静电纺丝法制备的钴酸镍微米带的磁性以及电化学性能
朱福良 赵景新 程永亮 李海宝 阎兴斌,*
(1兰州理工大学材料科学与工程学院,兰州730050; 2中国科学院兰州化学物理研究所,清洁能源化学与材料实验室,兰州730000)
静电纺丝法制备的钴酸镍微米带的磁性以及电化学性能
朱福良1,*赵景新1,2程永亮2李海宝1阎兴斌2,*
(1兰州理工大学材料科学与工程学院,兰州730050;2中国科学院兰州化学物理研究所,清洁能源化学与材料实验室,兰州730000)
通过直接退火静电纺丝前驱样品以及调节升温速率最终得到了钴酸镍(NiCo2O4)微米带.通过X射线衍射、扫描电镜、振动样品磁强计以及电化学工作站等分析手段对钴酸镍微米带的晶体结构、形貌、磁学性能以及电化学性能进行了研究.结果显示,以1°C·min-1的升温速率得到的NiCo2O4微米带属于立方尖晶石结构,高温处理后仍能保持一维结构.室温磁化结果显示制备的NiCo2O4微米带具有超顺磁性,在10 kOe时磁化强度为6.35 emu·g-1.此外,电化学测试结果显示,NiCo2O4微米带的电容特性是典型的赝电容,并且比电容随着放电电流密度的增加而减小.
NiCo2O4;微米带;磁学性能;电化学性能;倍率性能
1 Introduction
Recently,the synthesis of magnetic nanomaterials has become an important research field because of their various structures affording various novel properties,which should be useful in a variety of applications including catalysts,1,2information storage media,3electronic device,4medicine,5and gas sen-sors.6So far,many diverse methods have been reported for the preparation of magnetic nanomaterials including template method,7sol-gel,8co-precipitation,9-11and combustion synthesis,12which are interesting and attract much research attention. However,these preparation methods often suffer from tedious procedures and special conditions.Furthermore,agglomeration and wide particle size distribution are the drawbacks associated with most of these methods.From the viewpoint of fundamental research,the development of simple and efficient methods for fabricating magnetic nanostructures at low cost is a great challenge for further practical applications.
As one of the most important magnetic materials,the magnetic behavior of cobaltate nickel(NiCo2O4)has drawn much interest owing to its broad applications in many fields including electrochemical immunosensors,electrocatalysis,and magnetic hyperthermia.13-15To date,NiCo2O4nanostructural materials with various morphologies have been reported,such as nanoparticles,12,15nanopowders,16,17nanofibers,18nanoflakes,19nanoplates,20nanorods,19and nanowires.21However,NiCo2O4microbelts have never been reported in literature.
Recently,electrospinning has been widely used as a convenient and facile method to prepare various solid fibers with the diameter ranging from tens of nanometers to several micrometers.Also,by using tubes by fiber template(TUFT) process,22-24coaxial electrospinning,25-27and single nozzle coelectrospinning technique,28,29microbelts can be prepared.However,these methods usually need multi-steps process,strictly control the rate between core and shell solutions,and choose different polymers to generate phase separation.Therefore,it is necessary to develop a simple and one-step electrospinning method to prepare microbelts.
In this work,electrospinning synthesis route was successfully utilized to produce superparamagnetic crystalline NiCo2O4microbelts with high uniformity.Compared with above mentioned methods,only simple electrospinning and calcination are required.After that,the magnetic properties of as-made NiCo2O4microbelts calcined at 500°C were investigated using vibrating sample magnetometer in detail.Also,the electrochemical propertiesof theNiCo2O4sampleswereinvestigated.
2 Experimental
2.1 Materials
Polyvinylpyrrolidone(PVP,K-30,Mw=3×104)was purchased from Tianjin Tiantai Fine Chemical Co.,Ltd.Ni(NO3)2·6H2O (≥98.0%(w),purchased from Xiʹan Chemical Reagent Co., Ltd.),Co(NO3)2·6H2O(≥99.0%(w),purchased from Tianjin Fengyue Chemical Co.,Ltd)and ethanol(C2H5OH,analytical reagentgrade(AR),≥99.7%(w),purchased from Tianjin Rionlon BoHua Medical Chemistry Co.,Ltd.,China)were used as received.KOH(AR,≥85%(w))was otained commercially from BASF Chemical Co.,Ltd.of Tianjin.The conducting graphite(fixed carbon content≥99.9%(atom fraction))and the acetylene black(≥99.99%(w))were obtained commercially from SCM Industrial Chemical Co.,Ltd.Polytetrafluoroethylene(PTFE)emulsion(30%(w))purchased from Bu A Ze Industry&Trade Co.,Ltd.,Shanghai.Other reagents were commercially available and were of analytical reagent grade.
