Nicotinic acetylcholine signaling is required for motor learning but not for rehabilitation from spinal cord injury
Yue Li , Edmund R. Hollis II
Abstract Therapeutic intervention for spinal cord injury is limited, with many approaches relying on strengthening the remaining substrate and driving recovery through rehabilitative training. As compared with learning novel compensatory strategies, rehabilitation focuses on restoring movements lost to injury. Whether rehabilitation of previously learned movements after spinal cord injury requires the molecular mechanisms of motor learning, or if it engages previously trained motor circuits without requiring novel learning remains an open question. In this study, mice were randomly assigned to receive intraperitoneal injection with the pan-nicotinic, non-competitive antagonist mecamylamine and the nicotinic α7 subunit selective antagonist methyllycaconitine citrate salt or vehicle (normal saline) prior to motor learning assays, then randomly reassigned after motor learning for rehabilitation study post-injury. Cervical spinal cord dorsal column lesion was used as a model of incomplete injury. Results of this study showed that nicotinic acetylcholine signaling was required for motor learning of the single pellet-reaching task but it was dispensable for the rehabilitation of the same task after injury. Our findings indicate that critical differences exist between the molecular mechanisms supporting compensatory motor learning strategies and the restoration of behavior lost to spinal cord injury.
Key Words: acetylcholine; basal forebrain; corticospinal tract; dorsal column lesion; mecamylamine; methyllycaconitine; motor control; rehabilitation; rotarod; single pellet-reaching task
Introduction 364 Methods 365 Results 365 Discussion 366
Graphical Abstract
Nicotinic acetylcholine signaling is required for motor skill learning but not for recovery of the same skill after spinal cord injury

Introduction
Spinal cord injury (SCI) results in the lasting impairment of the motor and sensory functions that underlie movement. The majority of clinical cases of SCI are incomplete, allowing for a limited capacity for spontaneous or rehabilitation-mediated functional recovery (Fawcett et al., 2007). This capacity for partial recovery may be achieved through reinforcement of spared sensory and motor axons, compensatory activities of indirect pathways, or reorganization of supraspinal command centers (Hollis et al., 2016; Li and Hollis, 2017). The corticospinal tract is critical to restoring supraspinal command of voluntary movement. Following stroke, the extent of spared corticospinal tract is proportional to the amount of spontaneous recovery that individuals experience (Stinear et al., 2007). In chronic SCI, the use of rehabilitation and epidural electrical stimulation to restore voluntary locomotion likely leverages preserved corticospinal circuitry (Wagner et al., 2018). These spared circuits can be activated by cortical stimulation even in motor complete, chronically injured individuals (Edwards et al., 2013).
Both rodent and non-human primate models have been used to demonstrate the innate plasticity of corticospinal axons after injury (Rosenzweig et al., 2010; Mosberger et al., 2017). Within the injured spinal cord, corticospinal axons sprout locally and form novel axon collaterals, many of which are pruned back over time (Bareyre et al., 2004). The removal of intrinsic brakes on axon regeneration can enhance corticospinal axon plasticity and connectivity; however, the contribution of such connections to behavioral recovery is not always apparent (Liu et al., 2010; Hollis et al., 2016; Jayaprakash et al., 2016). Rehabilitative training drives recovery of previously trained, corticospinal tract-dependent, single pellet reach behavior in animal models of corticospinal tract injury (Wahl et al., 2014; Hollis II et al., 2016). Clinically, training after SCI can be used to either rehabilitate movements lost to injury, or to train compensatory strategies to improve mobility and independence (Behrman and Harkema, 2007). Animal models used to study recovery from SCI often rely on trained behavior; however, questions remain as to whether rehabilitation-mediated recovery represents novel learning, or a re-emergence of patterned movements using the remaining motor circuitry.
