Improving polyp detection at colonoscopy: Non-technological techniques
Ragul Rajivan, Sreedhari Thayalasekaran
Ragul Rajivan, Buckingham Medical School, Milton Keynes MK18 1EG, United Kingdom
Sreedhari Thayalasekaran, Department of Gastroenterology, University Hospitals of Leicester,Leicester LE1 5WW, United Kingdom
Abstract
Colonoscopy and polypectomy remain the gold standard investigation for the detection and prevention of colorectal cancer. Halting the progression of colonic adenoma through adequate detection of pre-cancerous lesions interrupts the progression to carcinoma. The adenoma detection rate is a key performance indicator. Increasing adenoma detection rates are associated with reducing rates of interval colorectal cancer. Endoscopists with high baseline adenoma detection rate have a meticulous technique during colonoscopy withdrawal that improves their adenoma detection. This minireview article summarizes the evidence on the following simple operator techniques and their effects on the adenoma detection rate; minimum withdrawal times, dynamic patient position change and proximal colon retroflexion.
Key Words: Colonoscopy; Minimum withdrawal times; Dynamic position change;Proximal colon retroflexion
INTRODUCTION
Colorectal cancer is the 3rdmost common cancer globally[1] occurring in 8% and 12% of all new cancer cases in the USA and UK, respectively[2]. Most colorectal cancers developviathe adenoma-carcinoma sequence[3,4]. Sessile serrated lesions are also recognised precursor lesions to colorectal cancerviathe CpG island-methylated pathway[5]. The polyp and adenoma detection rate is defined as the number of colonoscopies where at least one polyp and adenoma are detected respectively[6].
Colonoscopy remains the gold standard investigation for the detection and prevention of colorectal cancer[6]. The strength of colonoscopy lies in its ability to not only detect colorectal cancer but also prevent it through the early removal of polypsviapolypectomy, halting the progression of adenoma to colorectal cancer[7]. Despite, colonoscopy being the best investigative tool, colonic neoplasia is still missed at colonoscopy[8,9]. The reported miss rates of colorectal cancer and adenoma are 5% and up to 20% respectively[8-10]. The beneficial effects of colonoscopy are less obvious in the prevention of rightsided colonic cancers[11].
The adenoma detection rate (ADR) has been identified as a key performance indicator in colonoscopy[6]. The ADR is inversely proportional to the risk of interval colorectal cancer. In a large cohort study,endoscopists with an ADR ≥ 20% were found to have the lowest rates of interval colorectal cancer[12].Another pivotal study found a 3% reduction rate for interval colorectal cancers, with every 1% increase in the endoscopist’s ADR[13].
There has been significant research focusing on the identification of factors that could potentially improve the adenoma detection rate. Initial studies focused on evaluating simple operator techniques.More recently, research has been published with conflicting evidence evaluating both digital and dyebased chromoendoscopy, water-assisted colonoscopy, distal attachment devices, wide-angle colonoscopy, and artificial intelligence in the role of polyp detection.
This minireview gives an outline of the simple operator techniques (minimum withdrawal time,position change and proximal colon retroflexion) to improve polyp detection.
WITHDRAWAL TIMES
Withdrawal time is the time taken to inspect the colonic mucosa from the caecum to the anal canal after caecal intubation has been achieved[14]. The first study to show an association between a minimum withdrawal time and high-quality colonoscopy was a small study by Rexet al[15]. In this study 2 endoscopists (one with a greater adenoma miss rate than the other) had 10 consecutive colonoscopy withdrawals videotaped and evaluated by a group of 4 expert endoscopists who were blinded to which endoscopist had performed each procedure. Along with a minimum withdrawal time, each video was evaluated for adequacy of examination of proximal flexures and folds, washing, suctioning and luminal distension. The experts scored the colonoscopist with the lower miss rate much higher in each of the domains,P< 0.001[15]. The recommendation from the Multi-Society Task Force on Colorectal Cancer that a withdrawal time for colonoscopy should average 6-10 min (without the inclusion of time taken for polypectomy and biopsy) followed[16], but was based on limited scientific information[15].
In a landmark prospective study of 12 Gastroenterologists performing 7882 colonoscopies in a community-based setting by Barclayet al[17] over 15 mo, the adenoma detection rate in endoscopists with mean withdrawal times of < 6 min was compared to the adenoma detection rate in endoscopists with mean withdrawal times of > 6 min. Gastroenterologists with a mean withdrawal time of 6 min or more detected a greater number of adenomas (28.3%) compared to endoscopists with a mean withdrawal time of 6 min or less (11.8%),P< 0.001. This trend was also reflected in the greater detection of advanced neoplasia in 6.4% where withdrawal time ≥ 6 minvs2.6% where withdrawal times were ≤ 6 min,P= 0.005. The definition of advanced adenoma in this study included; ≥ 10 mm in size, villous component, high-grade dysplasia, or cancer. Most of the advanced lesions were ≥ 10 mm in size. 2 small polyps with high-grade dysplasia and invasive cancer were 5 mm and 7 mm in size respectively[17].
