Instability Mechanisms of Supported Liquid Membrane for Phenol Transport*
ZHENG Huidong (郑辉东), WANG Biyu (王碧玉), WU Yanxiang (吴燕翔) and REN Qilong (任其龙)**
Instability Mechanisms of Supported Liquid Membrane for Phenol Transport*
ZHENG Huidong (郑辉东)1,2, WANG Biyu (王碧玉)2, WU Yanxiang (吴燕翔)2and REN Qilong (任其龙)1,**
1National Laboratory of Secondary Resources Chemical Engineering, Zhejiang University, Hangzhou 310027, China2College of Chemistry and Chemical Engineering, Fuzhou University, Fuzhou 350108, China
The instability mechanisms of the supported liquid membrane using Celgard 2500 membranes as support and tributyl phosphate dissolved in kerosene as carrier for phenol transport was studied by electrochemical impedance spectroscopy. Emulsion formation is demonstrated to be one of the main causes for the instability of supported liquid membrane in the present system. The emulsion-facilitated conditions, such as higher membrane liquid concentration, faster stirring speed, lower salt concentration and higher HLB value, would accelerate the degradation of supported liquid membrane. Other mechanisms including solubility and osmotic pressure work together to increase the membrane liquid loss.
supported liquid membrane, phenol, instability mechanisms, emulsion formation
1 INTRODUCTION
Although supported liquid membranes (SLMs) have been widely studied for the separation and concentration of a variety of compounds and present many potential advantages over other separation methods, there have been very few applications of SLM with large scale due to the insufficient membrane stability [1]. According to literatures, the possible instability mechanisms are: (1) pressure difference over the membrane [2]; (2) mutual solubility of species from the aqueous phase and liquid membrane phase [3]; (3) progressive wetting of the pores in the membrane support by the aqueous phase [3, 4]; (4) emulsion formation in the liquid membrane phase [5, 6]; (5) blockage of membrane pores by precipitation of a carrier complex at the surface [7]. Although a number of mechanisms have been proposed, some of them are contradictory or still need further investigation [8].
Traditionally, two methods have been used to study SLM stability. The first method is based on measuring the change in mass transfer rate or permeability parameters of SLMs with time [9, 10]. The second one involves a direct determination of the quantity of membrane liquid (ML) lost [6, 11]. In the first method, the macro mass transfer properties of membrane are used to characterize the instability of membrane, however, it fails to detect the behavior of ML loss and instability mechanisms in micro scale. In the second method, membranes are weighted before and after experiment to measure the ML loss. Since the weighting method is destructive, the behavior of ML loss duringthe operation also could not be detected continually.
In this paper, a non-invasive method, electrochemical impedance spectroscopy (EIS) [12], was employed to study the ML loss in phenol extraction system with SLMs. The mechanisms governing the SLM stability was investigated experimentally and theoretically and the mechanisms of SLM instability for phenol extraction were further proposed.
2 Experimental
2.1 Reagents and membranes
The reagents included sodium hydroxide, phenol, Span-80 (sorbitan monooleate), Tween-80 (polyethylene sorbitan monooleate), tributyl phosphate (TBP) (Sinopharm Chemical Reagent Co. Ltd, China, analytical grade) and kerosene (Aldrich, for laboratory use only). All reagents were used without further purification. Aqueous solutions were prepared using deionized water. Celgard®2500 (microporous polypropylene; thickness: 25μm; porosity: 55%; pore dimensions: 0.21 μm×0.05μm) was used as support for all experiments described in this paper.
2.2 SLMs preparation
Supported liquid membranes were prepared by impregnating the polymeric support (4.5 cm×5 cm) in membrane liquid (ML), the organic solution (TBP in kerosene), for at least 12 h. Before use, the polymeric support was taken out from ML and the excess organic liquid attached to the surface of the membrane was wiped off gently by a tissue.
2.3 Experimental apparatus and method
2.3.1
The SLM cell consists of a cuboid chamber (4.5 cm×5 cm×10 cm) that is separated into two halves by a SLM, as shown in Fig. 1. The working electrode, auxiliary electrode and reference electrode were attached to the SLM cell.

