME. The resulting solution turned transparent and colorless, which is supposed to be the DME solution of biphenyl and can be recycled and reused. The resulting solid was yellowish white, then dried under vacuum at 100°C for 12 h to remove the remaining solvent. Finally,heat treatment at different temperatures for 5 h was carried out to promote the crystallization of Li2S,obtaining white powder. THF solvent was also employed using the same synthesis process to determine the proper solvent.
2.1.2. Synthesis of Li6PS5Cl
Li6PS5Cl powder was prepared by conventional solidstate synthesis.A stoichiometric mixture of Li2S,P2S5(Macklin,99%)and LiCl(99.9%,Aladdin)was mechanochemically milled for 20 h, followed by heat-treatment at 550°C for 5 h in a sealed quartz ampoule. Two types of Li2S were used to synthesis Li6PS5Cl, including Li2S synthesized in this work and commercial Li2S(Alfa,99.9%).
2.1.3. Synthesis of Li3PS4 via the two-step route and the one-step route
The synthesis processes of Li3PS4via the twostep route and the one-step route are illustrated in Fig. 1(b). First, lithium-based organic liquid metal solution Li1.0Bp1.0(DME)1.0was prepared as the lithium source,same as the first step of Li2S synthesis. Afterwards,S(Alfa,99.5%)and P2S5(Macklin, 99%) in stoichiometric proportions were immersed in the Li1.0Bp1.0(DME)1.0complex solution. For the two-step route, S and P2S5were added in batches. S was added first, forming a suspension of Li2S in Bp-DME solution after 12-h string at room temperature. Then, P2S5was added into the Li2S·Bp·DME suspension and allowed to react for 12 h during string at 80°C. For the one-step route,S and P2S5were added into the Li1.0Bp1.0(DME)1.0complex solution at one time, followed by a 12-h stirring at 80°C for adequate reaction. The reaction products via two routes were then centrifugated and washed with DME.The resulting solution turned transparent and colorless,which is supposed to be the DME solution of biphenyl and can be recycled and reused.The resulting solid was then dried under vacuum at 100°C for 12 h to remove the remaining solvent. Finally,heat treatment at different temperatures for 10 h was carried out to promote the crystallization of Li3PS4,obtaining grayish-white powder.

Fig.1. Schematic illustrations of the synthesis process of(a)Li2S and(b)Li3PS4.
2.2. Structure, morphology and chemistry characterization
The crystal structure of synthesized materials was investigated by x-ray diffraction (XRD, PERSEE XD2) equipped with CuKαradiation(λ=1.54178 ˚A)operated at 36 kV and 20 mA in the 2θrange of 10°-80°. A field emission scanning electron microscope (SEM, HITACHI, SU8100) was used to characterize the surface morphology of synthesized materials.
2.3. Electrochemical characterization
2.3.1. Ionic conductivities of solid electrolytes
For the measurement of ionic conductivity of solid electrolytes, the testing cells were assembled as follows: 100 mg of sulfide electrolyte was added into a Swagelok model cell and pressed under a pressure of 870 MPa into a pellet,directly using two stainless-steel(SS)rods as ion-blocking electrodes.The as-prepared testing cells were measured by alternatingcurrent (AC) impedance spectroscopy instrument (Zennium Pro Electrochemical Workstation) in the frequency range of 8 MHz-0.1 Hz with an AC amplitude of 20 mV at temperatures ranging from-30°C to 30°C with 15°C steps. The cell was kept at the specific temperature for 2 h prior to the measurement.
Ionic conductivities were calculated using the following equation:

whereσis the ionic conductivity(S·cm-1),Ris the resistance(Ω),lis the thickness (cm),Ais the area (cm2) of the coldpressing pellets of solid electrolytes.