In another study, the same group compared the detection of colonic neoplasia amongst 12 endoscopists following the implementation of a quality improvement intervention. The intervention incorporated techniques such as adequate air insufflation, washing the colonic mucosa, torque manoeuvres to flatten colonic folds, and repeated examination of colonic segments, within a minimum withdrawal time of 8 min. Following the intervention, endoscopists with mean withdrawal times of ≥ 8 min had greater rates of neoplasia detection (37.8%vs23.3%,P< 0.0001) and also advanced neoplasia detection (6.6%vs4.5%,P= 0.13)[18]. Advanced adenomas occur less frequently, and it is often difficult to make statistically significant conclusions from sub-group analysis. Larger studies are required to obtain adequate power, which is often not feasible. A limitation of this study was the comparison of a historical control group with the post-intervention group.
This study showed that the incorporation of a minimum withdrawal time into a quality intervention improves neoplasia detection. Evidence from this study is not enough to support minimum withdrawal times in isolation, without considering the implementation of other withdrawal techniques[18].
In a large study of 23910 colonoscopies, adherence to a departmental-wide policy of a 7-min minimum withdrawal time for negative colonoscopies (no polyps removed) showed no statistically significant improvement in the polyp detection rate. A limitation of this study is that the withdrawal times were only available as < 7 min or ≥ 7 min, which limited the ability to establish if there was a trend. Strengths of this study included the large size with the incorporation of 42 endoscopists with wide levels of experience, reflecting more widespread endoscopic practice[19]. Good withdrawal technique involves careful inspection behind folds and flexures, adequately distending the colonic lumen, washing the colonic mucosa, and suctioning excess fluid or faecal debris[19]. Endoscopists who perform high-quality colonoscopies are likely to take more time performing these manoeuvres than those that don’t. Longer withdrawal times are more likely to be a correlation between good colonoscopy technique, than causation. The study from Sawhneyet al[19] shows that simply implementing a mandatory departmental-wide policy of minimum withdrawal time, without incorporation of other high-quality colonoscopy manoeuvres, was not sufficient to increase neoplasia detection[19]. By contrast, the study by Barclayet al[18], showed that with a quality intervention program focusing on improving colonoscopy manoeuvres, coupled with a minimum withdrawal time, a significant improvement in neoplasia detection was noted.
More of the studies performed so far have focused on evaluating the effects of a minimum withdrawal time on experienced endoscopists[17,18,20]. Gromskiet al[21] performed a study evaluating the performance of four 1styear Gastroenterology trainees at a teaching centre who had to adhere to a 6-min minimum withdrawal time. Trainees that had withdrawal times > 10 min had an ADR of 32.3% compared to trainees with withdrawal times < 10 min who had an ADR of 9.5%,P< 0.001[21]. This study was limited in its ability to draw firm conclusions as it was a single-centre study, involving the analysis of only 4 trainees performing 1210 colonoscopies in total[21].
In the largest observational study to date, 31,088 screening colonoscopies in the National Health Service bowel cancer screening program performed by 147 colonoscopists in the United Kingdom were evaluated[20]. This study found that with a withdrawal time of < 7 min, the ADR was 42.5% compared to an ADR of 47.1% with a withdrawal time of ≥ 11 min,P< 0.001. The main increase was noted in subcentimetre or proximally located adenomas. No statistically significant difference was noted in the detection of advanced adenoma with longer withdrawal times. The entire study cohort had a positive faecal occult blood test[20]. The optimal withdrawal time suggested was 10 min[20], rather than 6-8 min as previously reported[17,18,22]. Beyond 10 min, there were minimal gains in the ADR[20]. The current minimum standard in bowel cancer screening programmes is 6 min, which is sufficient to detect advanced adenoma. The study by Leeet al[20] suggests that increasing it to between 6-10 min might increase the detection of small and proximal adenomas. The miss rate of proximal neoplasia is well recognised[10]. Proximal colorectal neoplasia is more difficult to detect; it can be flatter and quicker to progress to colorectal cancer[23]. A strength of the study by Leeet al[20] is that it did look at the prevalence of adenoma detected according to lesion location in the colon. The ability to make conclusions outside of a positive faecal occult blood cohort as in this study is a limitation[20].
In a prospective multi-centre Norwegian study by Moritzet al[24], no statistically significant difference was found in the detection of polyps between endoscopists with a withdrawal time of < 6 min compared to those with withdrawal times ≥ 6 min[24]. The overall withdrawal time, which includes time for polypectomy and biopsy, was separated from the visual withdrawal time, where therapy was not included. This methodological approach was a strength of their study design. In other studies[22,25]withdrawal times for negative colonoscopies were used for the analysis[24].