Motor learning requires contributions from various brain areas, including motor cortex, cerebellum, striatum, and brainstem. Basal forebrain cholinergic neurons release acetylcholine in distinct targets and modulate a diverse array of functions, such as motor control, attention, cognition, and perception coding (Zaborszky et al., 2018; Boskovic et al., 2019). The cerebral cortex receives cholinergic input from nucleus basalis of Meynert (NBM). Primary motor cortex (M1) depends upon these basal forebrain cholinergic neurons for the maturation of cortical motor representations, or motor maps (Ramanathan et al., 2015). Ablation of NBM cholinergic neurons in rats attenuates skill acquisition in the single pellet-reaching task as well as the corresponding expansion of cortical forelimb motor representations and dendritic spine remodeling of corticospinal neurons that control the distal forelimb (Conner et al., 2003; Wang et al., 2016). Following cortical injury, rehabilitative training of skilled forelimb movements results in reconstitution of affected movement representations within adjacent, ectopic cortical areas (Castro-Alamancos et al., 1992; Castro-Alamancos and Borrel, 1995). As with motor learning, cholinergic input is critical for motor map reorganization and functional recovery after cortical injury (Friel et al., 2000; Conner et al., 2005). Previously, we observed similar rehabilitation-dependent cortical reorganization and functional recovery after SCI (Hollis et al., 2016). Unlike following stroke, cortical structures remain intact after SCI. Cortical and other supraspinal motor centers are likely instrumental in driving rehabilitationmediated recovery, but it remains unknown what role the molecular and cellular mechanisms of motor learning play in this recovery.
Nicotinic acetylcholine receptors expressed in the central nervous system are important for synaptic excitation, attention, and cognition (Dani, 2001). In primary visual cortex, cholinergic innervation is required for experiencedependent plasticity during the critical period (Bear and Singer, 1986) and closure of the critical period is associated with reduced nicotinic signaling (Morishita et al., 2010). Here we tested the role of nicotinic cholinergic signaling in skilled motor task acquisition and rehabilitation after SCI.
Methods
Animals
All animal experiments and procedures were approved by the Weill Cornell Medicine Institutional Animal Care and Use Committee (protocol # 2015-0042) on May 12, 2018. All mice were housed on a 12-hour light/dark cycle from 9 a.m. to 9 p.m. at 25°C with free access to food and water. Twenty-four male and female C57BL/6J animals (8–12 weeks old) were purchased from Jackson Laboratory. For forelimb reaching task, animals were food restricted to 80–90% of their free-feeding bodyweight. Cervical spinal cord dorsal column lesion was used as a model of incomplete injury. Twelve mice used in this study performed the recessed single pellet-reaching task, the rotarod test, and the open field test. In each behavior test, six animals were injected with either nicotinic inhibitors or saline control. Experimental design is shown inFigure 1
.
Figure 1|Experimental design.
Drug administration
Mice were randomly assigned to drug or control groups prior to motor learning assays, then randomly reassigned after motor learning for rehabilitation study post-injury. Mice were injected intraperitoneally with the pan-nicotinic, non-competitive antagonist mecamylamine (MEC, 5 mg/kg; Tocris, Minneapolis, MN, USA, Cat# 2843) and the nicotinic α7 subunit selective antagonist methyllycaconitine citrate (MLA, 5 mg/kg; MilliporeSigma, Burlington, MA, USA, Cat # M168) or vehicle (normal saline) 30 minutes before behavioral testing (Grottick and Higgins, 2000; Shi et al., 2011; Kita et al., 2013).
Recessed single pellet-reaching task
To test the effect of nicotinic inhibition on skilled motor learning, we employed a recessed single pellet-reaching task as described previously (Li and Hollis, 2021). Animals were calorie restricted to 80–90% of their freefeeding bodyweight by being given 1–3 g food before training. An acrylic behavior box (length × width × height: 29.5 cm × 21.9 cm × 21.6 cm) with three slots (7 mm wide) on the left, middle, and right sides of the front wall was used to train the mice. A recessed hole (3 mm wide, 2 mm deep) at 12 mm from the inside wall of the box was used to hold a 20-mg flavored food pellet (Bio-Serv, Flemington, NJ, USA, Cat# F05301). The dominant forelimb was identified during a single test session. Once the dominant forelimb was determined, it was trained over a total of 14 daily sessions consisting of 25 trials each. A trial was counted as a success if the mouse grasped, retrieved, and ate the food pellet. Only trials with pellet contact were counted. The intensive rehabilitative training was performed 1 week after SCI. During the motor learning phase, animals were calorie-restricted to 80–90% of their freefeeding bodyweight before retraining in the pellet-reaching task. Mice were trained 25 trials daily and 15 sessions in total. The successful retrieval rate was defined as the percentage of trials with successful pellet retrieval and eating. To account for day-to-day variability in performance, the peak success rate from the last three sessions of skilled forelimb training was used for preinjury values in SCI experiment.