Figure 1 Experimental setup of-monitoring of SLM degradation
CHI660C electrochemical workstation of ShanghaiChenhua Instrument Company was used for EIS measurement. Frequencies were ranged from 5 Hz to 100 kHz (varying with the operating time) and the amplitude of the sinusoidal wave test signal was 10 mV. The measurements were carried out at room temperature.
2.3.2
The prepared SLM was placed between two chambers. Then simultaneously one chamber was filled with feed solution, phenol solution, and the other with stripping solution, sodium hydroxide solution. The magnetic stirrer was set to an appropriate stirring speed according to the chosen conditions. After that, the SLM cell was scanned for a certain period to obtain the EIS diagrams at different stages of ML loss.
These diagrams were analyzed by the equivalent circuit method in which the selected equivalent circuit wass(CmRm)according to the literature [13], as shown in Fig. 2.swas the resistance of the aqueous solution including the feed side and the stripping side (Ω).was constant phase-angle element (CPE) which caused by the ion transfer and the irregular surface of the electrode (F).mwas the membrane resistance of SLM, including the charge transfer resistance in the interface of the aqueous solution and the surface of membrane and in the membrane (Ω).mwas the membrane capacitance of the SLM (F). These parameters were calculated through fitting the obtained EIS diagrams by ZsimpWin software.

Figure 2 Equivalent circuit of membrane cell
During the process of ML loss, the empty space vacated by the loss of ML was gradually replaced by the aqueous solution [14]. Because the dielectric constant of ML (organic solution) was lower than that of aqueous solution, the membrane capacitance would gradually increase while the membrane resistance accordingly decreased. Thus, the method of EIS could provide the real-time information for the status of ML loss with more convenience.
3 Results and discussion
3.1 Effect of ML solubility on SLM stability
Many researchers [15, 16] have reported that solubility is one of the main reasons for the instability of SLM. The ML is not completely insoluble in an aqueous solution and a certain degree of solubility exists between the interface of ML and the aqueous solution. If the solubility of ML to the nearby aqueous solution is high, these influences are significant. The influence of solubility on the stability of SLM in this system is shown in Fig. 3.

Figure 3 Effects of solubility onmandm
By comparison with the loss behavior of the non-presaturated system, the change rate of the membrane resistance and capacitance with pre-saturated system, in which water for the aqueous solution preparation was saturated with ML by contacting with excess ML [17], has some but inconspicuous decrease. Due to the solubility of ML in the aqueous solution, the loss of ML could be slightly held down by using the pre-saturated aqueous solution. However, using pre-saturated aqueous solution could not completely eliminate the loss of ML. Thus, the solubility is not the main reason for the ML loss in the phenol extraction with SLM.
3.2 Effect of initial carrier concentration on SLM stability
The results concerned with the influence of initial concentration of the carrier in the ML on SLM stability are shown in Fig.4.
