青花菜与白菜间体细胞杂种获得与遗传特性鉴定
廉玉姬,林光哲,赵小梅
临沂大学 生命科学学院,临沂 267005
Introduction
The importance of Brassica vegetables such as Chinese cabbage, broccoli, cabbage, cauliflower, and kale have recently increased in terms of nutrient quality for human diet and processed foods. Among these Brassica vegetables, Chinese cabbage, the major ingredient in Kimchi, along with hot pepper and garlic, is the most important vegetable in Korea. The widespread popularity of Kimchi as a fermented food into other countries caused the increase in the production of the Chinese cabbage[1]. However, Chinese cabbage production is suffering heavy losses yearly because of its susceptibility to pathogens such as Xanthomonas carnpestris pv campestris (black rot), Ervinia carotovora (soft root), and Verticillium dahliae (vascular wilt).
Vascular wilt caused by Verticillium dahliae Kleb. is an important disease in many plant species, including many Brassica species, especially Chinese cabbage [B. campestris pekinensis (Lour.) Rupr.][2-4]. Presently, no desirable cultivars are resistant to Verticillium disease and great efforts have been made to exploit genetic resources to induce Verticillium dahliae Kleb resistance in B. campestris. Fortunately, Broccoli crops have Verticillium wilt resistance even in fields heavily infested with V. dahliae[4-5].
Even though rapeseed can be synthesized from its progenitors B. oleracea and B. campestris by sexual crosses[6], the number of hybrids obtained is highly variable and, occasionally, none are generated. This may be explained by variations in factors such as environment, physiological state of the material, and genotype. In addition, successful hybrid production usually depends on the use of B. oleracea as the female parent and on embryo rescue techniques[7]. The chances of improving genetic diversity in rapeseed by sexual crossings are therefore restricted[8].
Somatic hybridization provides a means to overcome sexual incompatibility and has been used to obtain many intraspecific and interspecific, intergeneric, intertribal, and even interfamilial somatic hybrids[9-13]. This technology allows not only intrageneric hybridizations, but also the production of intergeneric hybrids and cybrids[14]. Various desirable traits have been transferred from parents to hybrids and cybrids using this technique[15-22].
Aside from the recombination of nuclear genome between parents, cytoplasmic organelles such as mitochondria and plastids can be hybridized by protoplast fusion, thereby providing new genetic diversity and variations in the genome of these organelles[23]. In Brassica species, protoplast technology has been applied extensively through fusion of protoplasts from B. campestris and B. oleracea to widen their genetic diversity and so on[24-28]. Other examples of interspecific somatic hybridization between B. campestris and B. oleracea have been reported for the transfer of economic traits such as cytoplasmic male sterility[29-30]and disease resistance[31].
In this study, somatic hybrids that have valuable traits from both Chinese cabbage and broccoli were produced to improve crops. The somatic hybrids were verified by flow cytometry, random amplified polymorphic DNA (RAPD) analysis, and plant morphology.
1 Materials and methods
1.1 Plant materials
Inbred lines of B. oleracea L. var. italica (broccoli), B. campestris (Chinese cabbage) were used as plant materials for somatic hybridization. The seeds provided by the Choong Ang Seed Company of South Korea were surface-sterilized using 70% ethyl alcohol for 30 s followed by 15 min in 50% commercial Chlorox bleach solution. Then, two drops of Tween-20 were added and rinsed three times with sterile distilled water. The sterilized seeds were germinated and propagated in vitro on MS[32]medium supplemented with 1% sucrose and solidified with 0.8% agar under controlled conditions (25 °C, 16 h photoperiod, 84 μmol/(m2·s), white fluorescent light). Prior to protoplast isolation, the seedlings were placed in the dark for 1–2 days to reduce starch content.
1.2 Protoplast isolation, fusion, and culture
Protoplasts of the Chinese cabbage and broccoli were isolated from the cotyledons and hypocotyls of 10-day-old seedlings using as enzyme solution containing 0.4 mol/L mannitol, 50 mmol/L CaCl2, 1% cellulysin (Calbiochem, USA), and 0.5% macerozyme (Calbiochem, USA) at pH 5.8. Protoplast isolation and fusion were carried out as described by Lian and Lim[1]. The protoplasts of fusion partners were suspended in W5 (154 mmol/L NaCl, 125 mmol/L CaCl2, 5 mmol/L KCl, 2 mmol/L MES, pH 5.7) solution to adjust the final concentration of 1×105protoplasts/mL and mixed gently in a ratio of 1:1. Symmetric fusion was induced with a 40% polyethylene glycol (PEG, 1450) solution and dimethyl sulfoxide (DMSO).