2.3.2. Electronic conductivities of solid electrolytes
The electronic conductivities of solid electrolytes were measured via the direct-current(DC)polarization method using Zennium Pro Electrochemical Workstation. The testing cells are the same cells for ionic conductivity testing, which are SS/cold-pressing pellet/SS mold cells assembled under a pressure of 870 MPa. The cell was polarized at DC voltage(ΔV)of 50 mV,and the steady-state currentIsof the cell was recorded. The electronic resistances were obtained from ΔV/Isand the corresponding conductivities were calculated using Eq.(1).
2.3.3. Fabrication of ASSBs and electrochemical performance measurements
To prepare the composite cathode of ASSBs,LiNi0.8Co0.1Mn0.1O2(NCM811), sulfide solid electrolytes,and vapor grown carbon fiber (VGCF, Showa Denko K.K.)were mixed with a weight ratio of 50:45:5 for 30 min. Nano-Si, sulfide solid electrolytes and VGCF were mixed with a weight ratio of 50:45:5 for 30 min to obtain the composite anode powder. The lab-scale ASSBs were fabricated as follows:1)2.0 mg of mixed anode powder was uniformly spread onto the surface of the bottom of the Swagelok cell; 2) 80 mg of solid electrolyte powder was spread onto the anode powder layer, followed by a light pressing at 70 MPa; 3) 3.4 mg of mixed cathode powder was spread uniformly onto the pressed solid electrolyte layer; 4) the three-layer pellet was finally fabricated under a pressure of 870 MPa. The whole process was conducted in an argon-filled glove box. The galvanostatic charge-discharge tests were conducted in a voltage rang of 2.5-4.2 V at 0.1 C to measure the electrochemical performances of ASSBs via a battery test system(Land,CT2001A)at 30°C.

Fig. 2. (a) XRD patterns of the obtained Li2S samples prepared by DME and THF, and the indexed diffraction pattern of the Li2S phase(PDF#00-023-0369). (b)XRD patterns of the obtained Li2S samples before and after heat treatment at different temperatures from 180 °C to 400 °C.(c)-(f)Morphology of the obtained Li2S at 300 °C.
3. Results and discussion
3.1. Synthesis of Li2S
As illustrated in Fig. 1(a), Li2S was synthesized via liquid phase synthesis method, which is widely recognized as a cost-effective method for mass production with mild reaction conditions.[22]The Li metals can react with biphenyl in the DME solvent to form a dark green lithium solution at room temperature. This kind of solutions has been known as radical anions, acting as reducing agents in chemical synthesis since 1930s.[20,21]Because of its comparatively strong reducibility,it can react with sulfur at high reaction kinetics even at room temperature.
The XRD pattern of the as-synthesized Li2S with a heat treatment at 250°C is shown in Fig. 2(a), corresponding well with the indexed diffraction pattern of the Li2S phase(PDF#00-023-0369)without impurity phase.The solvent used in the Li2S synthesis was replaced by THF for comparison,with which the final product also exhibited the characteristic peaks of Li2S.However,the peak intensity is lower compared with that of Li2S mediated by DME solvent.
The Li2S samples using DME solvent before and after heat treatment at different temperatures are shown in Fig.2(b).The sample before heat treatment (after vacuum drying at 100°C) shows characteristic peaks of Li2S with low intensity, indicating the removal of DME at low temperature. For the samples after heat treatment at a series of temperatures from 180°C to 400°C,all diffraction peaks correspond well to the indexed diffraction pattern of the Li2S phase, and become sharper with higher intensity, indicating that the cubic crystalline Li2S phase has been formed. Interestingly,the particle size of the as-synthesized Li2S is extremely small, as illustrated in the SEM images of Li2S synthesized at 300°C(Figs.2(c)-2(f)). Figures 2(c)and 2(d)show the macroscopic morphology of Li2S particles. Big agglomerates were observed(approximately 5-40µm), which are composed of individual regular particles lower than 50 nm (Figs. 2(e) and 2(f)). The uniform and extremely small particle size should be attributable to the mediation of solvent during liquid-phase synthesis process.[15,23,24]The as-synthesized Li2S with extremely small particle size can be a promising cathode material for lithium-sulfur batteries,considering the critical role of particle size reduction in improving the reversibility and cycling stability of Li2S cathode with insulating nature.[25-27]
The cost of Li2S per kilogram (kg) synthesized via the liquid-phase synthesis route in this work and two conventional routes were roughly calculated and compared in Table 1, including materials cost and processing cost. It is noting that the cost of biphenyl and DME solvent in the liquid-phase synthesis route, and N-methyl-2-pyrrolidone (NMP) solvent in the synthesis route using LiOH and H2S were neglected, considering their recyclability. The cost of Li2S synthesized via the liquid-phase synthesis route in this work is calculated to be 947.885 RMB/kg,lower than that of the Li2S synthesized via two conventional routes.

