Kainate receptors in the CA2 region of the hippocampus
Yuniesky Andrade-Talavera, Antonio Rodríguez-Moreno
The hippocampus is involved in important brain functions such as learning and memory, spatial navigation, fear processing, and social behavior (Dudek et al, 2016). The most prominent areas of the hippocampus are typically denoted as the dentate gyrus and the three areas of the cornu ammonis (CA1, CA2, and CA3). Discovered by Lorente de Nó (1934), the CA2 region of the hippocampus is a relatively small area interposed between CA3 and CA1 that forms the nexus linking the input of the entorhinal cortex to the output of CA1 (Chevaleyre and Siegelbaum, 2010). Although little is known about the function of CA2 in detail (Hitti and Siegelbaum, 2014; Dudek et al., 2016), there is currently increasing interest in its physiology and cumulative evidence indicates that this region has important and unique properties as it participates in engram formation, neurodegeneration and information processing (Hainmueller and Bartos, 2018; Pang et al., 2019; Middleton and Mchugh, 2020; Lehr et al., 2021). Recent discoveries have revealed that CA2 is involved in the formation of social and spatio/temporal memories (Hitti and Siegelbaum, 2014; Dudek et al., 2016). In addition, the CA2 network seems to play a critical role in balancing levels of excitation and inhibition in the hippocampus (Boehringer et al., 2017). Importantly, excitation/inhibition imbalances have been implicated in the diverse brain and neurodevelopmental disorders.
The physiological presence of glutamate receptors of the kainate type (kainate receptors, KARs) in the hippocampal CA2 region has been recently demonstrated (Falcón-Moya et al., 2021). In the hippocampus, as in other brain regions, KARs are located postsynaptically participating in synaptic transmission and presynaptically modulating neurotransmitter release at different synapses (Sihra and Rodríguez-Moreno, 2013). Additionally, they participate in synaptic plasticity (Negrete-Díaz et al., 2007; Lyon et al., 2011) and their inadequate activation may produce brain alterations such as epilepsy (Falcón-Moya et al., 2018). KARs are involved in the control of GABA and glutamate release with well-known underlying mechanisms at CA1 and CA3 (Rodríguez-Moreno and Sihra, 2011, 2013; Negrete-Díaz et al., 2018). KARs have been described to mediate a depression of glutamate release at CA3 Schaffer collaterals (SC)-CA1 synapses and, for the moment, no mediated facilitation has been described at these synapses. At SC terminals, KARs-mediated depression of glutamate release is accompanied by a decrease of intracellular Calevels indicating that these terminals express KARs that operate in a metabotropic mode of action. This operational mode provokes a reduction of voltage-gated Cachannels (VGCC) activity, thereby inhibiting glutamate release. By contrast, at mossy-fibers (MF)-CA3 synapses they have a biphasic mode of action modulating glutamate release, with low agonist concentrations facilitating synaptic transmission, and higher concentrations mediating a depression of synaptic transmission (Rodríguez-Moreno and Sihra, 2013). Presynaptic KARsmediated facilitation of glutamate release at MFCA3 synapses involves a cytosolic Caincrease through Capermeable KARs that downstream triggers Ca-induced Ca-release from internal stores. The mechanisms involved in this facilitation imply the activation of an adenylyl cyclase (AC)/cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) cascade. The inhibitory action of KARs at the same synapses also involves an AC/cAMP/PKA cascade, with a fundamental difference: KAR-mediated decrease of glutamate release depends on G-protein activation, whereas release facilitation mediated by KARs is independent of G-protein activation. G-protein independent activation of AC/cAMP/PKA cascade by the increase of cytosolic Calevels following KARs activation involves the upstream formation of Ca-calmodulin complex that stimulates AC. In addition, KARs-mediated facilitation of glutamate release and the induction of longterm potentiation at MF-CA3 synapses have been found, at least in part, to share some intracellular signaling commonalities: a Ca-calmodulin/AC/cAMP/PKA-dependent mechanism.