In a single centre randomized controlled trial (RCT) with 1160 patients, Coghlanet al[26] compared colonoscopy with specified withdrawal times in different colonic segments (right colon, transverse colon, and left colon) to a minimum free colonoscopy withdrawal time of at least 6 min[26]. A strength of this study was the cessation of recording times when polypectomy was performed, with re-starting when re-examination of the colon continued. The overall ADR was 41% supporting other studies that withdrawal times of at least 6 min are associated with increased neoplasia detection. No significant statistical differences in ADR were seen when comparing the fixed withdrawal limb to the conventional free withdrawal limb; 42.1%vs39.8%,P= 0.43 respectively. This RCT was the first study to evaluate timed colonic segment withdrawal to conventional minimum withdrawal. It is, however, a single-centre study, so limited conclusions can be drawn in terms of widespread applicability[26].
An observational study by Gelladet al[27] was the first study to evaluate the association of withdrawal time to missed adenomas at subsequent colonic examination[27]. In this multi-centre study,1441 of 3121 patients in total had no polyps at baseline colonoscopy. 304/1441 subjects returned for follow-up colonoscopy within 5.5 years. 16.2% (49 people) of the study participants with no polyps seen initially had interval neoplasia, including 7 advanced adenomas and 1 invasive cancer. No association between the withdrawal time and risk of interval neoplasia was seen. A mean baseline withdrawal time of > 12 min was observed. The study findings did show a statistically significant association between the mean withdrawal time and adenoma detection rate at baseline,P= 0.03[27]. However, after a threshold between 5.2 and 8.6 min, no additional benefit was conferred to the detection of neoplasia[27]. Other studies have shown that increased withdrawal time led primarily to the detection of less clinically significant small and diminutive polyps[20,22].
Results from a population-based registry study showed a statistically significant increase in the polyp and adenoma detection rate when the withdrawal time was > 9 min. The PDR of 53.1% and ADR of 33.6% were found to be highest at 9 min. Endoscopists with median withdrawal times of < 6 min, were significantly worse than endoscopists with median withdrawal times of > 9 min; PDR was 10.5% less,and ADR was 9.8% less respectively. Serrated polyp detection rates were 4.5% higher amongst endoscopists with median withdrawal times of 9 min compared to those with median withdrawal times of 6 min. Roughly 10% of the data was missing, which could cause a degree of attrition bias[28].
A recent large multi-centre RCT of 1027 patients randomized to a 9-min or 6-min withdrawal showed a statistically significantly higher ADR in the 9-min limb compared to the 6-min limb, respectively this was 36.6%vs27.1%,P= 0.001. Similar improvements were noted in the sub-group analysis for the right colon; 9-min (21.4%) and 6-min (11.9%),P< 0.001. Small and diminutive adenoma detection also increased in the 9-min limb compared to the 6-min limb. Significant improvements in the ADR in less experienced endoscopists were noted when compared to experienced ones,P= 0.03[29].
The idea that the greater time spent evaluating the colonic mucosa would naturally increase polyp detection is a rationale one. However, simply spending more time without performing actions such as repeated examinations of colonic segments and adequate luminal distension might not make a significant improvement in polyp detection. It is difficult to evaluate minimum withdrawal time in isolation, as it is likely to be an indication of a superior operator technique, than a causal factor[30].
In general, most colonic polyps are benign and unlikely to transform into cancer[31]. Large polyps harbour the greatest risk of progression to colorectal cancer. Larger polyps are also more visible and harder to miss[32]. Two studies have shown that the association between minimum withdrawal times and polyp detection is less for larger polyps[22,25]. An obvious conclusion to make from these findings is that larger polyps are readily visible and unlikely to be missed in comparison to smaller polyps in the same amount of time. The infrequent occurrence of larger polyps means that much larger studies are needed to show statistical significance when a subgroup analysis is performed in the small cohort of larger polyps ≥ 20 mm[22,25].
Sessile serrated lesions have a subtle appearance and are more difficult to detect. Their prevalence varies between 7%-10%[33]. A registry-based study reported that the detection of sessile serrated lesions was higher with longer withdrawal times > 11 min compared to ≤ 6 min[5]. Most of the large studies evaluating minimum withdrawal times did not address sessile serrated lesion detection[17,18,20]. Two studies did report that the detection rates of sessile serrated lesions improved with increasing withdrawal times[5,28].
The 2 Largest studies, both observational in size showed conflicting evidence with one showing a positive effect of increased withdrawal time on the ADR[20] and the other showing no benefit[19]. A recent meta-analysis showed an improvement in the ADR with a 9-min colonoscopy withdrawal compared to withdrawal times between 6-9 min[34]. Overall, the evidence supporting the use of longer withdrawal times and increasing polyp/adenoma detection rates is conflicting[17-20,24].
Simply implementing minimum withdrawal times without the adoption of other mucosal inspection techniques is not likely to be as effective. This finding was highlighted in the study by Sawhneyet al[19]where a mandatory minimum withdrawal time was adopted without any benefit. In comparison, the study by Barclayet al[18] incorporated a minimum withdrawal time alongside a quality improvement intervention that included other operator techniques and reported a significant benefit (Table 1 and Table 2).