Table 1. Rough calculation of the cost of Li2S per kilogram (kg) synthesized via the liquid-phase synthesis route in this work and two conventional routes.a
To further investigate the feasibility of the as-synthesized Li2S in synthesizing sulfide electrolyte, Li6PS5Cl solid electrolytes were synthesized and compared via conventional solid-state synthesis method using Li2S synthesized at 300°C and commercial Li2S,respectively. As illustrated in Fig.3(a),the XRD patterns of Li6PS5Cl solid electrolytes using synthesized Li2S and purchased Li2S are virtually the same, corresponding well with characteristic peaks of Li6PS5Cl and without any obvious impurity.
The electrochemical properties of the synthesized Li6PS5Cl solid electrolytes were also compared, using SS/cold-pressing Li6PS5Cl pellet/SS cells with SS rods as the ion-blocking electrodes (Figs. 3(a) and 3(b), Table 2). The Nyquist plots at 30°C for two kinds of Li6PS5Cl are shown in Fig.3(b), exhibiting only one line in low frequency region for both plots, representing the Warburg diffusion impedance(W). The absence of semicircles in the high frequency region representing the bulk resistance (RSE,bulk) of solid electrolyte and in the intermediate frequency region representing the grain boundary resistance (RSE,gb) of solid electrolyte indicates the relatively small resistance of the solid electrolyte.The inset in Fig.3(b)shows the corresponding equivalent circuit of Nyquist plot. The ionic conductivity is calculated from the total resistance (RSE,total=RSE,bulk+RSE,gb) after coldpressing at 870 MPa, which is obtained from the intercept of line in the low frequency region ofx-axis. The as-calculated total ionic conductivity for Li6PS5Cl solid electrolytes synthesized using synthesized Li2S is 4.57×10-3S·cm-1at 30°C,slightly lower than that of Li6PS5Cl obtained using commercial Li2S (6.03×10-3S·cm-1). This might because of the XRD-undetectable amounts of impurity phases.

Fig.3. (a)XRD patterns of the synthesized Li6PS5Cl solid electrolytes via conventional solid-state synthesis method using synthesized Li2S at 300 °C and purchased Li2S.(b)Nyquist plots and(c)chronoamperometry profiles under a polarization voltage of 50 mV of SS/Li6PS5Cl/SS cells using synthesized Li6PS5Cl solid electrolytes via conventional solid-state synthesis method employing synthesized Li2S and purchased Li2S. The equivalent circuit is inserted in the Nyquist plots, in which RSE,bulk is the volume resistance of the solid electrolyte, RSE,gb is the grain boundary resistance of the solid electrolyte,and W is the Warburg impedance.
The DC polarization technique was employed to the SS/cold-pressing Li6PS5Cl pellet/SS cells to measure the electronic conductivity of Li6PS5Cl solid electrolyte. Figure 3(c)shows the time dependence of the current under a polarization voltage of 50 mV. The electronic resistances were obtained from the steady-state current value after the application of the voltage of 50 mV for 600 s. Based on the DC curves,the calculated electronic conductivities of synthesized Li6PS5Cl solid electrolytes using synthesized Li2S and commercial Li2S are 4.45×10-9S·cm-1and 6.67×10-9S·cm-1,respectively, consistent with the previously reported values of electronic conductivity for Li6PS5Cl solid electrolyte(10-9S·cm-1).[28-30]

Table 2. Summary of the ionic conductivity (σLi+) and electronic conductivity(σe-)at 30 °C of the synthesized Li6PS5Cl solid electrolytes via conventional solid-state synthesis method using Li2S synthesized at 300 °C and commercial Li2S.