What is the functional role of the decrease in glutamate release mediated by KARs at MFCA3 synapses? The decreased AC/cAMP/PKA signaling linked to the decrease of glutamate release at these synapses may underpin the synaptic plasticity observed therein. Thus, it has been shown that the induction of KARs-mediated synaptic depression at MF-CA3 synapses can be occluded by low-frequency stimulation-mediated long-term depression. In a reciprocal way, at MFCA3 synapses, KARs-mediated depression prevents long-term depression induction (Negrete-Díaz et al., 2007; Lyon et al., 2011). Much less is known about the physiological roles of KARs in the CA2 region, where synaptic transmission and plasticity at excitatory synapses appear subjected to tight control.
Very recently, by performing electrophysiological and pharmacological experiments in mouse hippocampal slices, Falcón-Moya et al. (2021) described for the first time that functional KARs are present at the CA2 region of the hippocampus. Interestingly, in this hippocampal region, activation of KARs exclusively mediates a depression of glutamate release. The results in this study show that the activation of KARs leads to a depression of glutamatergic synaptic transmission at SCCA2 synapses by decreasing glutamate release, indicating a presynaptic locus of action. The presynaptic presence of KARs at these synapses was determined by electrophysiological analysis of the paired-pulse ratio of consecutive evoked excitatory postsynaptic currents (eEPSCs) and the analysis of the coefficient of variation and the frequency of miniature EPSCs (mEPSCs). The different data obtained from different approaches are all supportive of a presynaptic mode of action for KARs. Altogether, three independent analyses mutually corroborate and emphasize a presynaptic locus of action of KARs functioning at SC-CA2 principal cells (PCs) synapses. It remains to be elucidated whether the presynaptic regulation by KA at SC-CA2 PC synapses reports the activity of KARs subcellularly localized at nerve terminal/axonal or at somatodendritic compartments. To elucidate the presynaptic compartmentalization of KARs, future work requires: (i) high-resolution immunolocalization (immunogold-based) of the receptor (contingent on the availability of highaffinity antibodies with appropriate KARs subunitspecificity); (ii) targeted blockade of KARs using caged-antagonists (contingent on the development of caged reagents) and (iii) paired-recordings (Rodríguez-Moreno et al., 2013).
Mechanistically, the analysis of the KARs-driven modulation suggests a coupling of KARs to PKA activity mediated by the activation of a G-protein at hippocampal SC-CA2 synapses involving cAMP-dependent signaling and excluding the requirement of protein kinase C. These findings denote that the mechanisms underlying KARsmediated modulation of glutamate release involving PKA is not restricted to the SC-CA1 or MF-CA3 synapses and is extended to the CA2 region of the hippocampus. Moreover, Gproteins have been previously involved in KARs signaling since the discovery of its role in KAR-mediated modulation of GABA release in the hippocampus (Negrete-Díaz et al., 2018). Notably, a role of G-protein in KARs-mediated modulation of glutamate release has also been described in the hippocampus at SC-CA1 and MF-CA3 synapses and in the cerebellum and more congruencies have been found in the mechanism described at CA2. Although the role of the protein kinase C pathway in some KARs actions is well recognized (Sihra and Rodríguez-Moreno, 2013), it has also been found that KARs activities may not be ionotropic and not mediated by protein kinases or may be mediated by PKA as observed in the hippocampus, amygdala and the cerebellum (Negrete-Díaz et al., 2018). The congruence of the KARs mechanism between these synapses is also highlighted in the current study, by observing the blockade of KARsmediated depression following the inhibition of the catalytic activity of PKA by H-89 (Falcón-Moya et al., 2021).
