Measurement of CO,HCN,and NO productions in atmospheric reaction induced by femtosecond laser filament
Xiao-Dong Huang(黄晓东) Meng Zhang(张梦) Lun-Hua Deng(邓伦华)Shan-Biao Pang(庞山彪) Ke Liu(刘珂) and Huai-Liang Xu(徐淮良)
1State Key Laboratory of Precision Spectroscopy,East China Normal University(ECNU),Shanghai 200062,China
2State Key Laboratory of Integrated Optoelectronics,College of Electronic Science and Engineering,Jilin University,Changchun 130012,China
3Chinese Academy of Sciences Center for Excellence in Ultra-Intense Laser Science,Shanghai 201800,China
Keywords: femtosecond laser filament,atmospheric reaction,CO,HCN,NO
1. Introduction
When the ultrashort laser pulses focus on the air, a plasma channel called filament is formed due to Kerr’s selffocusing and plasma defocusing effect. Filament provides a unique environment with high intensity. The atmospheric molecules present in the filament are ionized or dissociated into transient species,followed by chemical reactions,changing the atmospheric compositions and even forming aerosols and particles.[1]Filament-induced gaseous chemical reactions facilitate complex molecular synthesis. In humid air,the filament plasma promotes the production of nitric acid precursors and assists the synthesis of biopolymers thought essential for the emergence of life, providing further molecular complexity. On the other hand,the high-intensity filament can be generated in the atmosphere in the kilometer range and hundreds of meters long. These remote modifications to the air via filaments are ideal for atmospheric applications.[2-6]Therefore,the generation and evolution of the precursor species during filaments are essential to reveal the laser-induced molecular synthesis relating to atmospheric chemistry and filament application. Previous studies were mainly devoted to the discussion about the filament-induced atmospheric nitrogen chemistry. The nitrogen-containing products NOxin atmospheric filaments, acting as the precursors for nitric acid and helping the nucleation of H2O-HNO3in humid air,were monitored by analyzers and cavity-enhanced absorption spectroscopy.[7,8]However, the other stable molecules, such as CO and HCN,also crucial in atmospheric chemistry, especially in prebiotic chemistry,[9]have not been considered in filaments generated reactions. In this work the generation and evolution of NO,CO, and HCN generated by filaments in air are reported, involving the H, C, N, and O transformations of atmospheric molecules.
2. Experimental setup
Figure 1 shows the schematic diagram of the experimental setup. A Ti:sapphire laser supplying 35-fs,1-kHz pulses at the central wavelength of 808 nm was adopted, and the laser pulse energy was 7 mJ. Unless otherwise specified, the laser pulse energy used in this experiment is maintained at 1.2 mJ.A lens with focal lengthf=75 cm formed a filament in a quartz glass cylindrical absorption cell with 5 cm in diameter and 60 cm in length, which provides a volume for sufficient reactions. The laser beams of three mid-infrared tunable diode lasers (Nanoplus GmbH), operating at 4844 nm,5298 nm,and 3060 nm,passed through the absorption cell to measure the spectra of CO,NO,and HCN.The beams of the three lasers were switched through optical components. The sealed absorption cell sustained stable air-from lower pressure to an atmosphere. The sinusoidal signal provided by the lockin amplifier and the triangular signal provided by the function generator were first combined in an adder. Then the combined signal was sent to the laser controller to realize laser wavelength scanning and modulation. The power of the laser changes with the operating current, with a maximum power value being 6 mW. Several cooled IR detectors (VIGO system)were switched through optical components to receive the specific laser beam passed through the absorption cell. The received signals were sent to the lock-in amplifier for demodulation to obtain the wavelength modulated spectrum.