The fused protoplasts were cultured on modified K8p medium[33]supplemented with 0.2 mg/L 2,4-dichorophenoxyacetic acid (2,4-D), 0.5 mg/L 6-benzylaminopurine (6-BA), 0.1 mg/L 1-naphthaleneacetic acid (NAA), and 0.1 mg/L kinetin (Kin) for cell division. The protoplasts were first cultured in 6 mm plastic Petri dishes with 1 mL liquid culture medium. The Petri dishes were then sealed with Parafilm® and incubated at 25 °C in the dark. After 24 h of culture, two different culture methods were attempted.
In first method, another 1ml of fresh culture medium were added to the cultures, and then were maintained at liquid medium until colonies of 8–10 cells were observed. The cells were collected by centrifuging. The liquid culture medium was 50% renewed, at two weeks intervals, with the same culture medium with a 0.1 mol/L decrease in mannit ol concentration.
The second method, fused cells were tenderly resuspended, and then gently mixed with an equal volume of Kao’s basal medium[34]containing 0.3 mol/L sucrose, 0.2% agarose, 2 mg/L 6-BA, 2 mg/L ZEA, 1 mg/L NAA, and 0.5 mg/L Kin. The cultures were kept in the dark at 25 °C.
After 5 weeks, when calli were 2–3 mm in diameter, plating efficiency was investigated, then, they were transferred into regeneration medium containing 5 mg/L ZEA and 2 mg/L indole-3-acetic acid (IAA) and solidified by adding 8 g/L agar at pH 5.8 for shoot regeneration at 25 °C under fluorescent light at 84 μmol/(m2·s) and a 16 h photoperiod. The calli were transferred to new medium every 2–3 weeks and the resulting regenerated shoots were transferred to MS basal medium supplemented with 0.2 mg/L NAA for growth and rooting.
1.3 Flow cytometry
The fluorescence of the samples were measured on a Partec Flow Cytometer (Partec PA–I, Germany) equipped with a high-pressure mercury lamp. Up to 0.2 g of new fresh leaves from the five regenerated plants (selected randomly) and fusion parents were excised, chopped, and then incubated in 2 mL of nuclei extraction buffer (High-Resolution DNA Kit Type P, Solution A; Partec) for 1 min. Then, the resulting mixture was filtered for 30 min with Partec CelltricsTMand then stained for 2 min with 1 mL of Partec HR-B solution. The diploid B. oleracea and B. campestris were used as controls, against which the relative fluorescence intensities from the regenerated plants were compared.
1.4 Random amplified polymorphic DNA (RAPD) analysis
Total DNA was isolated from the leaves of greenhouse-grown parental lines and 11 regenerated plants following the protocols of the Cetyltrimethylammonium bromide (CTAB) method[35]. RAPD analysis was also carried out on 10 regenerated plants and protoplast fusion parents. In total, 42 primers (Operon Technologies, USA) were tested to find primers that could produce specific bands in both fusion parents. Amplification conditions were 35 cycles of 94 °C for 40 s, 40 °C for 60 s, and 72 °C for 60 s. PCR amplification was performed as described above.
1.5 Morphological and cytological analysis
To confirm the chromosome numbers of the somatic hybrids, root tips were pre-treated with 0.002 mol/L 8-hydroxyquinoline at room temperature for 1 h, fixed with 3:1 ethyl alcohol: acetic acid, and then with absolute alcohol at 4 °C for at least 24 h. The root tips were then washed with distilled water and macerated in enzyme solution (5% Cellulase Onozuka RS, 1% Pectolyase Y-23, 1 mmol/L EDTA; pH 4.52) for 40 min. After washing with distilled water, the macerated root tips were placed on a glass slide with a few drops of acetic alcohol solution, spread by tapping with fine forceps, and air dried under room temperature.
Morphological characteristics such as leaf shape, size, and flower color of the protoplast fusion plants were investigated and compared with those of the fusion parents. The morphologies and fertilities of the progenies from the first and second generations were also investigated.
1.6 Probe labelling and genomic in situ hybridization (GISH)
Genomic DNA of B. campestris was labelled with fluorescein-11-dUTP using a nick-translation kit (Boehringer-11-Mannheim, Roche, Germany) according to the manufacturer’s instructions. The slide preparations for genomic in situ hybridization (GISH) mainly followed Zhong et al[36]with minor modifications. In preparation of slides, an enzyme mixture decomposed the cell wall of root tip, at 37°C for approximately 40 min in the enzyme mixture. To prevent non-specific intergenomic cross-hybridization, a 30-fold excess of sheared genomic DNA was added to the hybridization solution. The DNA was sheared by autoclaving for 15 min, and then we performed the electrophoresis, identified genomic-blocking DNA fragment size below 100bp. In situ hybridization was carried out according to the methods of Leitch[37].