2.2 Preparation of NiCo2O4microbelts
In the preparation of the NiCo2O4microbelts,1.45 g of Ni(NO3)2·6H2O and 2.91 g of Co(NO3)2·6H2O were added into a mixture of 2.5 mL of ethanol and 7.5 mL of water,then 7.59 g of PVP was added into the above solution to increase viscidity.After the mixture was magnetically stirred for several hours at room temperature(RT),a viscous light-pink clear solution of Ni(NO3)2/Co(NO3)2/PVP was obtained.The schematic of electrospinning apparatus is shown in Fig.1.In the subsequent electrospinning process,the precursor solution was then delivered into a plastic syringe equipped with a 7#stainless steel needle.The metallic needle was connected to a high-voltage power supply,and a piece of grounded aluminum foil was placed 15 cm between the tip of the needle and collector.As a high voltage of 15 kV was applied,the precursor solution jet accelerated toward the aluminum foil,leading to the formation of Ni(NO3)2/Co(NO3)2/PVP composite fibers accompanied by rapid evaporation of solvent.For the following thermolysis process,as-spun fibers were placed in a muffe furnace and calcined at 500°C for 2 h with a heating rate of 1°C·min-1to remove PVP and obtain NiCo2O4samples.
2.3 Characterization

Fig.1 Schematic diagram of electrospinning apparatus
To analyze the crystal structures of the samples,X-ray diffraction(XRD)measurements were conducted on an X-ray diffractometer using Cu Kαradiation(XRD,Panalytical XʹPert Pro.,Holland)from 10°to 80°.The morphology and microstructures of the as-spun fibers and calcined samples were analyzed by the field-emission scanning electron microscope (FESEM,JSM-6701F,Japan).In addition,magnetic measurements were performed at RT using a vibrating sample magnetometer(VSM 7304,Lake Shore,USA)and the electrochemical measurements of each as-prepared electrode were carried out using an electrochemical working station(CHI660D,Shanghai, China)in a half-cell setup configuration at RT.The working electrodes were prepared according to the method reported in literature.30NiCo2O4powder(80%(mass fraction,the same below))was mixed with 7.5%of acetylene black(>99.9%)and 7.5%of conducting graphite in an agate mortar until a homogeneous black powder was obtained.To this mixture,5%of poly (tetrafluoroethylene)was added with a few drops of ethanol. After briefly allowing the solvent to evaporate,the resulting paste was pressed at 10 MPa to nickel gauze with a nickel wire for an electric connection.The electrode assembly was dried for 16 h at 80°C in air.Each NiCo2O4electrode contained about 5 mg of electroactive material and had a geometric surface area of about 1 cm2.Aplatinum gauze electrode and a saturated calomel electrode(SCE)served as the counter electrode and the reference electrode,respectively.The corresponding specific capacitance was calculated from the slope of each discharge curve,according to the equation SC=(I×Δt)/(ΔV×m),where SC is the specific capacitance,I is the constant discharge current,Δt is the discharge time,ΔV is the voltage difference in discharge,and m isthemass of NiCo2O4samplescoatedon eachwork electrode.
3 Results and discussion
3.1 XRD analysis
The XRD pattern of the as-prepared NiCo2O4microbelts synthesized by electrospinning method is shown in Fig.2.It shows that all the diffraction reflections can be indexed to a cubic spinel NiCo2O4(JCPDS.073-1702)and no impurity phase was detected.Therefore,single phase NiCo2O4can be obtained by directly calcining electrospun composite samples.In addition, the average crystallite sizes calculated by the Scherer formula:
D=0.9λ/(βcosθ)
where D is the crystallite size(nm),β is the full width of the diffraction line at half the maximum intensity measured in radians,λ is the X-ray wavelength,and θ is the Bragg angle.From the formula,the NiCo2O4crystallite size is found to be around 15.74 nm.