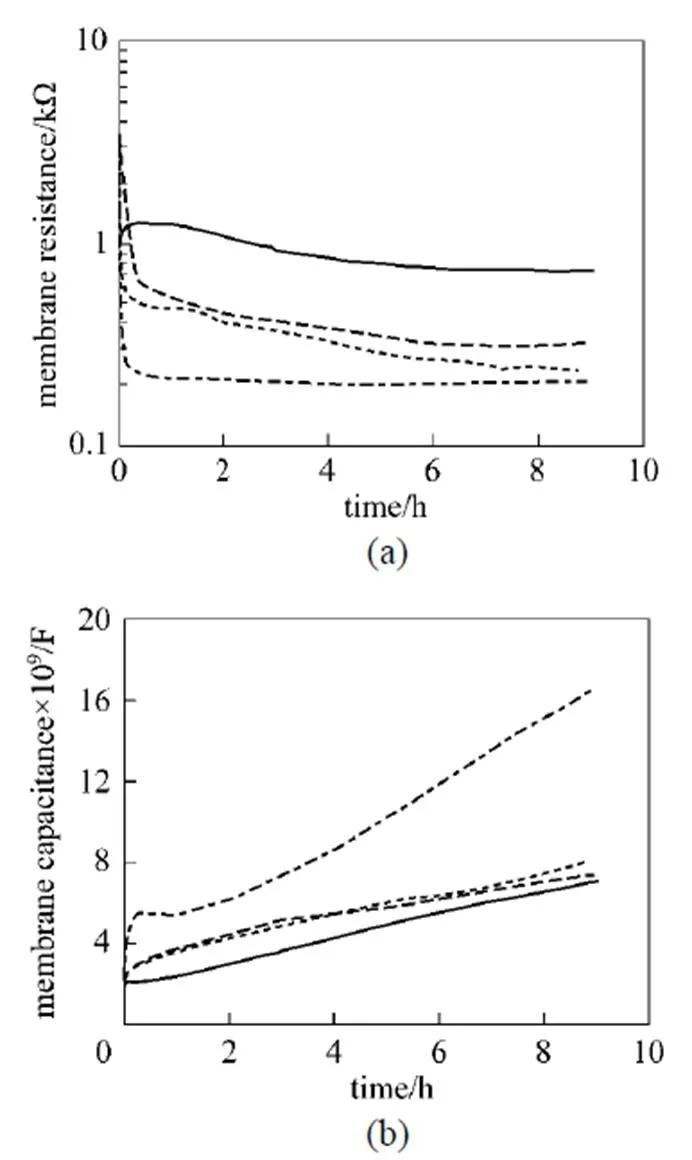
Figure 4 Effects of carrier concentration onmandm
It is found that a higher initial carrier concentration leads to a faster change of the membrane resistance and capacitance, which also indicates increased loss of ML. An increase in ML carrier concentration increases the solubility of carrier in the nearby aqueous solution and reduces the interfacial tension between the aqueous phase and the ML phase [18]. As a result, the aqueous solution can wash the ML away more easily, leading to an acceleration of the ML loss.
3.3 Effect of initial phenol concentration in the feed solution on SLM stability
From Fig. 5, the decrease speed of membrane resistance and the increase speed of membrane capacitance have little change as the phenol concentration in feed solution decreases. Phenol is a kind of surface tension neutral substance which means the surface tension of aqueous solution and ML has little change as the phenol concentration in feed solution increases. Furthermore, most of phenol in the feed solution exists as molecules instead of ions, which has little influence on the ion concentration in the feed solution. Thus, the change of the initial phenol concentration in the feed solution has little influence on the chemical and physical properties of SLM system.

Figure 5 Effects of initial phenol concentration onmandm
3.4 Effect of stirring speed on SLM stability
ML loss is accelerated by increasing the stirring speed in the aqueous solution (see Fig. 6), which is in agreement with results reported by Neplenbroek. [19] and Takeuchi[20]. The increase of stirring speed not only enlarges the tangent velocity at the interface between the phases of the aqueous solution and the ML, but also increases the disturbance in the aqueous solution, which also in turn puts more shear force at the interface between the phases of the aqueous solution and the ML, and thus quickens the ML loss. According to the mechanism of emulsion formation [5], the strength of the shear force induced by the stirring in the aqueous solution has significant impact on the formation of emulsion and the simplest method to eliminate the emulsion is reducing the shear force.

Figure 6 Effects of stirring speed onmandm
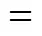
Also, the membrane resistance and capacitance still gradually change during the operation and the loss of ML is quite noticeable even without stirring as shown in Fig. 6. This reveals that the gradient of osmotic pressure or/and solubility also contributes to the loss of ML and shortens the lifetime of SLM.
3.5 Effect of salt concentration on SLM stability
To investigate the influence of electrolyte strength in the aqueous solution on the ML loss, certain quantity of sodium chloride was added to the feed solution or the stripping solution. Results are plotted in Fig. 7.