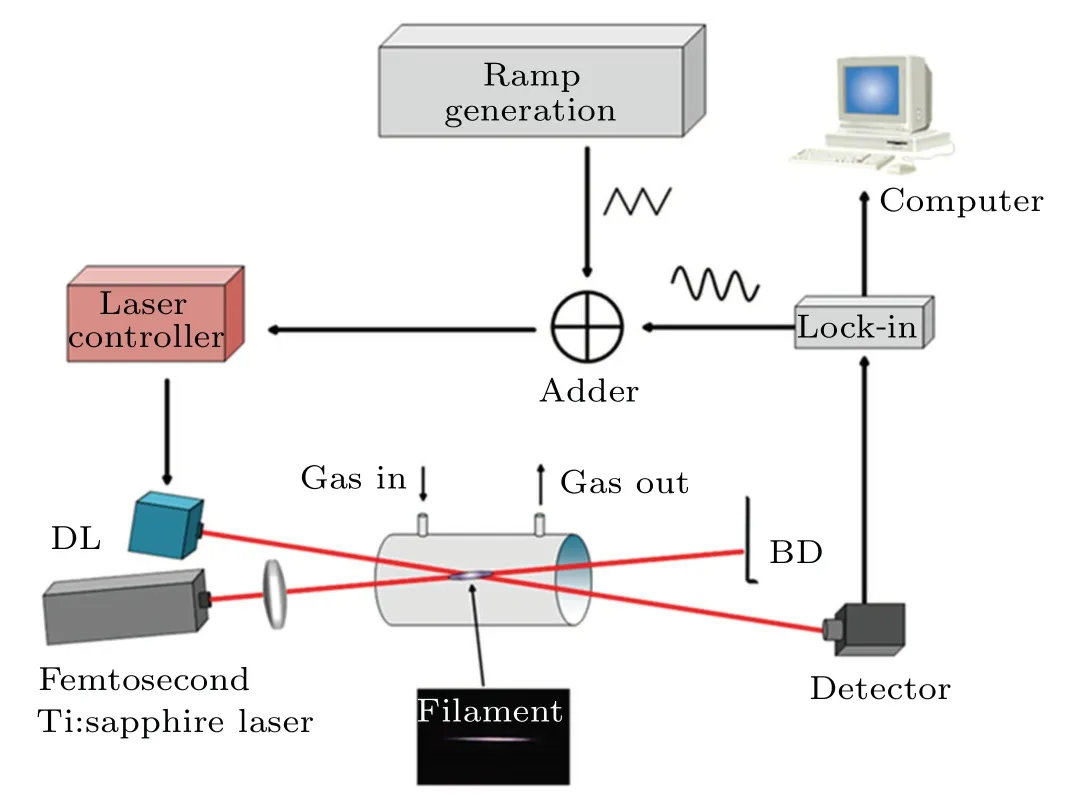
Fig. 1. Experimental setup for femtosecond laser filament-induced atmospheric reaction and absorption spectra measurement. DL:diode lasers,and BD:beam dump.
3. Experimental results and discussion
The production and evolution of CO, NO, and HCN are monitored by using laser absorption spectroscopy. The stronger absorption line intensities in the mid-infrared region provide high detection sensitivity for the generated products. Figure 2 shows the spectra measured by direct absorption spectroscopy for determining the absolute concentration and the supplemented wavelength modulation absorption spectroscopy with enhanced sensitivity for trace products monitoring. The P19transition of CO fundamental vibration band (υ'= 1)←(υ''= 0) at 2064.3969 cm-1is used to measure CO. The P13transition in (υ'= 1 000)←(υ''=0 000) vibration band at 3271.443160 cm-1is used to measure HCN. The isolated molecular absorption peaks for CO and HCN measurements are shown in Figs.2(a)and 2(b),respectively. The frequency intervals of the absorption lines are too close to be resolved and the mixed absorption peaks of NO molecules are shown in Fig. 2(c). The transitions of NO are assigned to the fundamental vibration bands in X2Π1/2(υ'=1)←X2Π1/2(υ'=0)and X2Π3/2(υ'=1)←X2Π3/2(υ''=0)systems. The generated products gradually accumulate and evolve with reaction time in the absorption cell. The spectral intensities of CO,HCN,and NO,defined as the peak values of wavelength modulation absorption spectra illustrated in Fig.2 are continuously monitored with the reaction time at varying air pressures.

Fig.2. Measured direct absorption(DA)spectra(red lines)and wavelength modulation absorption spectra(black lines)of(a)CO,(b)HCN,and(c)NO produced from filaments-induced atmospheric reaction.
Figure 3(a)shows that the CO spectral intensity increases gradually with reaction time increasing in 10-Pa air. In contrast, the spectral intensities first increase, then decrease slowly in 150-Pa and 250-Pa airs, reaching their corresponding maximum values at 10 min and 5 min. A decrease of the time to reach the maximum spectral intensity of CO with increasing air pressure is observed. The phenomena in Fig.3(a)can be explained below. The generated CO molecules suffer the rotation excitation induced by filaments that enhance the population at the lower level,resulting in the enhancement of absorption spectra. However,the continuous excitation by the filaments may also depopulate the low level,causing the spectral intensity to decrease with time going by. Another reason for the spectral intensity decreasing with time going by is that the consumption of CO due to its reacting with other products surpasses the generation of CO,which will be further described below. Therefore, we use the spectral intensities to represent the CO accumulation because the rotation excitation enhanced absorption spectra do not accurately describe the CO number density. The maximum spectral intensities of CO at other air pressures are normalized to the maximum value at 10 Pa as shown in Fig. 3(b). The CO spectral intensity decreases dramatically with air pressure increasing.