Identification of CA2 principal cells does not appear trivial, but they can be identified by their location along the transverse axis of the brain slice and supported by their distinctive intrinsic electrophysiological properties (Chevaleyre and Siegelbaum, 2010; Sun et al., 2014). Compared to CA1, CA2 pyramidal neurons exhibit lower input resistance, have longer delays from the onset of depolarizing pulses to the firing of action potentials and display differences in the input/output curves evidenced by differential EPSCs magnitudes recorded in CA2 and CA1 pyramidal neurons in response to increasing stimulation intensity. In addition, the resting membrane potential appears hyperpolarized compared to CA1 pyramidal neurons. Thus, CA2 seems to have different properties in comparison to cells from other regions related to death and survival from injury or aging. CA2 neurons appear similar in size to neurons from the CA3 region but with some differences in the dendritic tree (Lorente de Nó, 1934). These cells are more resistant to injury than those from CA1 and CA3. The reasons for this are not known for the moment but may be related to molecular and genetic differences as they exhibit distinctive gene expression patterns. Additionally, and interestingly, synapses onto CA2 neurons do not normally show plasticity, or at least long-term potentiation and long-term depression observed are normally quite reduced in magnitude. This is not the case for CA3 and CA1 regions where several forms of synaptic plasticity have been described.
In addition to the above-mentioned unique features of neurons from the CA2 region of the hippocampus, they are also different from the CA3 and CA1 neighbors according to their elevated Cabuffering ability (Dudek et al., 2016). This inherent capacity probably underlies the low success in finding plasticity with classical inducing protocols and remarks how intriguing this hippocampal region, flanked by two highly plastic ones is. Interestingly, at SC-CA2 synapses, KARs are not calcium permeable and do not require Caentering into the cytoplasm from the extracellular side nor calcium from internal stores to mediate their function modulating glutamate release. To what extent this is due to or is a consequence of CA2 neurons properties needs to be determined.
Interestingly, the depression of glutamate release at SC-CA2 PC synapses described by Falcón-Moya et al. (2021) does not require calcium-permeable L-type VGCCs nor release from intracellular stores. The role of Cain KARs-induced modulation of synaptic transmission has been the subject of debate and controversy, for instance, at hippocampal MF-CA3 synapses. To examine the requirement of Caat SC-CA2 synapses to depress glutamate release, the authors investigated the effects of nimodipine, which selectively blocks L-type VGCCs, ryanodine that prevents Ca-induced Carelease, and thapsigargin that depletes intracellular Castores. All these experimental approaches failed to prevent KARsmediated decrease of glutamate release at SCCA2 synapses. This confirms that Capermeation through L-type VGCCs and Camobilization from internal stores have no role in the synaptic depression observed at SC-CA2 synapses following KAR activation (Figure 1