In the aqueous solution the ion strength increases as the increase of salt concentration. Before adding the electrolyte to the stripping solution its ion strength is zero. Therefore, when some salt (NaCl) is added to the stripping solution, the difference of ion strength between the stripping solution and the feed solution becomes greater, which results in the increase of osmotic pressure between the two sides of SLM. According to the osmotic pressure mechanism [15, 21], the SLM would be more unstable as the osmotic pressure between two sides of the membrane increases. However, the results (see Fig. 7) show that adding the electrolytes to the feed side or the stripping side can significantly reduce the ML loss and help to improve the stability of the SLM. Consequently, it is suggested that the mechanism of osmotic pressure is not the major reason for the SLM instability in this system.

Figure 7 Effects of salt concentration onmandm
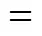
Although an increase of salt concentration can improve the interface tensions between aqueous solutionand ML phases [6], only slight increase is found. Anyway, these results agree with the mechanism of emulsion wherein adding the electrolytes hinders the formationof emulsion and effectively reduces the ML loss [5].
Fig. 8 shows the distribution of emulsion droplets size of kerosene in different salt concentrations prepared under the same operating conditions [6]. The distribution of droplet size was analyzed by Nanophox particle size analyzer (Sympatec Corp., Germany) shortly after the preparation of the emulsion. It is obvious that the sizes of emulsion droplet significantly increase with the increase of salt concentration in the aqueous solution. Under the same energy input from outside, the emulsion droplet with bigger size would be hard to form than the smaller ones, which leads to less ML loss. Obviously, emulsification mechanism is the main reason of SLM degradation.

Figure 8 Distribution of emulsion droplets size in different salt concentrations
salt concentration/mol·L-1:■ 0;▲ 0.10;● 0.25
3.6 Effect of hydrophile-lipophile balance (HLB) value of membrane liquid on SLM stability
The formation and stability of emulsion are affected by many factors. Among them, the most important parameter is the HLB value of ML, which decides the possible type of emulsion. The surfactant could generally be sorted into two types according to its HLB value. The surfactant with HLB value ranging from 3 to 6 normally forms water in oil (W/O) emulsion, while the surfactant with HLB value in the range of 8 and 15 would likely form emulsion of oil in water (O/W) [22]. Span 80 with an HLB value of 4.3 belongs to the first type, whereas Tween 80 with an HLB value of 15.0 to the second type. Some Span 80 or Tween 80 was added to the ML to evaluate the HLB value of ML on SLM stability as shown in Fig. 9.

Figure 9 Effects of HLB value onmandm
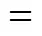
The results show that SLM becomes more unstable with the increase of the HLB value of the ML, which is represented by a quicker loss of ML. The reason is that ML containing 5% Span 80 is inclined to form W/O emulsion, which improves the stability of SLM. On the other hand, because the emulsion formed by ML containing 5% Tween 80 and water is O/W type, which is more soluble in water solution. This type of SLM seems to be more unstable even compared with ML containing no surfactant. This is another evidence to prove that the emulsification mechanism is the main instability mechanism of this SLM system.
4 Conclusions
EIS method, in which the membrane resistance,m, and membrane capacitance,m, can directly reflect the status of SLM degradation, is an effective tool to study the instability of SLM. By employing this method, the influence of different experimental conditions on SLMs stability was investigated in order to reveal the underlying instability mechanism of SLMs for phenol transport.
Results show that the mechanism of emulsification is the main instability mechanism of SLMs in this system. The emulsion-facilitated conditions, such as higher ML concentration, faster stirring speed, lower salt concentration and higher HLB value, would stimulate the emulsification of ML in the nearby aqueous solution. The mechanisms of solubility and osmotic pressure are proved not to be important instability mechanism, although they are also contributed to the loss of ML.
1 Kocherginsky, N.M., Yang, Q., Seelam, L., “Recent advances in supported liquid membrane technology”,..., 53 (2), 171-177 (2007).
2Zha, F.F., Fane, A.G., Fell, C.J.D., Schofield, R.W., “Critical displacement pressure of a supported membrane”,..., 75, 69-80 (1992).
3Danesi, P.R., “Separation of metal species by supported liquid membranes”,..., 85, 857-894 (1984).