Rotarod test
To test the effect of nicotinic inhibition on coordinated motor learning, mice were habituated on the rotarod (Med Associates, St. Albans, VT, USA, Cat# ENV-577M) at a speed of 4 r/min for 60 seconds before testing. For each trial, the rotarod accelerated from 4 to 40 r/min over 300 seconds, and then remained at 40 r/min for an additional 300 seconds, as necessary. Animals were tested 30 minutes after intraperitoneal injection of MEC and MLA or vehicle, and one trial was performed per day. The latency to fall after the onset of acceleration during each trial was recorded by the rotarod for each mouse. Individual trials were stopped, and the duration was recorded, if mice could not run with consecutive rotations or failed to stay on the rotarod. If animals successfully completed 600 seconds on the rotarod, the latency was recorded as 600 seconds.
Open field test
To test animal activity, mice were placed in a chamber (length × width × height: 30 cm × 22.5 cm × 25 cm) and allowed to explore for 5 minutes. Behavior was recorded from the top at 48 frames per second (GoPro camera, San Mateo, CA, US; Model HERO3) and total walking distance was analyzed by MATLAB software (MathWorks, Natick, MA, USA) (Autotyping15.04) (Patel et al., 2014).
C5 dorsal column spinal cord lesion
Two days after finishing the washout session of forelimb reach task, SCI was performed in both control and drug-treated animals. Mice were anesthetized with 4% isoflurane (VetOne, Boise, ID, USA) during surgery with 1.5–3% isoflurane, and their body temperature was maintained at 37°C using a SomnoSuite small animal anesthesia system (Kent Scientific, Torrington, CT, USA). Subcutaneous injection of the analgesic buprenorphine (0.1 mg/kg) was given immediately following anesthesia. Spinal level C5 was exposed by laminectomy and the dorsal columns were lesioned at a depth of 1 mm with Vannas spring scissors (Fine Science Tools, Foster City, CA, USA), as previously performed (Hollis et al., 2016). The dorsal musculature was sutured with 6-0 suture and the skin was closed with wound clips. Mice were allowed to recover from surgery for 1 week before rehabilitative training, allowing recovery from the effects of laminectomy and the hyporeflexic phase of spinal shock (Ditunno et al., 2004).
Histolo
gyTo confirm SCI, after finishing all behavioral trainings, animals were anesthetized with ketamine/xylazine cocktail (ketamine, 150 mg/kg, McGuff, Santa Ana, CA, USA; xylazine, 15 mg/kg, VetOne) and transcardially perfused with ice-cold PBS followed by 4% paraformaldehyde (PFA). Spinal columns were postfixed in 4% PFA overnight at 4°C, cryoprotected by immersion in 30% sucrose in 0.1 M PBS for 2 days. Samples were then sagittally cryosectioned at 20 µm using a Leica cryostat and directly mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA, USA). Sections were blocked with 10% donkey serum for 1 hour, and incubated with rabbit anti-PKCγ (a marker for corticospinal axons; 1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA, sc-211, RRID: AB_632234) and mouse anti-GFAP (a marker for astrocytes; 1:750, Abcam, Cat# ab10065, RRID: AB_296804) antibodies for 2 days at 4°C. Sections were then washed three times with PBS and incubated with fluorescently conjugated donkey anti-rabbit/mouse secondary antibody (1:200, Jackson ImmunoResearch, Cat# AB_2340854, RRID: AB_2313584) for 1.5 hours at room temperature. Images were acquired on a Leica SP8 confocal microscope with 10× objective.