3.2 SEM characterization

Fig.2 XRD patterns of NiCo2O4microbelts calcined at 500°C
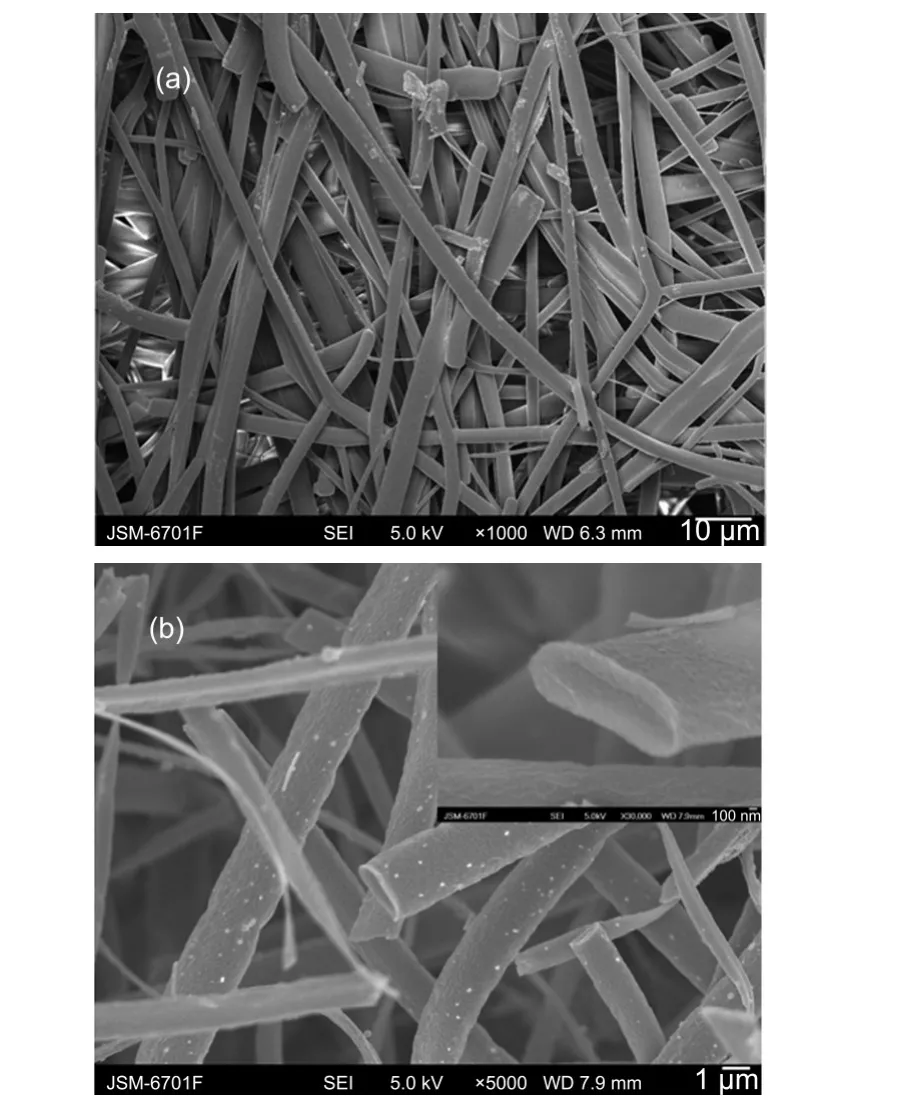
Fig.3 FESEM images of(a)precursor samples and(b)calcined NiCo2O4microbelts at 500°CInset in(b)shows the cross-section image of calcined NiCo2O4microbelts.