4Takeuchi, H., Nakano, M., “Progressive wetting of supported liquid membranes by aqueous solutions”,..., 42, 183-188 (1989).
5Neplenbroek, A.M., Bargeman, D., Smolders, C.A., “Mechanism of supported liquid membrane degradation: emulsion formation”,..., 67, 133-148 (1992).
6Zha, F.F., Fane, A.G., Fell, C.J.D., “Instability mechanisms of supported liquid membranes in phenol transport process”,..., 107, 59-74 (1995).
7Makoto, N., Takahashi, K., Takeuchi, H., “A method for continuous operation of supported liquid membranes”,..., 20 (3), 326-328 (1987).
8Kemperman, A.J.B., Bargenman, D., van den Boomgaard, T., Strathmann, H., “Stability of supported liquid membranes: State of the art”,..., 31, 2733-2762 (1996).
9Szpakowska, M., Nagy, O.B., “Stability of supported liquid membranes containing Acorga P-50 as carrier”,..., 129, 251-261 (1997).
10Danesi, P.R., Reichley-Yinger, L., Rickert, P.G., “Lifetime of supported liquid membranes: The influence of interfacial properties, chemical composition and water transport on the long-term stability of the membranes”,..., 31 (2/3), 117-145 (1987).
11Yang, X.J., Fane, T., “Effect of membrane preparation on the lifetime of supported liquid membranes”,..., 133 (2), 269-273 (1997).
12Mark, O.E., Bernard, T., Impedance Spectroscopy: Theory, Experiment, and Applications, Wiley Interscience, San Francisco (2005).
13Zheng, H.D., Wu, Y.X., Xue, H.Y., Ren, Q.L., “Study on the instability of supported liquid membrane by electrochemical impedance spectroscopy”,..., 29 (4), 28-32 (2009).
14Xue, H.Y., “Performance and stability of supported liquid membranes for copper transport”, Master Thesis, Fuzhou University, Fuzhou (2008). (in Chinese)
15Danesi, P.R., Reichley, L., Rickert, P.G., “Lifetime of supported liquid membranes: The influence of interfacial properties, chemical composition and water transport on the long term stability of the membranes”,..., 31, 117-145 (1987).
16Chiarizia, R., “Stability of SLMs containing long-chain aliphatic amines as carriers”,..., 55, 65-77 (1991).
17Fortunato, R., Afonso, C.A.M., Reis, M.A.M., Crespo, J.G., “Supported liquid membranes using ionic liquids: Study of stability and transport mechanisms”,..., 242, 197-209 (2004).
18Malmary, G., Faizal, M., Albet, J., Molinier, J., “Liquid-liquid equilibria of acetic, formic, and oxalic acids between water and tributyl phosphate + dodecane”,..., 42, 985-987 (1997).
19Neplenbroek, A.M., Bargeman, D., Smolders, C.A., “Supported liquid membranes: Instability effects”,..., 67, 121-132 (1992).
20Takeuchi, H. , Takahashi, K., Goto, W., “Some observations on the stability of supported liquid membranes”,..., 34, 19-31 (1987).
21Fabiani, C., Merigiola, M., Scibona, S., Casgnola, A., “Degradation of supported liquid membranes under osmotic pressure gradient”,..., 30, 97-104 (1987).
22Grayson, M., Encyclopedia of Emulsion Technology, Wiley-Interscience, New York (1979).
2008-12-16,
2009-03-06.
the National Natural Science Foundation of China (20676023).
** To whom correspondence should be addressed. E-mail: renql@zju.edu.cn
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Molecular Simulation of CO2/H2 Mixture Separation in Metal-organic Frameworks: Effect of Catenation and Electrostatic Interactions*
- Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
- Corrosion Behavior of TP316L of Superheater in Biomass Boiler with Simulated Atmosphere and Deposit
- Influence of A-type Zeolite on Methane Hydrate Formation*
- Effects of Sintering Atmosphere on the Microstructure and Surface Properties of Symmetric TiO2Membranes*
- Improvement of Isomerization Process of Crude Isoamylene with Tertiary-amyl-alcohol Addition