Fig.4. (a)Charge and discharge profiles at the 1st cycle of NCM811/Li6PS5Cl/Si lab-scale ASSBs using the synthesized Li6PS5Cl via conventional solidstate synthesis method using liquid-phase-synthesized Li2S and purchased Li2S,respectively. (b),(c)Cycling performances of NCM811/Li6PS5Cl/Si labscale ASSBs using the synthesized Li6PS5Cl via conventional solid-state synthesis method using liquid-phase-synthesized Li2S.(b)Cycling performances(0.1 C,1 C=200 mA·g-1,in the voltage range of 2.5-4.2 V).(c)Charge and discharge profiles of ASSBs at the typical cycles.
NCM811/Li6PS5Cl/Si lab-scale ASSBs using the synthesized Li6PS5Cl via conventional solid-state synthesis method using liquid-phase-synthesized Li2S and purchased Li2S were assembled and compared to evaluate the feasibility of the assynthesized Li2S in synthesizing Li6PS5Cl sulfide solid electrolytes. As illustrated in Fig. 4(a), the two batteries exhibit comparable charge and discharge profiles. The ASSBs using the Li6PS5Cl synthesized via liquid-phase-synthesized Li2S delivers a reversible discharge capacity of 174.2 mAh·g-1and a Coulombic efficiency of 66.33% at the 1stcycle, while the ASSBs using the Li6PS5Cl synthesized via purchased Li2S delivers a reversible discharge capacity of 170.0 mAh·g-1and a Coulombic efficiency of 65.50% under the same test conditions. Furthermore,the battery with the Li6PS5Cl synthesized via liquid-phase-synthesized Li2S delivers good cycling performance,as shown in Figs.4(b)and 4(c). The overpotentials of profiles increase gradually during cycling,corresponding to discharge capacities of 167.7, 161.6, 154.3, 134.7, 113.9 and 104.6 mAh·g-1at the 5th, 10th, 20th, 50th, 80thand 100thcycle, respectively. To conclude, the as-synthesized Li6PS5Cl exhibits satisfactory compatibility with NCM811/Si system.Therefore, the as-synthesized Li2S can be successfully employed in synthesizing sulfide solid electrolytes.
3.2. Synthesis of Li3PS4
The application of Li1.0Bp1.0(DME)1.0lithium solution in Li2S synthesis has been proved to be feasible in synthesizing sulfide solid electrolytes (such as the solid-state synthesis of Li6PS5Cl)in the front part. The direct employment of lithium solution in Li3PS4solid electrolytes via liquid-phase synthesis was also investigated in this work,in which the centrifugation and heat treatment processes of Li2S are not necessary, providing simplified production process.
For this purpose,two synthesis routes can be possible,as illustrated in Fig.1(b). For the two-step route, sulfur first reacted with lithium solution to form the suspension of Li2S in Bp-DME solution,followed by the reaction between P2S5and Li2S with the addition of P2S5.For the one-step route,the synthesis process is further simplified, in which sulfur and P2S5reacted with lithium solution at the same time.

Fig.5. (a)XRD patterns of the obtained Li3PS4 samples prepared by DME and THF,and the indexed diffraction pattern of the Li3PS4 phase(PDF#01-076-0973)and the S8 phase(PDF#00-053-1109). (b)XRD patterns of the obtained Li3PS4 samples before and after heat treatment at different temperatures from 160 °C to 250 °C.