Table 1 Summary of studies evaluating colonic withdrawal times

Table 2 Results of studies evaluating colonic withdrawal times
POSITION CHANGES ON WITHDRAWAL
An essential component of the colonoscopy technique is adequate luminal distension on withdrawal to provide enhanced endoscopic fields of view[35]. Position change during colonoscopy results in the elevation of gas to the highest position with fluid moving away from the area of interest, facilitating improved distension of the lumen[36]. Although prolonged insufflation may improve colonic distension, it does not move the fluid away and may not automatically improve the ADR as position changes, which provides a different field of view[37].
The use of changing the patient’s position during the withdrawal phase of colonoscopy has shown mixed results[38-40]. Adoption of the technique of position change during the withdrawal phase of colonoscopy is often done at the discretion of the endoscopist and not routinely performed.Endoscopists may be unaware or not convinced of the benefit, given the conflicting evidence to position change during colonic withdrawal. It may simply be technically easier and faster to perform the colonic withdrawal in one position than incorporate position change in colonic segments, especially in heavily sedated patients[38].
Dynamic position change is often adopted in the following fashion; Left lateral position for the cecum, ascending colon, and hepatic flexure; Supine position for the transverse colon; Right lateral position for the splenic flexure, descending colon, and sigmoid colon[36,37].
In a tandem-design RCT of 130 patients, dynamic position change compared to the left lateral position alone was evaluated[37]. The colonic examination was performed segmentally: (1) Caecum,ascending colon, and hepatic flexure; (2) transverse colon (TC); and (3) splenic flexure and descending colon (DC). Each segment was examined for 2 min in both the left lateral position and position changes.Polypectomy was performed only after examination in both comparison arms. The definition of position changes used in the study are outlined; accordingly: (1) Caecum, ascending colon, and hepatic flexure =left lateral position; (2) transverse colon = supine position; and (3) splenic flexure and descending colon= right lateral position. The ADR improved by 11% in the cohort where position change other than left lateral (TC, splenic flexure and DC) was adopted when compared to left lateral position change alone,P= 0.01[37]. This was more noticeable in the transverse colon where a supine position was adopted; left lateral position limb 15%vsposition change limb 24%,P= 0.02. Similarly, there was an 18% increase in the PDR in position changes that were not in the left lateralvsleft lateral position only,P< 0.001. The median size of polyps that were detected in the position change limb was 3 mm (range 1-10 mm). A strength of this study is the RCT design. However, as it is a single-centre, single-endoscopist study,there are limitations in the widespread applicability of the findings[37].
In a tandem design 102 patient RCT, colonoscopic withdrawal in the left lateral position compared to dynamic position change was evaluated[41]. In concordance with the findings of East[37], this RCT also showed positive findings with dynamic position change on colonoscopic withdrawal. This single-centre study was performed in a Turkish hospital and adopted the following examination pattern; right colon(left lateral twice), transverse colon (left lateral and supine), and left colon (left lateral, right lateral and supine). The PDR in the left lateral position compared to the dynamic position limb was 30.3% and 43.1% respectively,P< 0.001. The ADR in the left lateral position was 23.5% and 33.3% in the dynamic position limb,P= 0.002 respectively. The increase in the ADR was more noticeable in the transverse and left colon[41].
2) 无需复杂后置处理,灵活运用,通用性强,可支持卧车、立车,Fanuc系统、Siemens系统的仿真;
In a multi-centre RCT (parallel design) study, 1072 patients were randomized to either the left lateral position or the dynamic position change on withdrawal. Dynamic position change was followed accordingly: (1) Caecum, ascending colon, and hepatic flexure = left lateral position; (2) transverse colon= supine position; and (3) splenic flexure and descending colon = right lateral position. A higher ADR was found in the dynamic position change limb; 42.4%vs33.0% in the left lateral position,P= 0.002. An increase in the number of adenomas per patient was evident in the intervention limb 0.9vs0.67,P=0.01. Furthermore, in the transverse colon, the increase in adenoma in the intervention limb was 0.22vs0.13,P= 0.016 and in the left colon 0.37vs0.27,P= 0.045 respectively. The mean size of the adenomas in both limbs was 5mm. This study showed that endoscopists with a lower baseline ADR (< 35%) had a significant increase in their ADR when position change was adopted compared to endoscopists with a higher baseline ADR (> 35%). The detection of sessile serrated adenoma was also greater in the position change limb 2.3%vsleft lateral position 0.8%, but this did not reach statistical significance. No statistically significant improvement in the detection of advanced adenoma was shown in the intervention limb. This RCT is the largest study conducted so far, with the additional merit of being a multi-centre trial[36].
The tandem design (130 patient) RCT by Ballet al[42], had a different methodology in their evaluation of position change to previous studies[36,37]. Each colonic segment was evaluated twice; right colon(left lateral and supine), transverse colon (supine twice), and left colon (supine and right lateral position). In this single-centre study in a large teaching hospital, a statistically significant increase in the polyp detection rate in the right colon when withdrawal was performed in the left lateral position rather than supine was noted; 26.2%vs17.7% respectively,P= 0.01[42]. In contrast to other studies[37,41], the study by Ballet al[42], found no significant difference in PDR in the left colon when comparing the right lateral and supine position adoption[42].