).CA2 neurons are more resistant to injury than CA3 and CA1 neurons that are quite sensitive and vulnerable to seizures, traumas, and ischemic insults. Epilepsy normally produces loss of pyramidal neurons, but CA2 cells are resistant with less cell loss observed after epilepsy. The presence of KARs at SC-CA2 synapses highlights the need to further research dissecting the putative role of the described KARs in the CA2 region mediating a decrease in glutamate release after exposure of increasing glutamate concentrations and their role promoting neuronal network stability as opposed to the known involvement of KARs in epilepsy (Falcón-Moya et al., 2018).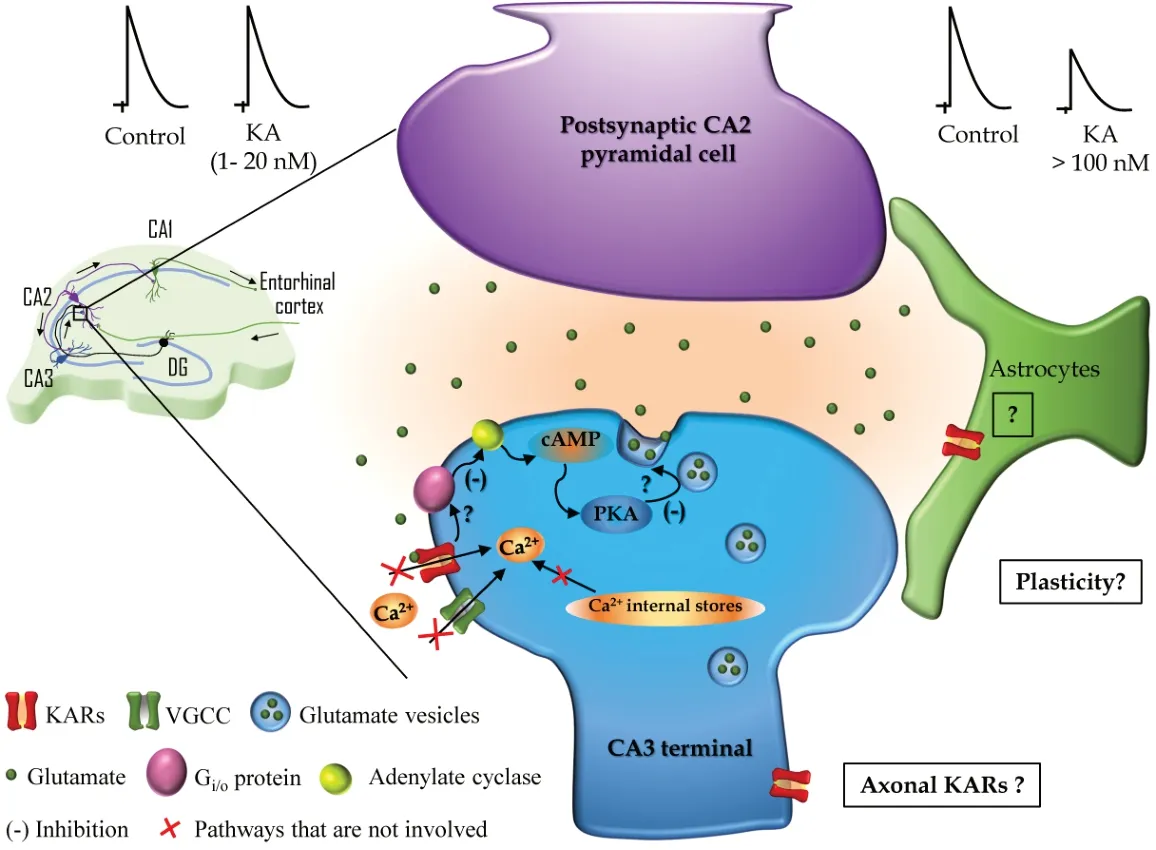
Figure 1|Kainate receptors (KAR) in the CA2 region of the hippocampus. Left: Schematic representation showing the connections of a pyramidal cell from the CA2 region within the classical trisynaptic hippocampal circuit. Black arrows indicate the direction of the information flow along CA3, CA2, and CA1 including the input from the entorhinal cortex and the dentate gyrus (DG). Synaptic contacts are represented along the dendritic arbor and the synapse studied by Falcón-Moya et al. (2021) (CA3–CA2 synapse) is marked with a little box and magnified on the right. Right: Schematic representation of the mechanism described by Falcón-Moya et al. (2021) showing that relatively low doses of KA (1–20 nM, upper left) do not affect N-methyl-D-aspartate (NMDA)-mediated excitatory postsynaptic currents (EPSCs), while higher doses (> 100 nM, upper right) induces a decrease of NMDAEPSCs amplitude. In the KAR-mediated decrease of glutamate release at CA3–CA2 synapses the postsynaptic neuron (CA2 pyramidal cell in magenta) is not involved and in the presynaptic neuron (CA3 terminal in blue), KAR activation leads to inhibition (-) of the adenylyl cyclase mediated by a Gi/o-protein and the downstream regulation of cAMP and protein kinase A activity that ultimately decreases glutamate release. How KAR interacts with the Gi/o-protein and the substrate of PKA leading to a decrease in glutamate release at CA3-CA2 synapses are represented with question marks and remain to be elucidated. In addition, the described mechanism does not involve Ca2+ mobilization from the internal stores, neither Ca2+ influx through voltage-gated calcium channels (VGCC) nor calcium-permeable KARs. Question marks in the boxes represent future directions based on the recent findings by Falcón-Moya et al. (2021): Are KARs present in astrocytes at these synapses? Are KARs located at the axonal compartment far from the axonal button? Do KARs participate in synaptic plasticity at CA3–CA2 synapses?