Hybridization signals of the B. campestris probe was detected using fluorescein isothiocyanate (FITC) -anti-avidine. Chromosomes were counterstained with propidium iodide (PI) (Roche, Basel, Switzerland), mounted in anti-fade solution (Vector Laboratories, Cambridge, MA, USA) and examined using fluorescence microscopy.
2 Results and discussion
2.1 Protoplast fusion and plant regeneration
Isolated protoplasts (Fig. 1A) were fused using 40% PEG, and the fused protoplasts were cultured in liquid medium. Periodic microscopic examination revealed that the first division of protoplasts occurred within 48 h. In the first 7 days of culture, colonies of 8–10 cells were observed (Fig. 1B), and about more than 50% of the plated cells had divided at least once. After 14 days of culture, significantly higher division frequency was observed in the protoplasts cultured in liquid culture compared to semi-solid agarose medium. However, when the formed calli reached 0.5–1 mm in diameter, browning gradually occurred in the liquid culture. When agarose culture was compared with that of the liquid medium culture for protoplasts, cell division and colony formation were more active in semi-solid agarose culture than cultured in liquid medium. Five weeks after culture initiation, the plating efficiency attained 0.66% (Fig. 2).
It means semi-solid agarose culture method was more effective than liquid culture, and it may also protected the cells from browning caused by polyphenolic compound released during protoplast culture. Dons and Colijn-Hooymans referred that culture in agarose presents several advantages over liquid culture[38]. One of them is the fact that protoplasts remain immobilised, which allows the culture medium renewal without protoplast damage and facilitates the follow up of protoplast proliferation.
Thus, the semi-agarose embedding culture prevented cell aggregation and necrosis. Moreover, it efficiently avoided an attack by toxic substrates secreted from necrotic or non-divided old cells. Another one is an improved plating efficiency. The superior ability of agarose to support protoplast culture may relate to the essentially neutral characteristic of the polymer[39].
Agarose has produced the best results in terms of retention of viability and secondary product production[40]. A total of 300 calli (Fig. 1C) were obtained from fused protoplasts, were transferred to MS basal medium containing 5 mg/L ZEA, and 2 mg/L IAA. Eleven plants were obtained from the calli (Fig. 1D), plant regeneration frequency was 3.7%. All of regenerated plants were transferred to pots (Fig. 1E), for morphological comparison.

Fig. 1 Morphologies traits of somatic hybrids and their fusion parents. (A) Isolated protoplast from cotyledons. (B, C) Cell division. (D) Regenerated plants from calli. (E) Regenerated plants from fusion derived calli, and their fusion partners. (a) Chinese cabbage. (b) Broccoli. (c) Regenerated plants. (F) Flowering of broccoli. (G) Flowering of Chinese cabbage. (H) Flowering of somatic hybrids. (I) Bolting behaviour of broccoli. (J) Chinese cabbage. (K) Bolting behaviour of somatic hybrid.
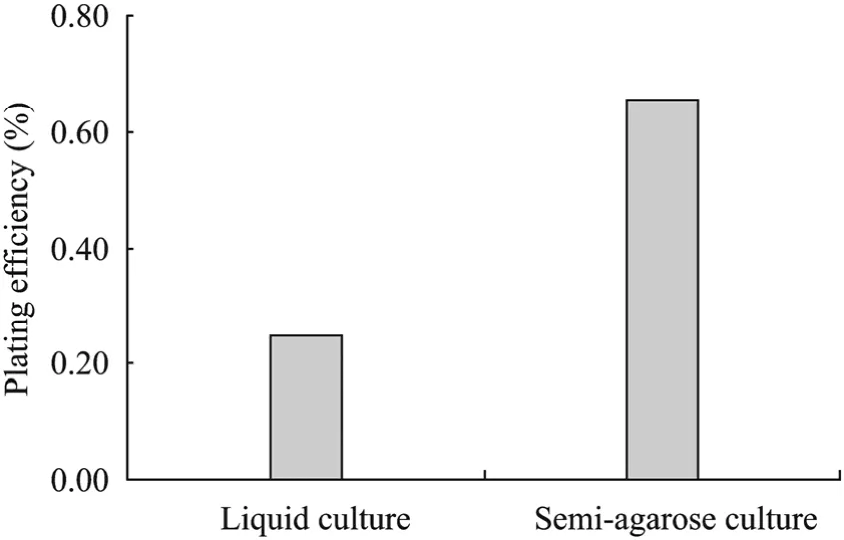
Fig. 2 Plating efficiency of fused protoplasts after 5 weeks culture by different culture methods. Plating efficiency was investigated as the percentage of plated protoplasts that formed microcalli after 5 weeks of initial culture.