In a parallel design RCT of 776 patients, randomization to the endoscopist’s usual adopted position change or dynamic position change failed to show any improvement in the PDR and ADR. Deviation from prescribed position changes in the dynamic limb was allowed if the endoscopist deemed it clinically necessary[40]. This study was unique, in that the control limb contrary to other studies[37,41]was not limited to performing withdrawal solely in the left lateral position. It is noteworthy that because of this, roughly half of the patients in the usual practice limb underwent right colon examination in the left lateral position and transverse colon examination in the supine position. This would reduce any possible advantage of the position change.
The study by Ouet al[40] was the only RCT to show no benefit in ADR with prescribed position changes. A significant feature of the methodology of this study was the adoption of the endoscopist's usual position change as the control limb. As a result, almost half the patients underwent a right colonic examination in the left lateral position and a transverse colon examination in the supine position. The potential advantages of position change would be reduced due to the lack of a single, standard position serving as a control limb[40].
The studies by Eastet al[37], Köksalet al[41] and Leeet al[36] found a more noticeable increase in the ADR in the dynamic position limb in the transverse colon, splenic flexure and descending colon. These 3 studies adopted a very similar definition of dynamic position change in their methodology. Heterogeneity in the study design makes it difficult to compare all RCTs as the other 2 studies by Ballet al[42]and Ouet al[40] adopted a different position as their control limb. Other than the study by Leeet al[36]which was a multi-centre one, the remaining studies[37,40-42] were all single-centre studies in academic units. The widespread applicability of these studies to routine community practice is therefore limited.A strength of the mentioned studies is all were randomized controlled trials[36,37,40-42].
A recent meta-analysis showed that dynamic position change during colonic withdrawal increased ADR. The recommendations from the meta-analysis for position change adoption were; left lateral position for the right colon, supine for the transverse colon and right lateral position for the left colon[43] (Table 3 and Table 4).

Table 3 Summary of studies evaluating dynamic position change
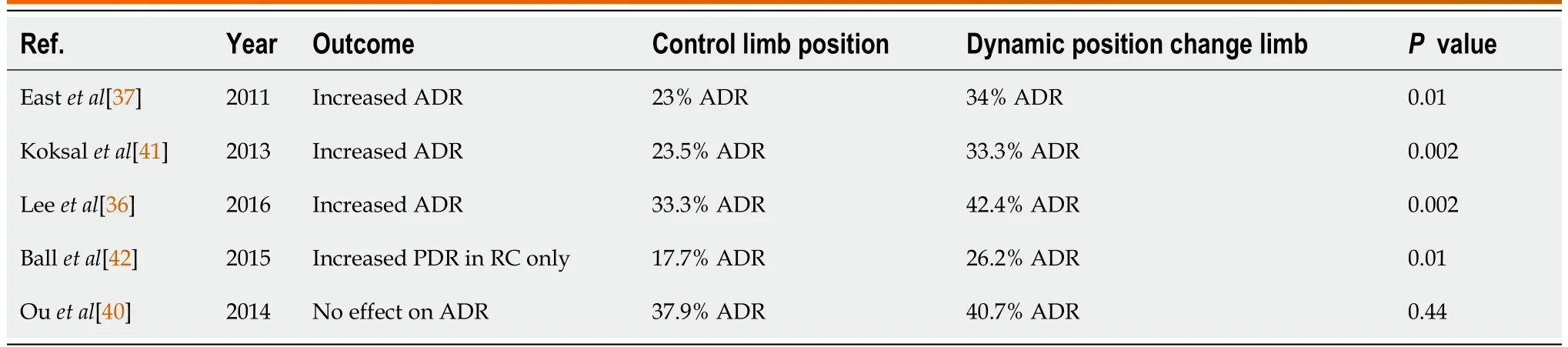
Table 4 Results of studies evaluating dynamic position change
PROXIMAL COLON RETROFLEXION
Retroflexion is thought to improve the detection of polyps in blind spots (behind the proximal aspect of folds). Proximal colon retroflexion involves the following manoeuvres: Maximum up deflection,maximum left wheel deflection and left torque. Colonoscopy is less beneficial in the detection of rightsided colonic neoplasia[11]. The theory that polyps located on the proximal sides of folds or flexures are missed because they are not within the endoscopic field of view is plausible[44]. Retroflexion has been speculated to assist the visualization of the posterior aspect of haustral folds and is more commonly performed in the rectum[45]. Theoretically, proximal colon retroflexion as a technique may expose polyps located on the proximal haustra.
In a randomized controlled trial, one of two 2ndyear Gastroenterology fellows performed colonic withdrawal and polypectomy from the caecum to the splenic flexure. The attending physician reintubated the caecum and was randomized to perform colonic withdrawal to the splenic flexure in either a forward or retroflexed view. This study failed to show a statistically significant benefit in the adenoma miss rates between the standard forward view and retroflexed view in the 2ndexamination,with a lower adenoma miss rate in the standard forward view (33.3%) compared to the retroflexed view(23.7%),P= 0.31[44]. Withdrawal in retroflexion can be technically challenging; the colonoscope may fall back more in the retroflexed view, increasing the likelihood of missed adenoma. Furthermore, the colonoscope shaft may conceal a small part of the mucosa, which could be another explanation for the negative findings. The first withdrawal was performed by trainees in forward view, whereas the second withdrawal was performed by the attending physician. This is a small single-centre study so has significant limitations in its ability to draw conclusions in widespread clinical practice[44].