Following the two-step route, Li3PS4solid electrolytes were synthesized employing different solvents (DME and THF)and different heat treatment temperatures(160-250°C)to optimize the synthesis parameters. First, different solvents were respectively employed in the synthesis of Li3PS4at heat treatment of 250°C to determine the suitable solvent. Figure 5(a) displays the XRD patterns of resulting products using DME and THF. The XRD pattern of the resulting product using DME solvent corresponds well with the indexed diffraction pattern of the Li3PS4phase (PDF#01-076-0973)without any impurity phase,indicating the successful synthesis of Li3PS4. For the THF solvent, which is widely employed in the synthesis of Li3PS4via liquid-phase synthesis method,[12,14,31]except for the Li3PS4phase,the S8phase was also detected. This might be due to the insufficient dissolution or reactivity of lithium in Bp-THF solution. Therefore,DME solvent is suitable for the synthesis of Li3PS4. Figure 5(b)shows the XRD patterns of Li3PS4samples using DME solvent before and after heat treatment at a series of temperatures from 160°C to 250°C.The sample before heat treatment(after vacuum drying at 100°C for 12 h)is well-crystallized because of the complex formation with DME,[32]and the DME solvent can not be removed after heat treatment at 160°C.For the samples after heat treatment at a series of temperatures from 170°C to 250°C,all diffraction peaks correspond well to the indexed diffraction pattern of the Li3PS4phase without any impurity or formation of new phase. Considering the almost unchanged peak sharpness and intensity from 180°C,180°C was fixed as the optimal heat treatment temperature for further study.

Fig.6. (a)XRD patterns of the obtained Li3PS4 samples prepared by two-step and one-step routes, and the indexed diffraction pattern of the Li3PS4 phase(PDF#01-076-0973). (b), (c) Morphology and corresponding particle size distribution of the obtained Li3PS4 samples prepared by (b) two-step and (c)one-step routes.
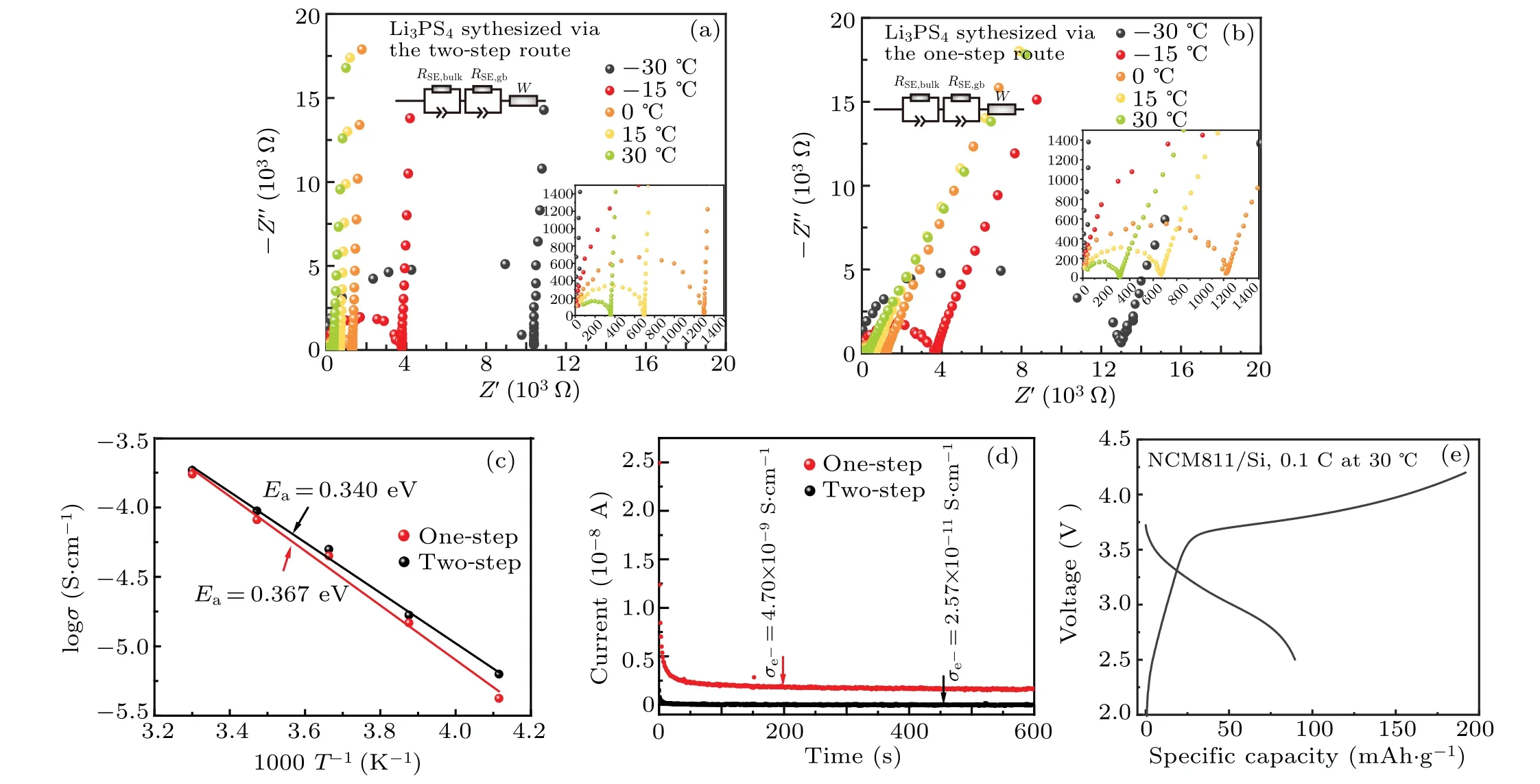
Fig. 7. (a) Nyquist plots of SS/Li3PS4/SS cells at temperatures from -30 °C to 30 °C using synthesized Li3PS4 solid electrolytes via (a) two-step route and(b)one-step route. Insert: the magnified region and the corresponding equivalent circuit of the Nyquist plots. (c)Arrhenius plots of synthesized Li3PS4 solid electrolytes via two-step and one-step routes. (d)Chronoamperometry profiles under a polarization voltage of 50 mV of the SS/Li3PS4/SS cells using synthesized Li3PS4 solid electrolytes via two-step and one-step routes. (e)Charge and discharge profiles at the 1st cycle of the NCM811/Li3PS4/Si lab-scale ASSB using the synthesized Li3PS4 via the two-step route.