2.2 Flow cytometry analysis
Flow cytometry is also very helpful in the detection of variation in ploidy status among genotypes of the same species, e.g. Brassica napus[41], as well as in interspecific hybrids[42-43]. Flow cytometry is also widely used to study the genome size and stability in different plant materials cultured in vitro[44-46]. Regenerated plants derived from two fusion partners were used to confirm the somatic hybrids using a ploidy analyzer. Estimation of the nuclear DNA content of the somatic hybrids and fusion parents was done using flow cytometry as previously described by Arumuganathan and Earle[44]. The typical position of the histograms of the fluorescence peak was obtained using flow cytometry. B. campestris (Chinese cabbage) (Fig. 3A) showed one peak with a diploid approximately at channel 60, according to the Partec User Manual. The peak of the diploid B. oleracea (broccoli) (Fig. 3B) was located around channel 89. The peak of regenerated plant after the protoplast fusions appeared at the channel near 170, indicating that the somatic hybrid (Fig. 3C–G) was derived from a combination of the B. campestris and B. oleracea genomes. All of regenerated plants were tetraploid. Similar results were also reported by Hansen and Earle[31].
2.3 Identification of somatic hybrids using RAPD
According to morphological and chromosomal observations, RAPD analysis using 42 random Operon (Operon Technology, USA) OPR primers further confirmed the somatic hybrid status of the plants. The products amplified from both parents using the primer OPB8 (5'-GTCCACACGG-3') were clearly polymorphic, with putative hybrid plants showing specific bands from both parents (Fig. 4). Thus, genetic materials from both parents were successfully incorporated into the somatic hybrids. RAPD analysis indicated that the somatic hybrids included partial genomes of both parents. RAPD DNA analysis is a quick and simple method for determine the hybridity of fusion products[47]. The appearance of new bands in the hybrid could be attributed to DNA rearrangements subsequent to the somatic hybridization. Wang et al. made similar observations for somatic hybrids in Brassica species[48].
2.4 Morphological and cytological characterization of the somatic hybrids
To compare the morphological traits between the regenerated plants and fusion parents, these were simultaneously transferred into pots and cultivated in a green house. All of the plants derived from protoplast fusion were determined as somatic hybrids based on their morphology. No parental plants were obtained in this study, as in the studies by Yamagishi and Glimelius[49]and Tu et al[12]. Wide variations in morphology were not seen and the hybrid plants grew vigorously during the cell culture, division, and cultivation in pots. Several studies regarding somatic hybrids of Brassica species reported similar results[50-51].
In this study, the most striking difference between the fusion parents and the somatic hybrids was in leaf morphology. Generally, broccoli has emerald green dense cluster of flower buds with narrow petioles, arranged in a tree-like fashion on branches sprouting from a thick, edible stalk. The mass of flower heads is surrounded by leaves. Chinese cabbages have broad green leaves with white, large petioles, tightly wrapped in a cylindrical formation and usually forming a compact head. The leaves of regenerated plants emerald green and thick, covered with a powder or waxy coating similar to broccoli. The petioles of regenerated plants were intermediate to those of the parents; Chinese cabbage (Fig. 1E-a) has enlarged petioles, whereas broccoli has narrow petioles. All regenerated hybrid leaves were crenate or lyrate, deep green and thick, and covered with a waxen powder similar to those of broccoli (Fig. 1E-b). The regenerated plants exhibited morphology intermediate to those of the two parents (Fig. 1E-c). The basal leaves were parted, similar to those of broccoli. The plant was similar to that of broccoli, but the trichomes on the leaves were similar to those of Chinese cabbage. The flower buds were 0.4 cm in diameter, 0.6 cm in long, and the flowers were larger than those of both parents (Fig. 1F–H). The flowers were yellow, similar to Chinese cabbage (Fig. 1H). In some reported cruciferous plants, the morphology of the hybrids had been described as intermediate of those of the parents[52-54].

Fig. 3 Histogram of the fluorescence intensities for isolated cells from chopping leaves of fusion partners and somatic hybrids. (A) B. oleracea (broccoli). (B) B. campestris (Chinese cabbage). (C-G) Somatic hybrids.

Fig. 4 RAPD analysis of parental lines and somatic hybrids. M: DNA marker. P1: B. campestris; P2: B. oleracea; 2–11: somatic hybrids; 8: missing band.