In a large observational study (1000 patients), Hewettet al[46] performed an initial withdrawal to the hepatic flexure in the standard forward view (SFV), with a repeat 2ndexamination in the retroflexed view (RV). This was a single-centre study (without randomisation) and performed by only 2 endoscopists limiting its generalisability. Furthermore, the 2 endoscopists that were evaluated were also experts with considerable experience. An adenoma miss rate (AMR) of 9.8% in the 2nd examination in the retroflexed view was found[46], which is comparable to the AMR of studies with a 2ndexamination in the standard view[47,48].
In a large observational study, (1351 consecutive patients) a comparison between ADR in the forward viewvsADR in the retroflexed view from the caecum to the hepatic flexure was performed. The study found that in the forward view, the ADR was 24.6% compared to the retroflexed view with an ADR of 26.4%,P< 0.001. The increase in ADR was small but did reach statistical significance. The limitations of this study are the lack of randomisation. As a double-take procedure was performed, the mere fact that a 2ndlook examination was performed could account for the increased ADR, rather than because it was performed in retroflexion. The strengths of this study are that it was multi-centre (5 hospitals). In this study, the detection of polyps in the forward view was the only single predictor for the detection of additional polyps in the retroflexed view (odds ratio 4.13; 95% CI: 2.43-7.09;P< 0.001)[45]. This might add weight to the theory that if polyps are detected in the right colon on forward view, then a 2ndexamination should be performed in retroflexion. The strengths of this study are the multi-centre design, representing both tertiary and private centres. However, the lack of randomization is a significant limitation[45].
In a randomized controlled study (parallel blind design), 850 patients were randomized to a 2ndright colon examination in either the forward view or retroflexed view. No statistically significant difference in the ADR was observed between the SFV and RV in the 2ndexamination. Retroflexion may not be exposing all aspects of the colonic mucosa. The lack of difference between SFV and RV might also be explained by the lack of an endoscopist's ability to detect sessile serrated lesions (SSL), which are flatter,more difficult to detect and occur more commonly in the right colon. Interestingly this study did show a 20% adenoma miss rate in the right colon on the 2ndexamination. Furthermore, the shape of colonic folds and colonic distension vary between each examination, so more polyps are exposed on 2ndview.This study was performed in 2 academic units, so although multi-centred only involved 2 centres. This does pose some limitations in the applicability of this in the widespread community. However, as 10 endoscopists with varying levels of experience participated, this did lessen any effect[49].
A large (1020 patient) observational study by Leeet al[50] where 3 colonic withdrawal examinations were performed; the first 2 in forward view and the 3rdin retroflexed view, demonstrated a statistically significant increase in the ADR in retroflexion than the ADR with the combined forward view examinations; forward view (25.5% ADR)vstotal examination (27.5% ADR),P< 0.001. A transparent cap was used for each of the examinations. Polyps detected at each examination were then resected. In contrast to other studies[46,49], retroflexion was only successful in 82.4% of cases here. Leeet al[50] found that proximal colon retroflexion improved the ADR, despite 2 forward-view examinations beforehand.Caution should be used in the interpretation of this study as it has the confounding factor of the transparent cap in the colonic examination. The cap probably flattened the folds on subsequent examinations, with alteration in the shape of the haustra and the degree of luminal distension. Most of the adenomas identified in the retroflexed view were < 5 mm in size. The clinical significance of diminutive polyps is still undetermined and not likely to be very relevant[50].
A recent multi-centre RCT of 692 patients with a positive FIT test[51] randomized patients to a repeat right colon examination in standard forward view or retroflexed view. The repeat examination increased the ADR by 11%, with no statistically significant difference between SFV and RV; 12% and 9% respectively,P= 0.21. The detection of sessile serrated lesions in the right colon at the second examination was 11.1%, with no significant difference between SFV and RV. The success of retroflexion was only 83%. This study backs existing evidence that a repeat examination improves the ADR,whichever, view (SFV or RV) is adopted[46,47]. The strengths of this study are that it is a parallel blinded RCT across 3 Spanish centres. However, a limitation, in this case, is the lack of blinding of the endoscopists which could potentially incorporate more operator bias[51].
In an RCT of 205 patients randomly assigned to SFV or RV on 2ndexamination of the whole colon, not just the right colon. The initial withdrawal was always in SFV. An increased adenoma detection rate was noted in the 2ndexamination, despite whether there was randomization to either the SFV or the RV. A reasonable assumption to make is that the increased detection is related to the factor of a 2ndexamination itself, rather than the examination technique. Most adenoma detection on the 2ndexamination regardless of the limb of randomization was in the transverse and left-sided colon[52]. This is a relatively small study, limiting the opportunities for firm conclusions to be drawn[52].