The two synthesis routes including two-step and one-step routs were compared,using DME as the solvent and heat treatment at 180°C.In both cases,pure-phase Li3PS4was obtained via the detection of XRD(Fig.6(a)). The difference in particle size was observed,as illustrated in Figs.6(b)and 6(c). The two-step synthesized Li3PS4exhibits uniform particle size of 1.65±0.32 µm, smaller that of one-step-synthesized Li3PS4(3.26±0.75 µm). This might be attributable to the relatively adequate and uniform chemical reaction during solvent-solid interactions in the two-step synthesis process.
The electrochemical performances of the synthesized Li3PS4via two routes were also compared(Fig.7 and Table 3).Figures 7(a)and 7(b)show the Nyquist plots of SS/Li3PS4/SS cells at temperatures from-30°C to 30°C using synthesized Li3PS4solid electrolytes via two routes. The plots bothe consist of a semicircle and a spike. The line in the low frequency region represents the diffusion impedance(W),and the incomplete semicircle contains the the grain boundary resistance (RSE,gb) of the solid electrolyte in the intermediate frequency region and the bulk resistance (RSE,bulk) of the solid electrolyte in the high frequency region. The corresponding equivalent circuit is illustrated as the insert in Nyquist plots. The intercept of the semicircle in the intermediate frequency region ofx-axis, recognized as the total resistance(RSE,total=RSE,bulk+RSE,gb) after cold-pressing at 870 MPa,gradually decreases with the increase of temperature,indicating the increase of Li+conductivity.As illustrated in Fig.7(c),the as-calculated Li+conductivities of the synthesized Li3PS4via two routes all conform to the Arrhenius equation

whereEais the activation energy for Li+conduction,Ais the pre-exponential factor, andkis the Boltzmann constant.The two-step synthesized Li3PS4exhibited a Li+conductivity of 1.85×10-4S·cm-1at 30°C and an Arrhenius activation energy of 0.340 eV, while the one-step synthesized Li3PS4exhibited a Li+conductivity of 1.74×10-4S·cm-1at 30°C and an Arrhenius activation energy of 0.367 eV,consistent with the previously reported values of liquid-phase synthesized Li3PS4.[31,33]Figure 7(d) shows the time dependence of the current under a polarization voltage of 50 mV,and the calculated electronic conductivities of the synthesized Li3PS4solid electrolytes via two-step and one-step routes are 2.57×10-11S·cm-1and 4.70×10-9S·cm-1,respectively.To conclude, although the two-step synthesis route requires longer synthesis time compared with the one-step route, the as-synthesized Li3PS4exhibited better electrochemical performances and small particle size, which is supposed to provide ASSBs with preferable electrochemical performances.NCM811/Li3PS4/Si lab-scale ASSBs using the synthesized Li3PS4via the two-step route were assembled and tested to evaluate the compatibility of the as-synthesized Li3PS4with NCM811/Si system (Fig. 7(e)). The battery exhibits a reversible capacity of 89.5 mAh·g-1with large polarization for the 1stcycle, which should be attributable to the relatively lower ionic conductivity of Li3PS4compared with Li6PS5Cl.