Under green house conditions, all regenerated plants and broccoli parent began bolting without vernalization after two months of cultivation. Typically, B. campestris requires vernalization prior to bolting and flowering[55]. This result indicates that the bolting behavior of the regenerated plants were similar to that of broccoli. The floral apex branching patterns was intermediate of broccoli and Chinese cabbage with loosely branched small terminal heads. Broccoli has a compact head of florets attached by small stems to a larger stalk. In contrast, Chinese cabbage has loosely branched small terminal heads (Fig. 1I–K).
Regenerated plants had very poor pollen fertility with 0.13 seeds per pod after self-pollination, even though the plants produced many pollen grains. The hybrid plants produced seeds of various sizes ranging from 0.7–3.1 mm by self-pollination. The plants produced no seeds after backcrossing with Chinese cabbage and only two seeds per pod after pollination with broccoli. Similar results have been reported by Yamagishi et al.[56]and Chen et al[10]. Thus, somatic hybrids between Chinese cabbage and broccoli have low fertility, as reported by Sundberg[57]. This may be due to somatic incompatibility. The incorporation of the total genomes of two very distantly related species in a hybrid through somatic hybridization has two obvious disadvantages: the introduction of too much exotic genetic material accompanying the expected gene (s) and the genetic imbalance leading to somatic incompatibility[58].
2.5 Chromosome counting and GISH analysis
Eleven of the regenerated plants were presumably somatic hybrids based on their morphology and was further confirmed by their chromosome number. The diploid chromosome number was 38, the sum of two fusion parental chromosomes (Fig. 5A–B) similar to data from the flow cytometry described above.
GISH analysis was performed using the labelled probes. The B. campestris probes were clearly distinguished in the mitotic cells of the hybrids. As expected, the mitotic GISH analysis of the hybrids showed that the hybrid chromosomes was 2n=38; the DNA of B. campestris origin, fluorescing yellow, were well-mixed on the hybrid chromosome (Fig. 5C). GISH enables not only the distinction of parental chromosomes in a large number of inter-specific and inter-generic hybrids, but also the detection of genomic constitutions and chromosome behaviour[59-60], and it has been applied effectively by other researchers to identify Brassica inter-generic hybrids[61].
2.6 The first and second generation by selfpollination or backcrossing
The first progeny derived by self-pollination had no morphologic difference from the somatic hybrids. No variations were also observed in the individual progenies from both the seedlings (Fig. 6A) and the mature plants (Fig. 6B). The first progenies obtained by backcrossing with Chinese cabbage or broccoli produced 0.02–0.06 and 0.03–0.05 seeds per set, respectively (Table 1). Seeds obtained by selfpollination and open pollination produced 0.12 and 0.24 seeds per pod, respectively.
In the second progenies, fertility gradually recovered. Seeds obtained by self-pollination ranged from 0.23–1.02 seeds per pod whereas open pollination produced 1.35–0.98 seeds per set. The seeds obtained by backcrossing with Chinese cabbage showed low growth and fertility with only 0.03 seeds per pod. On the other hand, backcrossing with broccoli produced a twofold higher fertility than with cabbage (Table 1).
The seeds were of various sizes and shapes. Seeds produced by backcrossing with Chinese cabbage had different sizes, but all germinated to normal plants and normal-sized of seeds were obtained in next generation. The morphological traits of the second progenies also showed intermediate characteristics after maturation (Fig. 6C). For production more suitable progenies, the crossing progress was carried using the second progenies (Fig. 6D).

Fig. 5 Cytology of somatic hybrids between B. oleracea and B. campestris. (A, B) Mitotic cells of somatic hybrids (2n=38). (C) Genomic in situ hybridisation (GISH) images of somatic hybrids between B. campestris and B. oleracea (2n=38), yellow signals (allows) are from the labelled B. campestris probe, chromosome counterstained by propidium iodide (PI).

Fig. 6 Plant morphologies of somatic hybrids and their progenies. (A) Yong seedlings of the first progenies of somatic hybrids. (B) Mature plant of the first progenies. (C) Mature plant of the second progenies. (D) Crossing progress was carried using the second progenies.

Table 1 Fertility test of somatic hybrids and their progenies (Chinese cabbage× broccoli, Unit: seeds/set)
3 Conclusion
The somatic hybrids obtained between B. campestris and B. oleracea were fertile. Furthermore, hybrids were backcrossable with Chinese cabbage and broccoli, making the hybrids accessible to advanced utilization for breeding purposes. Therefore, the hybrids are useful breeding materials for B. campestris. Further studies are needed to identify the source of vascular wilt resistance in the progeny using pathological and molecular biological analyses.
Acknowledgement The authors are grateful to Nam Kwon Baek for providing seed.