A smaller (655-patient) observational study by Michopouloset al[53] had a similar study design to Leeet al[50]. In this observational study, 2 withdrawal examinations were performed in the forward view and a 3rdin the retroflexed view. The transparent cap was not used in this study. A statistically significant improvement in the ADR in the retroflexed view was noted, in comparison to the forward view 22.75%vs14.2%,P< 0.01. The improvement was more noticeable with diminutive adenomas and in the proximal 1/3 of the ascending colon[53]. This recent study showed the largest benefit of retroflexion. Polypectomy was performed after completion of the inspection, not immediately after detection. Most additional polyps noted in this study were diminutive and close to the hepatic flexure[53].
In a single-centre prospective observational study in a tertiary hospital, 463 patients were evaluated.When retroflexion was performed, additional adenoma was identified in 6.7% of patients, showing some benefit. In this study, the degree of right colon retroflexion was recorded as follows; grade 1; 1-2 haustra exposed and grade 3; ≥ 5 haustra exposed. A strength of this study was the evaluation of the degree of adequate mucosal exposure on retroflexion as most of the additional polyps (73.5%) were detected when a grade 3 right colon retroflexion (RCR) was recorded. This sub-group analysis was not reported in many of the other studies[54].
Studies have shown that retroflexion is relatively easy to perform with success rates ranging between 82.4%-96%[46,49-51,53,54]. Most studies have found no complications with proximal colon retroflexion[44,45,49,53]. One observational study found that 3% of patients had a minor bleed, 0.8% a mucosal tear and no cases of perforation with proximal colon retroflexion[55].
The evidence for proximal colon retroflexion is conflicting with some studies showing a benefit[45,50,53] and others showing none[44,49]. A previous meta-analysis supported the idea that a 2ndstandard forward view was equally successful in improving the ADR as a 2ndexamination in the retroflexed view[56]. A more recent meta-analysis found that the additional detection of adenoma was lower in the retroflexed view in 4 RCTs than with SFV colonoscopy. This meta-analysis also found that in 6 observational studies, the ADR was marginally higher in combined examinations with a retroflexed view than in both single-pass and double-pass forward view examinations[57].
The evidence supports the role of a 2ndinspection of the right colon[56], especially when polyps are found in the 1stwithdrawal[45]. A repeat colonic evaluation in a standard forward view is easier to perform than a retroflexed view. One should consider a repeat right colon examination, especially if right colonic polyps are noted on the initial withdrawal. Further information is needed before recommendations can be made to support the role of a repeat right colon examination in the retroflexed view (Table 5 and Table 6).

Table 5 Summary of studies evaluating proximal colon retroflexion

Table 6 Results of studies evaluating proximal colon retroflexion
CONCLUSION
The performance of colonoscopy is highly variable amongst endoscopists. Evidence has shown that increasing the ADR can reduce the risk of interval colorectal cancer[12,13]. A considerable amount of research has focused on the skills and technologies that could potentially improve the ADR[30]. Skilled endoscopists use several withdrawal techniques to increase their adenoma detection rate. One single technique in isolation is unlikely to make a significant impact. For this minireview, we focused on evaluating the literature on the following aspects of operator technique; minimum withdrawal times,dynamic position change on withdrawal and proximal colon retroflexion. The evidence supporting each technique is conflicting.
Most of the available literature on the role of simple operator techniques in adenoma detection during colonoscopy are from retrospective and prospective studies. This poses a limitation on the conclusions that can be drawn from the findings, as the lack of randomization in these study designs introduces inherent bias. There are only a few large, multi-centred RCTs addressing this area.
The study designs discussed in this minireview have some limitations that apply across all forms of endoscopic trials. In most instances, it is not possible to blind the endoscopist to the intervention limb.The endoscopist is instructed to follow a particular technique or use a device and will instantly know what is being evaluated. This can introduce a degree of investigator bias. Most endoscopic trials are performed by enthusiastic endoscopists in academic hospitals. The translation of this evidence into widespread clinical practice can therefore be challenging. Single-centre studies pose a similar limitation.
Studies are often not adequately powered to detect differences between sub-groups. The adoption of various endoscopic techniques and technologies may be more effective in different endoscopists and different patient cohorts. One technique may be more beneficial to 'low adenoma detector' endoscopists in comparison to those with a high baseline ADR. The non-technological techniques outlined in this minireview may help endoscopists with a lack of experience to improve their ADR.
Similarly, techniques may have more of a role in the detection of diminutive polyps than larger polyps. The infrequent occurrence of larger polyps ≥ 1 cm poses a challenge in obtaining statistically significant data that show a difference in the intervention limb, as a very large trial will need to be performed. The practicality of arranging large multi-centre-controlled trials is often not possible in realworld research settings.
Studies showed a trend towards greater detection of small and diminutive adenomas in comparison to larger polyps ≥ 1cm across all the 3 operator techniques outlined[20,29,36,42,46,50]. Although the clinical significance of small polyps remains unclear[31], as data shows that ADR reduces the interval risk of colorectal cancer[12,13], even if this is more pronounced in small polyps, cancer prevention is likely to be improved.