Statistical analysis
We used power analysis with a 40% effect size, an alpha of 0.05 and beta of 0.2, to generate our estimated group sizes based on our previous study (Li and Hollis, 2021). All procedures and analysis were performed blind to treatment assignment. Skilled pellet-reaching and rotarod tests were analyzed using twoway repeated measures analysis of variance withpost hoc
Sidak’s comparison test using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA, www.graphpad.com). The differences between two groups were compared by twotailed unpairedt
-tests. The intra-group differences were analyzed using pairedt
-test.P
< 0.05 was considered statistically significant.Results
Systemic inhibition of nicotinic receptors impairs motor learning
We used a pharmacological approach to study the contribution of nicotinic receptors to motor learning in adult mice. MEC and methyllycaconitine were injected intraperitoneally 30 minutes prior to behavioral training. Control mice were injected with normal saline. Single pellet reaching is a skilled behavior used to measure dexterity of a single forelimb (Whishaw and Pellis, 1990). We employed a modified recessed single pellet-reaching task in which the food pellet is retrieved from a concave depression (Figure 2A
); we previously found that this modification allows for a consistent learning curve in C57BL/6J mice (Li and Hollis, 2021). We found that systemic blockade of nicotinic receptors significantly attenuated skilled motor learning in the single pellet-reaching task (Figure 2B
andC
). MEC and MLA-treated mice showed smaller improvements over the course of training than controls. Washout of MEC and MLA over 5 days enabled the mice to learn the task with the same proficiency as controls (Figure 2B
andC
).The effects of systematic inhibition of nicotinic acetylcholine signaling were not specific for skilled forelimb motor learning. MEC and MLA administration also impaired coordinated motor learning on the accelerating rotarod task. Mice trained over nine trials (4 to 40 r/min, constant acceleration over 5 minutes) exhibited worse performance following intraperitoneal injection with MEC and MLA compared to control mice. MEC and MLA inhibition of nicotinic signaling resulted in significantly reduced latency to fall (Figure 2D
). As with single pellet reaching, washout of MEC and MLA over 5 days enabled the mice to learn the rotarod with the same proficiency as controls (Figure
2D
). Additionally, MEC and MLA significantly reduced total walking distance in an open field test (Figure 2E
).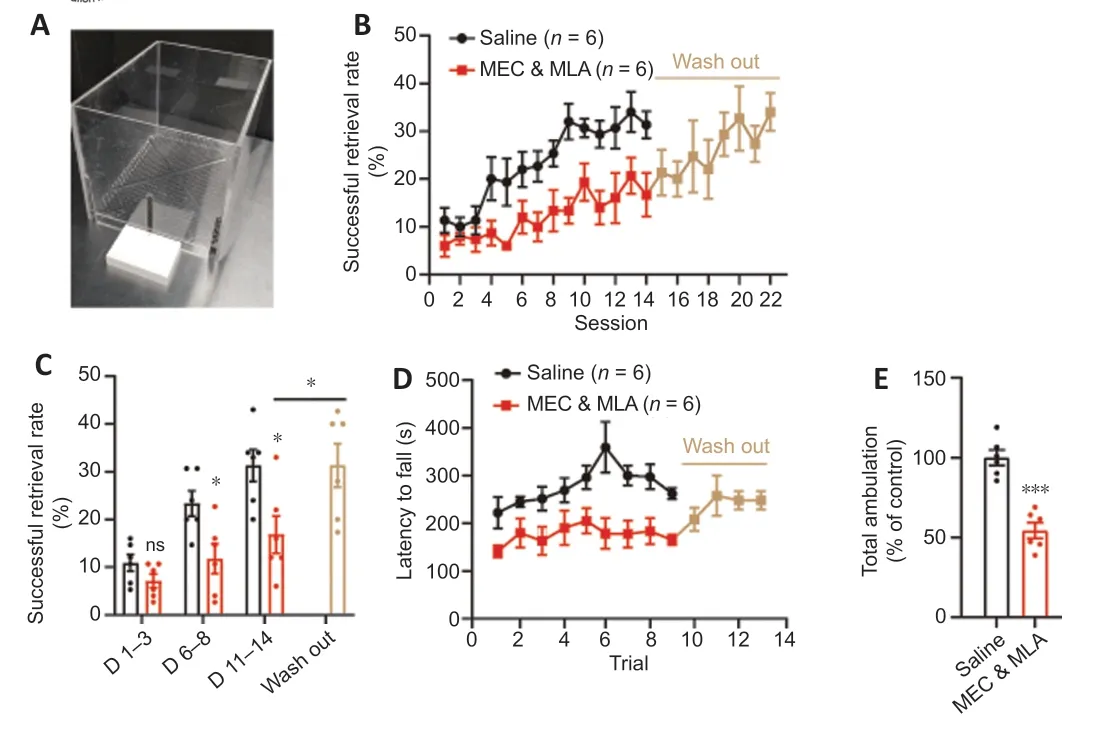