Fig.3. Reaction-time-dependent wavelength modulation absorption spectral intensities at different air pressures for(a)CO,(c)HCN,and(e)NO.Pressuredependent maximum spectral intensities for(b)CO,(d)HCN,and(f)NO.
The generated HCN also suffers rotation excitation by filaments. Like the case of CO, the relative spectral intensity other than absolute number density is adopted to represent the accumulation process of HCN.Figure 3(c)shows that the HCN spectral intensity increases with reaction time increasing under different air pressures. The spectral intensities of HCN after 30-min reaction at different air pressures are normalized as indicated in Fig. 3(d). Like the case of CO, the generated HCN decreases sharply with air pressure increasing. The spectral intensity of HCN will still increase with a reaction time, longer than 30 min. Then the lower level will suffer a depopulation by the filament,resulting in the spectral intensity decreasing with time increasing, which is similar to the scenario of CO in Fig.3(a).
The generations of CO and HCN are related to the CO2conversion since CO2is the dominant carbon-containing molecule in the air. Figures 3(b)and 3(d)reveal that the spectral intensity of CO and HCN decrease with air pressure increasing,consistent with the CO2decomposition in sealed-off CO2laser.[10]The number density of CO and HCN can be determined from their absorption spectra to deduce the conversion ratios from CO2. However, the obtained density of CO and HCN are close to or even higher than that of CO2due to the filament-induced rotation excitation. The deduced conversion ratio from CO2to HCN and from CO2to CO are much overestimated in the reduced pressure air. When CO and HCN are produced in the reactor and the laser is blocked,the spectral intensity of HCN and CO remain stable within tens of hours of continuous observation. This spectral stability means that the de-excitation and reaction of the excited HCN molecules and CO molecules are negligible without the continuously inducing the laser filaments.
On the other hand, the generation and evolution of NO are entirely different from that of CO and HCN. The inset in Fig.3(e)shows that the NO accumulates to a maximum value after 15-min reaction and remains stable in 100-Pa air. When in 104-Pa air,it takes much longer time for NO to reach the stable concentration,and the spectral intensity increases linearly with reaction time increasing within 30 min. The spectral intensity of NO increases with reaction time increasing within longer than 30 min untill a stable value is reached,but the obvious depopulation by the filament is not observed for CO and HCN. Figure 3(f) shows the NO number density determined from the direct absorption spectra after one-hour reaction at different air pressures. The NO density shows good linearity in reduced pressure air with pressure less than 104Pa, corresponding to a mixed concentration of 445 ppm. However, it takes more time to reach a comparable concentration at the higher air pressure.
The ambient air is sealed in the absorption cell, and the chemical reaction induced by laser filament lasts for 5 h to estimate the concentration of stable products. The accumulated number density of NO is 3.62×1015cm-3,corresponding to a mixed concentration of 134 ppm. The concentration of CO and HCN are 80 ppm and 1.6 ppm,corresponding to 20%and 0.4%of CO2conversion ratio,respectively. However,the deduced conversion ratio of CO2to CO and CO2to HCN may still be overestimated due to the rotation excitation induced by laser filament.
Figure 4 shows the schematic diagram of the decomposition of CO2and the generation of CO, HCN, and NO in air. The CO generated in air filaments comes from the decomposition of CO2. The CO2can be converted into the more reactive species, CO and O in filaments through direct electrons impact dissociation,i.e.,e+CO2→CO+O+e. In addition, the filament plasma transfers its energy to the vibrationally excited states of CO2. The electron impact causes CO2to dissociate from the excited states and promote the CO generation. This mechanism is called excitation dissociation through the pathway e+CO2→e+CO∗2→CO+O.[11,12]Besides, the electrically excited states of N2molecules, such as the A3Σ+umetastable state, are generated through collisions between energetic electrons and neutral N2by ultrashort laser pulses. The collision between metastable N2molecules and excited CO2molecules also promotes the dissociation of CO2molecules and increases the formation of CO through N2(A3Σ+u)+CO2→CO+O+N2.[13]We do find that the CO2in the reduced pressure air is dramatically vibrationally excited by filaments, by monitoring its absorption spectra. The enhanced dissociation from the excited states of CO2should significantly contribute to the CO formation,especially in the reduced pressure air with less than 1000 Pa.

Fig.4. Schematic diagram of decomposition of CO2 and generation of CO,HCN,and NO in air.
The HCN formation involves the reactions between the molecular fragments coming from H2O,CO2,and N2.The CN radicals, mainly coming from the reactions between N atoms and CO molecules,act as the precursor and react with H,H2O,H2, and OH to form HCN as shown in Fig. 4. The available hydrogen atoms,mainly from H2O decomposition,are essential for HCN generation. The relative humidity of the ambient air is 29%.We observed that the filaments in humid air greatly enhance the HCN generation. The H and N atoms are abundant in the humid-reduced pressure air plasma benefiting from the collisions between the higher energetic electrons and the neutral molecules,promoting the HCN generation.