[1] Lian YJ, Lim HT. Plant regeneration of B. juncea through plant tissue and protoplast culture. J Plant Biotechnol, 2001, 3(1): 27−31.
[2] Ciccarese F, Frisullo S, Cirulli M. Severe outbreaks of verticillium wilt on Cichorium intybus and Brassica rapa and pathogenic variations among isolates of Verticillium dahliae. Plant Dis, 1987, 71(12): 1144−1145.
[3] Subbarao KV, Chassot A, Gordon TR, et al. Genetic relationships and cross pathogenicities of Verticillium dahliae isolates from Cauliflower and other crops. Phytopathology, 1995, 85: 1105−1112.
[4] Bhat RG, Subbarao KV. Reaction of broccoli to isolates of Verticillium dahliae from various hosts. Plant Dis, 2001, 85(2): 141−146.
[5] Koike, ST, Subbarao KV, Davis RS, et al. Verticillium wilt of cauliflower in California. Plant Dis, 1994, 78(11): 1116−1121.
[6] UN. Genome-analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn J Bot, 1935, 7: 389−452.
[7] Namai H, Sarashima M, Hosoda T. Interspecific and intergeneric breeding in Japan// Tsunoda S, Hinata K, Gómz-Campo C, eds. Brassica Crops and Wild Allies. Tokyo: Japanese Science Society Press, 1980: 191−203.
[8] Glimelius K, Fahlesson J, Landgren M, et al. Gene transfer via somatic hybridization in plants. Tibtech, 1991, 9(1): 24−30.
[9] Waara S, Glimelius K. The potential of somatic hybridization in crop breeding. Euphytica, 1995, 85: 217−233.
[10] Chen HF, Wang H, Li ZY. Production and genetic analysis of partial hybrids in intertribal crosses between Brassica species (B. rapa, B. napus) and Capsella bursa-pastoris. Plant Cell Rep, 2007, 26(10): 1791−1800.
[11] Zhao ZG, Hu TT, Ge XH, et al. Production and characterization of intergeneric somatic hybrids between Brassica nupus and Orychophragmus violaceus and their backcrossing progenies. Plant Cell Rep, 2008, 27(10): 1611−1621.
[12] Tu YQ, Sun J, Liu Y, et al. Production and characterization of intertribal somatic hybrids of Raphanus sativus and Brassica rapa with dye and medicinal plant Isatis indigotica. Plant Cell Rep, 2008, 27(5): 873−883.
[13] Du XZ, Ge XH, Yao XC, et al. Production and cytogenetic characterization of intertribal somatic hybrids between Brassica napus and Isatis indigotica and backcross progenies. Plant Cell Rep, 2009, 28(7): 1105−1113.
[14] Dudits D, Maroy E, Praznovszky T, et al. Transfer of resistance traits from carrot into tobacco by asymmetric somatic hybridization: regeneration of fertile plants. Proc Natl Acad Sci USA, 1987, 84(23): 8434−8438.
[15] Menczel L, Morgan A, Brown S, et al. Fusion-mediated combination of Ogura-type cytoplasmic male sterility with Brassica napus plastids using X-irradiated CMS protoplasts. Plant Cell Rep, 1987, 6(2): 98−101.
[16] Chatterjee G, Sikdar SR, Das S, et al. Intergeneric somatic hybrid production through protoplast fusion between Brassica juncea and Diplotaxis murlis. Theor Appl Genet, 1998, 76(6): 915−922.
[17] Jourdan PS, Earle ED, Mutschler MA. Synthesis of male sterile, triazine-resistant Brassica napus by somatic hybridization between cytoplasmic male sterile B. oleracea and atrazine-resistant B. campestris. Theor Appl Genet, 1989, 78: 445−455.
[18] Walters TW, Mutschler MA, Earle ED. Protoplast fusion-derived Ogura male sterile cauliflower with cold tolerance. Plant Cell Rep, 1992, 10(12): 624−628.
[19] Arumugam N, Mokhopadhyay A, Gupta V, et al. Somatic cell hybridization of 'oxy' CMS Brassica juncea (AABB) with B. oleracea (CC) for correction of chlorosis and transfer of novel organelle combinations to allotetraploid Brassicas. Theor Appl Genet, 2000, 100(7): 1043−1049.
[20] Ren JP, Dickson MH, Earle ED. Improved resistance to bacterial soft rot by protoplast fusion between Brassica rapa and B. oleracea. Theor Appl Genet, 2000, 100(5): 810−819.