Figure 2| Inhibition of nicotinic acetylcholine signaling impairs motor learning. (A) Forelimb reaching box. (B) Intraperitoneal injection of MEC and MLA 30 minutes prior to training significantly attenuated learning on the recessed single pellet-reaching task (P = 0.009, repeated measures analysis of variance, F(1,10) = 10.6). After 14 days of forelimb reach training, MEC and MLA treatment was stopped. Five days later, mice were trained for an additional 8 days without treatment. (C) Mean value analysis of successful retrieval rate. Day 1–3 is the initial phase of motor learning, day 6–8 is the rising phase, and day 11–14 is the plateau phase. MEC and MLA treatment had no effect during the initial phase, but the mean values of successful retrieval rate during the rising and plateau phases were significantly lower in drug-injected animals than those in controls (*P < 0.05, unpaired t-test). Following wash out of MEC and MLA, the mice were able to learn the task (mean success rate over days 19–22 vs. days 11–14, *P < 0.05, paired t-test). (D) Administration of MEC and MLA impaired rotarod training performance (P = 0.007, repeated measures analysis of variance, F(1,10) = 11.62). Following wash out, mice were able to learn the rotarod task. (E) Total walking distance was shorter in mice injected with MEC and MLA (n = 6/group, ***P < 0.001, unpaired t-test) compared with that in mice injected with saline. MEC: Mecamylamine; MLA: methyllycaconitine citrate.
Nicotinic signaling is not required for functional recovery following SCI
To test whether nicotinic signaling is also required for functional recovery, we randomly reassigned animals after motor learning, performed a cervical SCI, and tested the effects of MEC and MLA on intensive rehabilitative training on the single pellet-reaching task. We performed a dorsal column lesion at cervical spinal cord segment 5 (C5) to transect the corticospinal and ascending dorsal column tracts, leaving most gray matter, lateral white matter, and ventral spinal cord intact (Figure 3A
andB
). One week after C5 dorsal column lesion, intensive rehabilitative training was carried out in which animals were tested daily on the recessed pellet-reaching task. SCI significantly impaired task success (Figure 3C
). Intensive rehabilitative training promoted recovery to levels similar to pre-injury, regardless of treatment group; MEC and MLA delivery had no effect on the recovery of function (Figure 3C
).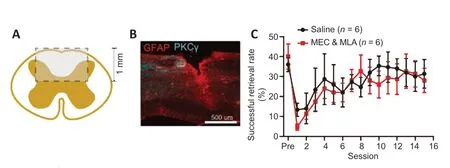
Figure 3| Inhibition of nicotinic receptors does not prevent functional recovery after spinal cord injury. (A) Illustration depicting a transverse view of the spinal cord lesion (rectangular box: lesion area). (B) Representative image of injured cervical spinal cord with PKCγ-labeled corticospinal axons and GFAP-labeled astrocytes. (C) Functional recovery through rehabilitation after spinal cord injury. Animals injected with MEC and MLA show similar functional recovery on the single pellet-reaching behavior to vehicle-treated control animals (P = 0.76, repeated measures analysis of variance, F(1,10) = 0.1026). GFAP: Glial fibrillary acidic protein; MEC: mecamylamine; MLA: methyllycaconitine citrate; PKCγ: protein kinase C gamma.