Table 3. Summary of the ionic conductivity(σLi+)and electronic conductivity (σe-) of the synthesized Li3PS4 via two-step and one-step routes at 30 °C.
4. Conclusion and perspectives
In conclusion, a new synthesis route of Li2S via liquidphase synthesis method was developed in this work, using lithium solution with strong reducibility as lithium precursor.This new synthesis route of Li2S starts with cheap precursors of lithium,sulfur,biphenyl and DME solvent,and the only remaining byproduct(DME solution of biphenyl)after the collection of Li2S product can be recycled and reused. Besides,the reaction can proceed effectively at room temperature with mild condition, reducing energy cost to a great extent. The as-synthesized Li2S owns uniform and extremely small particle size, proved to be feasible in synthesizing sulfide solid electrolytes (such as the solid-state synthesis of Li6PS5Cl).Spontaneously,this lithium solution can be directly employed in the synthesis of Li3PS4solid electrolytes via liquid-phase synthesis method, in which the centrifugation and heat treatment processes of Li2S are not necessary,providing simplified production process. The synthesis parameters of Li3PS4, including the solvent type,heat treatment temperature and route were systematically investigated. The as-synthesized Li3PS4with the optimal parameters (using DME solvent, with heat treatment at 180°C via two-step route) exhibits typical Li+conductivity of 1.85×10-4S·cm-1at 30°C.
Acknowledgements
This work is supported by Key R&D Project funded by Department of Science and Technology of Jiangsu Province(Grant No. BE2020003), Key Program-Automobile Joint Fund of National Natural Science Foundation of China(Grant No.U1964205),General Program of National Natural Science Foundation of China(Grant No.51972334),General Program of National Natural Science Foundation of Beijing (Grant No. 2202058), Cultivation Project of Leading Innovative Experts in Changzhou City (Grant No. CQ20210003), National Overseas High-level Expert Recruitment Program (Grant No. E1JF021E11), Talent Program of Chinese Academy of Sciences, “Scientist Studio Program Funding” from Yangtze River Delta Physics Research Center and Tianmu Lake Institute of Advanced Energy Storage Technologies(Grant No. TIES-SS0001), and Science and Technology Research Institute of China Three Gorges Corporation (Grant No.202103402).
- Chinese Physics B的其它文章
- Characterizing entanglement in non-Hermitian chaotic systems via out-of-time ordered correlators
- Steering quantum nonlocalities of quantum dot system suffering from decoherence
- Probabilistic quantum teleportation of shared quantum secret
- Spin–orbit coupling adjusting topological superfluid of mass-imbalanced Fermi gas
- Improvement of a continuous-variable measurement-device-independent quantum key distribution system via quantum scissors
- An overview of quantum error mitigation formulas