[21] Hu Q, Andersen SB, Dixelius C, et al. Production of fertile intergeneric somatic hybrids between Brassica napus and Sinapis arvensis for the enrichment of the rapeseed gene pool. Plant Cell Rep, 2002, 21(2): 147−152.
[22] Hu Q, Hansen LN, Laursen J, et al. Intergeneric hybrids between Brassica napus and Orychophragmus violaceus containing traits of agronomic importance for oilseed rape breeding. Theor Appl Genet, 2002, 105(67): 834−840.
[23] Yamagishi H, Mohammad MH, Katsuei Y. Production of somatic hybrids between southern type Chinese cabbage“Kenshin” and Cabbage “Yoshin” and their flowering Characteristics. J Jpn Soc Hort Sci, 1992, 16(2): 311−316.
[24] Terada R, Yamashita Y, Nishiyashi S, et al. Somatic hybrids between Brassica oleracea and B. cmapestris. selection by the use of iodoacetamide inactivation and regeneration ability. Theor Appl Genet, 1987, 73(3): 379−384.
[25] Robertson D, Palmer JD, Earle ED, et al. Analysis of organelle genomes in a somatic hybrid derived from cytoplasmic male-sterile Brassica oleracea and atrazine-resistant B. campestris. Theor Appl Genet, 1987, 74(3): 303−309.
[26] Sundberg E, Langgren M, Glimelius K. Fertility and chromosome stability in Brassica napus resynthesised by protoplast fusion. Theor Appl Genet, 1987, 75(1): 96−104.
[27] Rosén B, Halldén C, Heneen WK. Diploid Brassica napus somatic hybrids: characterizationof nuclear and organellar DNA. Theor Appl Genet, 1988, 76(2): 197−203.
[28] Ozminkowski RH, Jourdan PS. Expression of selfincompatibility and fertility of Brassica napus L. resynthesized by interspecific somatic hybridization. Euphytica, 1993, 65(2): 153−160.
[29] Cardi T, Earle ED. Production of new CMS Brassica oleracea by transfer of ‘Anand’ cytoplasm from B. rapa through protoplast fusion. Theor Appl Genet, 1997, 94(2): 204−212.
[30] Sigareva MA, Earle ED. Direct transfer of a cold tolerant Ogura male sterile cytoplasm into cabbage (Brassica oleracea ssp. capitata) via protoplast fusion. Theor Appl Genet, 1997, 94(2): 213−220.
[31] Hansen LN, Earle ED. Transfer of resistance of Xanthominas campestris pv campestris into Brassica oleracea L. by protoplast fusion. Theor Appl Genet, 1995, 91: 1293−1300.
[32] Murashige T, Skoog F. A revised medium for rapid grown and bio-assays with tobacco tissue cultures. Physiol Plant, 1962, 15(3): 473−497.
[33] Glimelius K, Djupsjöbackaa M, Fellner-Feldegg H. Selection and enrichment of plant protoplast heterokaryons of Brassicaceae by flow sorting. Plant Sci, 1986, 45(2): 133−141.
[34] Kao KN, Michcharyluk MR. Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta, 1975, 126(2): 105−110.
[35] Doyle JJ, Doyle JI. Isolation of plant DNA from fresh tissue. Focus, 1990, 12(1): 13−15.
[36] Zhong XB, Hans JJ, Zabel P. Preparation of tomato meiotic pachytene and mitotic metaphase chromosomes suitable for fluorescence in situ hybridization (FISH). Chromosome Res, 1996, 4(1): 24−28.
[37] Leitch AR, Schwarzacher T, Jackson D, et al. In situ hybridization: a practical guide (Microscopy Handbook No. 27). Oxford: Bios Scientific, 1994.
[38] Dons JJM, Colijn-Hooymans CM. Agarose plating of protoplasts and its applications// Bajaj YPS, ed. Plant Protoplasts and Genetic Engineering I. Biotechnology in Agriculture and Forestry, Vol 8. Berlin, Heidelberg, Springer-Verlag, 1989: 50−62.
[39] Lörz H, Larkin PJ, Thomson J, et al. Improved protoplast and agarose media. Plant Cell Tissue Organ Cult, 1983, 2(3): 217−226.
[40] Brodelious P, Nilsson K. Entrapment of plant cells in different matrixes: a comparative study. FEBS Lett, 1980, 122(2): 312−316.
[41] Takahira J, Cousin A, Nelson MN, et al. Improvement in efficiency of microspore culture to produce doubled haploid canola (Brassica napus L.) by flow cytometry. Plant Cell Tiss Organ Cult, 2011, 104(1): 51−59.
[42] Arumuganathan K, Earle ED. Estimation of nuclear DNA Content of plants by Flow cytometry. Plant Mol Bio Rep, 1991, 9(3): 229−233.