Fig.3 shows the SEM images of as-spun precursor fibers and NiCo2O4microbelts prepared at 500°C.As shown in Fig.3(a), precursor samples exhibit ultra-fine belt morphology and the surfaces of the belts appear to be smooth and uniform due to amorphous nature.31,32The average length of most belts is up to tens of micrometers with the average diameter of approximately 3.31 μm.For the NiCo2O4belts,when the calcined temperature reaches 500°C(Fig.3(b)),compared with the precursor samples,although the NiCo2O4belts still maintain the original zonal morphology,the average diameter decreases to about 1.70 μm and the surfaces of the belts become rougher.The obvious shrinkage in diameter is mainly due to the removal of PVP and the decomposition of nitrate salts.The formation of rough surface is contributed to the growth of NiCo2O4nanocrystals at higher temperature.Moreover,the thickness of microbelts is also an important parameter.The cross-section image of NiCo2O4microbelts is shown in the inset of Fig.3(b),it is clearly seen that the thickness of NiCo2O4microbelts reaches 150 nm.All of the results indicate that the prepared NiCo2O4is a zonal structure.
3.3 Magnetic property
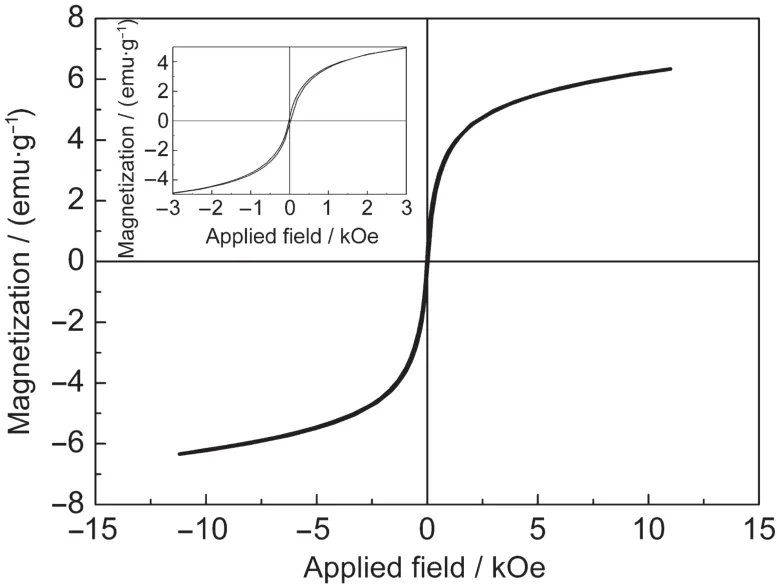
Fig.4 Room temperature magnetic hysteresis loops of NiCo2O4 microbelts calcined at 500°CInset shows the magnification at lower applied field.
It is well-known that the magnetic behavior of nanomaterials is strongly affected by their morphology and size.31Fig.4 displays the RT hysteresis loops for the NiCo2O4microbelts calcined at 500°C.It is observed that the NiCo2O4microbelts reveal a typical superparamagnetic behavior.The microbelts calcined at 500°C have a magnetisation value of 6.35 emu·g-1at 10 kOe.Because the microbelts are composed of nanoparticles,the structural property of NiCo2O4nanoparticles and their interaction have significant influence on the magnetic property of the NiCo2O4microbelts.For magnetic nanoparticles,the structures of inner and surface are different,the former is usual spin arrangement,however,the latter atomic moment arrangement is disorder,which could change the surface coordination and break surface exchange bond,leading to the decrease of saturation magnetization(Ms)of nanoparticles.33

Fig.5 Electrochemical properties of NiCo2O4microbelts calcined at 500°C(a)CV curves at different scan rates;(b)GCD curves at different current densities;(c)Nyquist plots
3.4 Electrochemical property
The electrochemical properties of the NiCo2O4microbelts were studied by cyclic voltammetry(CV),galvanostatic charge/ discharge(GCD),and electrochemical impedance spectroscopy (EIS).The CV curves of NiCo2O4microbelt electrodes taken between 0 and 0.5 V in 1 mol·L-1KOH electrolyte at different scan rates are shown in Fig.5(a).Non-rectangular form of CV curves is due to the pseudocapacitive contribution of NiCo2O4. Meanwhile,the current density increases with the increase of the voltage sweep rate.Fig.5(b)presents the typical GCD curves of the NiCo2O4electrodes in the potential range of 0 and 0.4 V in 1 mol·L-1KOH electrolyte at various current densities as indicated.The specific capacitance(SC)for NiCo2O4electrodes at various current densities can be calculated based on the galvanostatic discharge curves in Fig.5(b).Remarkably,the NiCo2O4microbelt electrode manifests acceptable values of SC of 245,240, 235,231,225,220,and 212 F·g-1at current densities of 1,2,4, 5,6,8,and 10A·g-1,respectively.EIS measurements were performed on the NiCo2O4electrodes and Nyquist plots are shown in Fig.5(c),which contain an arc in the high-frequency region and a sloped line in the low-frequency region.At the high-frequency,the arc is mainly caused by the formation of a solid electrolyte interface film and the charge transfer reaction at the electrode/electrolyte interface,respectively.At the low-frequency,a straight sloping line represents the diffusive resistance(Warburg impendence)of the electrolyte in electrode pores and the proton diffusion in the host material.34The spectrum shows a higher angle than 45°,suggesting the low diffusive resistance of the electrolyteinNiCo2O4materialelectrode.35,36

Fig.6 Specific capacitance of NiCo2O4electrodes as a function of discharging current density
Fig.6 reveals that the values of the specific capacitance for the NiCo2O4electrodes are strongly dependent on the current density.In detail,the specific capacitance slightly decreases with the increase of the current density.Up to a relatively large current density of 10 A·g-1,86.53%of the initial value remains for the NiCo2O4material.This indicates that the rate capability of the NiCo2O4in KOH electrolyte is nice,indicating that KOH is suitable for the NiCo2O4electrodes.