The formations of CO and HCN in air filament plasma are accompanied by consumption due to reactions. The consumption is more significant at higher pressure,resulting in a minor cumulative concentration with the same reaction time as at lower pressure. As shown in Fig. 4, one consumption pathway of CO is transformed into that of HCN through the CN radicals by reacting with N atoms. Other consumption of CO is the reaction with other free radicals or collision-induced recombination to produce CO2.
The OH radicals, mainly generated by dissociating H2O molecules in the air filaments,consume many CO molecules.Figure 5 shows the CO spectral intensity in 200-Pa air mixed with different quantities of water vapors. The accumulation time to reach the maximum is shortened, and the maximum spectral intensity is reduced with the increase of water vapor added. Besides, the survival time of CO is also significantly shortened due to the enhanced OH concentration coming from the added water vapor. The CO generated by filaments in the mixed gases is almost entirely consumed in 4 min when 100-Pa water vapor is mixed in 200-Pa air. As a comparison,adding the same amount of N2and O2to 200-Pa air has no such significant effects as adding H2O on the formation and evolution of CO.

Fig. 5. Wavelength modulation absorption spectral intensities versus reaction time of CO generated by filaments in 200-Pa air mixed with different amounts of water vapor.

Fig. 6. Influence of O2 on generated HCN in ultrashort laser filamentinduced atmospheric reaction.
Reactions with O dominate the consumption of HCN in the filament-induced reactor.[14]Figure 6 shows the HCN generation and evolution by filaments in 50-Pa humid air influenced by adding O2. First,the HCN accumulate for 10 min to obtain sufficient concentration in the reactor. Then, the laser is blocked, and 10-Pa O2is added for mixing. The spectral intensity of HCN shows a slight increase within 100 min during oxygen mixing, meaning that the generated HCN is not consumed by the added O2and other molecules in the reactor. When the laser functions again, the spectral intensity decreases rapidly due to the reactions between HCN and the products, mainly the O atoms, induced by laser filament. As shown in Fig. 4, the HCN can also react with OH to form HNCO,the isocyanic acid related to prebiotic chemistry.[14]
The CO2is the primary carbon-containing molecule in the air,dominating the atmospheric carbon chemistry,providing the carbon for CO and HCN generations by filaments. In reduced pressure air,the collision between high energetic electrons and the ground state CO2improves the vibration excitation of CO2, promoting the conversion of CO2into CO and HCN via the excited states of CO2. The N2is the primary nitrogen-containing molecule in the air,dominating the nitrogen chemistry initialized by ultrashort laser filaments. The N2provides N atoms for forming nitrogen oxides and promotes the dissociation of CO2in filament plasma. The highconcentration NO is produced under various air pressures,which is the precursor of other nitrogen oxides.The very complex chemistry occurring in ultrashort laser-induced reactions limits the entire understanding of the generation and synthesis of the products. We quantitatively measure the generation and evolution of CO, HCN, and NO in the reactor by utilizing mid-infrared absorption spectroscopy. Many other stable products generated in the filaments,such as O3and other NOx,need to be included in the filament-induced atmospheric chemistry.On the other hand,filament-produced atoms and radicals play significant roles because they facilitate complex molecular synthesis to provide the precursors for synthesizing more complicated molecules,such as biopolymers.[15]
4. Conclusions
In this work,we experimentally demonstrate that the laser filament initializes the chemical reactions and generates CO,HCN, and NO directly from the air. The vibration excitation of CO2and rotation excitation of the generated CO and HCN induced by laser pulses are observed in the reduced pressure air. The mechanisms and paths of chemical reactions induced by ultrashort laser pulse still need further studying. By optimizing the power consumption and increasing the net conversion rate of carbon dioxide, the ultra-short laser-induced atmospheric reaction may transform atmospheric carbon dioxide into commercially valuable products while maintaining environmental benefits.
Acknowledgements
Project supported by the National Natural Science Foundation of China(Grant Nos.1625501 and 62027822)and the Research Funds of Happiness Flower ECNU, China (Grant No.2021ST2110).
- Chinese Physics B的其它文章
- Characterizing entanglement in non-Hermitian chaotic systems via out-of-time ordered correlators
- Steering quantum nonlocalities of quantum dot system suffering from decoherence
- Probabilistic quantum teleportation of shared quantum secret
- Spin–orbit coupling adjusting topological superfluid of mass-imbalanced Fermi gas
- Improvement of a continuous-variable measurement-device-independent quantum key distribution system via quantum scissors
- An overview of quantum error mitigation formulas