In conclusion, KARs are functionally present at SC-CA2 excitatory synapses and the presynaptic activation of KARs by KA at these synapses produces a depression of synaptic transmission and a decrease in the amount of glutamate released. Mechanistically, KAR-mediated presynaptic depression involves the activation of a G-protein that would signal the activation of AC to reduce cAMP levels to mediate a decrease in glutamate release and, therefore, in synaptic transmission at the SC-CA2 PC synapses of the hippocampus. Whether KARs present in the CA2 region of the hippocampus have a role in social behavior or spatial or temporal memories requires further experimentation, but modulating glutamate release, KARs may directly control or affect excitation/inhibition ratio that may be involved in different functions including the proper network performance and their behavioral outcomes.
Future directions:
The determination of the presence of functional KAR in the CA2 region of the hippocampus advances our understanding of the widespread presence of KARs in pivotal brain structures, subregions, and synapses. Although this study by Falcón-Moya et al. (2021) brings a kind of starting cornerstone in the field of KARs functions in this overlooked but relevant hippocampal region, it reveals that much research is needed in this field to provide answers to remaining questions such as 1) Do KARs have a role in plasticity in the CA2 region of the hippocampus? 2) Are glial cells in the CA2 region involved in the resistance to damage of this region and, should it be the case, are KARs involved? 3) Do KARs play a role in CA2 during development? In addition, in future studies, a possible facilitation of glutamate release and the mechanism involved should be investigated in this synapse to determine whether the aforementioned bimodal regulation of neurotransmitter release by KARs is a general mechanism or not. Moreover, in adults, modulation involves PKA and the AC/cAMP/PKA pathway. The substrate for PKA-mediated phosphorylation that underpins the decrease in glutamate at this area remains to be determined.The work performed in ARM laboratory received support from the Agencia Estatal de Investigación and FEDER (BFU2015-68655-P).
Yuniesky Andrade-Talavera, Antonio Rodríguez-Moreno
Laboratorio de Neurociencia Celulary Plasticidad, Departamento de Fisiología, Anatomía y Biología Celular, Universidad Pablo de Olavide, Sevilla, Spain
Correspondence to:
Antonio Rodríguez-Moreno, PhD, arodmor@upo.es.https://orcid.org/0000-0002-8078-6175
(Antonio Rodríguez-Moreno)
Date of submission:
February 2, 2022Date of decision:
March 2, 2022Date of acceptance:
March 16, 2022Date of web publication:
July 1, 2022https://doi.org/10.4103/1673-5374.343912
How to cite this article:
Andrade-Talavera Y, Rodríguez-Moreno A (2023) Kainate receptors in the CA2 region of the hippocampus. Neural Regen Res 18(2):320-321.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Advances in quantitative analysis of astrocytes using machine learning
- Nicotinic acetylcholine signaling is required for motor learning but not for rehabilitation from spinal cord injury
- Understanding the timing of brain injury in fetal growth restriction: lessons from a model of spontaneous growth restriction in piglets
- MicroRNA-based targeting of the Rho/ROCK pathway in therapeutic strategies after spinal cord injury
- Intrathecal liproxstatin-1 delivery inhibits ferroptosis and attenuates mechanical and thermal hypersensitivities in rats with complete Freund’s adjuvant-induced inflammatory pain
- Commentary on “PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons”