[43] Tiwari JK, Sarkar PD, Pandey SK, et al. Molecular and morphological characterization of somatic hybrids between Solanum tuberosum L. and S. etuberosum Lindl. Plant Cell Tissue Organ Cult, 2010, 103(2): 175−187.
[44] Clarindo WR, de Carvalho CR, Araújo FS, et al. Recovering polyploid papaya in vitro regenerants as screened by flow cytometry. Cell Tissue Organ Cult, 2008, 92(2): 207−214.
[45] Makowczyńska J, Andrzejewska-Golec E, Sliwinska E. Nuclear DNA content in different plant materials of Plantago asiatica L. cultured in vitro. Plant Cell Tiss Organ Cult, 2008, 94(1): 65−71.
[46] Mallón R, Rodríguez-Oubiňa J, González ML. In vitro propagation of the endangered plant Centaurea ultreiae: assessment of genetic stability by cytological studies, flow cytometry and RAPD analysis. Plant Cell Tiss Organ Cult, 2010, 101(1): 31−39.
[47] Xu YS, Clark MS, Pehu E. Use of RAPD markers to screen somatic hybrids between Solanum tuberosum and S. brevidens. Plant Cell Rep, 1993, 12(2): 107−109.
[48] Wang MQ, Zhao JS, Peng ZY, et al. Chromosomes are eliminated in the symmetric fusion between Arabidopsis thaliana L. and Bupleurum scorzonerifolium Willd. Plant Cell Tissue Organ Cult, 2008, 92(2): 121−130.
[49] Yamagishi H, Glimelius K. Somatic hybrids between Arabidopsis thaliana and cytoplasmic male-sterile radish (Raphanus sativus). Plant Cell Rep, 2003, 22(1): 52−58.
[50] Schoenmaker HC, Nobel EM, Koornneef M. Use of leaky nitrate reductase-deficient mutants of tomato (Lycopersicon esculentum Mill.) for selection of somatic hybrid cell lines with wild type potato (Solanum tuberosum L.). Plant Cell Tissue and Organ Cult, 1992, 31(2): 151−154.
[51] Borgato L, Conicella C, Pisani F, et al. Production and characterization of arboreous and fertile Solanum melongena + Solanum marginatum somatic hybrid plants. Planta, 2007, 226(4): 961−969.
[52] Gleba YY, Hoffmann F. ‘Arabidobrassica’: a novel plant obtained by protoplast fusion. Planta, 1980, 149(2): 112−117.
[53] Schenk HR, Robbelen G. Somatic hybrids by fusion of protoplasts from Brassica oleracea and B. campestris. Z Pflanzenzuchtg, 1982, 89: 278−288.
[54] Wang YP, Sonntag K, Rudloff E, et al. Production and characterization of somatic hybrids between Brassica napus and Raphanus sativus. Plant Cell Tiss Organ Cult, 2006, 86(2): 279−283.
[55] Yui S, Yoshikawa H. Breeding of bolting resistance in Chinese cabbage-critical day length for flower induction of late bolting material with no chilling requirement. J Japan Soc Hort Sci, 1992, 61(3): 565−568.
[56] Yamagishi H, Terachi T. Molecular and biological studies on male-sterile cytoplasm in the Cruciferae. I. The origin and distribution of Ogura male-sterile cytoplasm in Japanese wild radishes (Raphanus sativus L.) revealed by PCR-aided assay of their mitochondrial DNAs. Theor Appl Genet, 1994, 87(8): 996−1000.
[57] Sundberg E. Somatic hybrids and cybrids within Brassicacea: studies focused on refining production methods and identifying factors influencing the genetic composition of somatic hybrids [D]. Uppsala: Swedish University of Agricultural Sciences, 1991: 7−44.
[58] Liu JH, Xu XY, Deng XX. Intergeneric somatic hybridization and its application to crop genetic improvement. Plant Cell Tissue Org Cult, 2005, 82(1): 19−44.
[59] Wang YP, Zhao XX, Sonntag K, et al. Behaviour of Sinapis alba chromosome in a Brassica napus background revealed by genomic in-situ hybridization. Chromosome Res, 2005, 13(8): 819−826.
[60] Ji YF, Pertuze R, Chetelat RT. Genomic differentiation by GISH in inter specific and intergeneric hybrids of tomato and related nightshades. Chromosome Res, 2004, 12(2): 107−116.
[61] Benabdelmouna A, Guéritaine G, Abirached-Darmency M, et al. Genome discrimination in progeny of interspecific hybrids between Brassica napus and Raphanus raphanistrum. Genome, 2003, 46(3): 469−472.