SSLs are increasingly recognized as important precursor lesions to colorectal cancer. The evidence supporting the role of colonoscopy withdrawal techniques in this sub-group is limited. Data supporting the role of minimum withdrawal times[5,28], dynamic position change[36] and proximal colon retroflexion[51] show a positive trend towards increasing detection of SSLs. Further studies adequately powered to perform sub-group analysis for small polyps and sessile serrated lesions are required.
Studies have shown that interventions that focus on improving endoscopist technique have improved endoscopists’ performance[58-62]. The initial QIC (Quality Improvement in Colonoscopy) study evaluated the outcomes of endoscopists following a training intervention that included withdrawal times of ≥ 6 min, supine position in the transverse colon, use of hyoscine butylbromide and rectal retroflexion. The study participants were evaluated 3 mo before and 9 mo after the implementation.17508 colonoscopies were evaluated in total[58]. A 2.1% absolute increase in the ADR (P= 0.002) was noted after the training. The improvement was more noticeable amongst the lower-performing endoscopists. A limitation of this study is that bundle compliance was determined by the uptake of hyoscine butylbromide alone and might not reflect the uptake of all the other parameters. A strength of this study is that 12 community hospitals participated, which is more representative of widespread clinical practice. The follow-up study found that the training from the initial QIC study still maintained the ADR 3 years after, with a statistically significant improvement maintained amongst the poorerperforming endoscopists[59].
In the Endoscopic Quality Improvement Program (EQUIP) study[61] the baseline ADR of 15 endoscopists were calculated before 8/15 were randomized to a training intervention and 7/15 were not. An ADR of 47% was noted in the group that was randomized to the training intervention, in comparison to an ADR of 35% in those that did not receive training,P= 0.0013. The educational interventions consisted of the following: Withdrawal time, careful inspection behind folds, and adequate cleansing of the colonic mucosa. Video recordings were utilized as training. An NBI learning module was also used to teach differentiation between neoplastic and non-neoplastic polyps with the use of still images. The limitation of this study is that it was performed in a tertiary academic unit, so the results are not generalizable to routine widespread clinical practice. In this study, only 8 endoscopists received the training interventions. This is a relatively small number and more endoscopists need to be evaluated for the results to be more applicable[61]. The follow-up study 5 mo after the initial study showed that the ADR improvements were maintained in the EQUIP-trained group at 46%[62].
A plethora of evidence evaluating the use of technologies such as distal attachment devices,chromoendoscopy, and wide-angle colonoscopes has been published, with conflicting results[2,30,63].Evidence shows that endoscopists with a low baseline ADR gain more from the use of distal attachment devices[2,64,65]. The use of these devices, is, however, seldom performed outside of academic institutions. The purpose of continuing to evaluate technologies that are not widely used by most Gastroenterologists should be questioned.
Meticulous technique by a skilled operator could be the most important factor. Instead of researching endoscopy technologies that are rarely used outside of a trial setting, perhaps the focus should be on evaluating quality intervention programs that focus on improving endoscopists ‘performance with simple operator skills.
Gathering the resources required to remove high-performing endoscopists from their day-to-day work to train lesser-performing endoscopists would pose significant challenges. Another option would be to encourage ‘low adenoma detector’ endoscopists to undergo a colonoscopy training course. A recent study did show a sustained improvement in the ADR amongst screening centre leaders who undertook a ‘Train the Colonoscopy Leaders ‘course with improvement in the performance of the overall centre, sustained over 1.5 years[60]. Further work is required in this area.
FOOTNOTES
Author contributions:Rajivan R contributed to the acquisition and interpretation of the data, drafted and made critical revisions to the manuscript; Thayalasekaran S designed the study, made critical revisions to the manuscript and approved the final version of the manuscript to be published.
Conflict-of-interest statement:The authors have no conflict of interest to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United Kingdom
ORCID number:Ragul Rajivan 0000-0002-3605-3978; Sreedhari Thayalasekaran 0000-0002-6290-2144.
Corresponding Author's Membership in Professional Societies:British Society of Gastroenterology, No. BSG61606.
S-Editor:Wang JL
L-Editor:A
P-Editor:Cai YX
 World Journal of Gastrointestinal Endoscopy2023年5期
World Journal of Gastrointestinal Endoscopy2023年5期
- World Journal of Gastrointestinal Endoscopy的其它文章
- Recent advances in endoscopic management of gastric neoplasms
- Unlocking quality in endoscopic mucosal resection
- Rectal neuroendocrine tumours and the role of emerging endoscopic techniques
- Effect of modified ShengYangYiwei decoction on painless gastroscopy and gastrointestinal and immune function in gastric cancer patients
- Expanding endoscopic boundaries: Endoscopic resection of large appendiceal orifice polyps with endoscopic mucosal resection and endoscopic submucosal dissection
- Effect of music on colonoscopy performance: A propensity scorematched analysis