Discussion
In this study, we used pharmacological tools to demonstrate that nicotinic acetylcholine signaling is required for the acquisition of motor skills but not the rehabilitation-mediated recovery of the previously trained skills after SCI. Recently, we have found that mice, unlike rats, do not require basal forebrain cholinergic input to cortex for the acquisition of skilled motor learning on the forelimb reach task (Li and Hollis, 2021). This leaves open the question of the locus of nicotinic activity during motor learning. In our previous study, we targeted both NBM cholinergic neurons directly, through targeted toxin, genetic, and optogenetic means, as well as the projections of NBM cholinergic neurons to motor centers in medial prefrontal cortex and primary motor cortex, leaving cholinergic innervation of striatum, brainstem, cerebellum, spinal cord, and periphery intact. Nicotinic signaling is likely active in one of these other motor loci during motor learning.
During rehabilitation from SCI, the extent to which rehabilitation either relies on the execution of previously encoded motor programs or else leverages the cellular and molecular mechanisms of motor learning is not known. Motor cortex plasticity occurs alongside the acquisition of skilled motor learning and we previously found that rehabilitation on the single pellet-reaching task after SCI shapes both behavioral recovery and cortical plasticity (Hollis et al., 2016). During motor learning, the role of motor cortex diminishes with the development of task proficiency. Inactivation of primary motor cortex early in training of a forelimb lever press task impaired performance; however, cortical silencing after an extensive training period had little effect on task success or movement kinetics (Hwang et al., 2019). In fact, the execution of a similar trained temporally precise lever press task is essentially unperturbed by the bilateral aspiration of the entire motor cortex (Kawai et al., 2015). The declining role of motor cortex in execution of learned behavior is reflected in the absence of a role for cholinergic signaling following training. We previously found that ablation of cholinergic innervation of motor cortex after coordinated motor learning of rotarod behavior had no effect on task execution (Li and Hollis, 2021), similar to the absence of effects on single pellet-reaching task success in rats when cholinergic neurons were ablated after training (Conner et al., 2003). Thus, when animals become proficient in a motor skill, motor cortex disengages from the behavior and subcortical structures (such as basal ganglia, red nucleus, brain stem, and cerebellum) are sufficient for maintenance of previously learned motor skills (Hikosaka et al., 2002).
The spinal cord receives multiple motor inputs and these supraspinal circuits are likely to control different aspects of movement execution. Our dorsal column SCI was limited to transection of the main body of the descending corticospinal tract and the ascending dorsal column-medial lemniscal sensory circuit, leaving other supraspinal pathways intact, including rubrospinal, reticulospinal, and the lateral, minor corticospinal tracts. It may be that the remaining supraspinal motor circuits retain the motor patterns encoded through training needed to compensate for the loss of dorsal column circuitry, or that nicotinic signaling plays no role in the shaping of these alternate motor pathways. Others have implicated a role for spared ventral corticospinal axons in rats and dorsolateral corticospinal axons in mice in the restoration of corticospinal-dependent behaviors, with limited effect on cortical motor representations (Weidner et al., 2001; Hilton et al., 2016). While we found no role for nicotinic signaling during intensive rehabilitative training to restore a previously trained, stereotyped movement, nicotinic signaling is likely to play a role in the learning of novel, compensatory movement strategies employed by individuals to regain independence after SCI.
Acknowledgments:
The authors are grateful to the Burke Neurological Institute Structural and Functional Imaging Core for providing the resources used in image acquisition.
Author contributions:
YL and ERH designed the study and wrote the manuscript. YL performed the experiments and analyzed data. Both authors approved the final version of the manuscript.
Conflicts of interest:
The authors declare that there are no conflicts of interest associated with this manuscript.
Availability of data and materials:
The complete dataset is available at doi.gin.g-node.org/10.12751/g-node.atjjnn/.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Advances in quantitative analysis of astrocytes using machine learning
- Commentary on “PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons”
- Kainate receptors in the CA2 region of the hippocampus
- MicroRNA-based targeting of the Rho/ROCK pathway in therapeutic strategies after spinal cord injury
- Intrathecal liproxstatin-1 delivery inhibits ferroptosis and attenuates mechanical and thermal hypersensitivities in rats with complete Freund’s adjuvant-induced inflammatory pain
- Understanding the timing of brain injury in fetal growth restriction: lessons from a model of spontaneous growth restriction in piglets