The cycle life of the NiCo2O4electrode at the current density of 1 A·g-1in 1 mol·L-1KOH electrolyte was depicted in Fig.7. As shown in Fig.7,the specific capacitance of the NiCo2O4electrodes decreased gradually with the increase of the cycle number.It was seen that about 73.7%of original capacitance was retained after 500 cycles,exhibiting acceptable cycle ability for the NiCo2O4microbelts.The reduction of the specific capacitance was caused by the pseudocapacitance from the oxide.
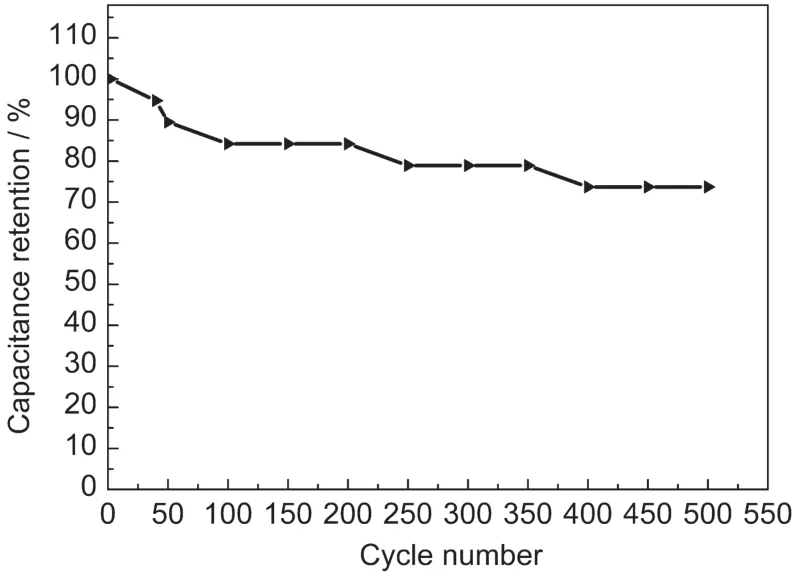
Fig.7 Cycle life of the NiCo2O4electrode at the current density of 1A·g-1
4 Conclusions
In summary,NiCo2O4have been successfully fabricated by direct calcination of electrospun precursor samples.Compared with other methods for preparing microbelts by electrospinning,only simple calcination of precursor samples is required. After high temperature treatment,the fibers still remain one-dimensional texture of 1.70 μm in diameter.Besides,the microbelts show a superparamagnetic behavior at room temperature with the magnetisation value of 6.35 emu·g-1at 10 kOe.Moreover,the electrochemical capacitances of NiCo2O4microbelts show the typical pseudocapacitive capacitance and the specific capacitance gradually decreases with the increase of discharge current density.The material has an acceptable rate capability in 1 mol·L-1KOH electrolyte,showing about 86.53%of its maximal capacitance at a current density of 10 A·g-1in GCD experiments.The highest specific capacitance of 245 F·g-1is observed in 1 mol·L-1KOH electrolyte at 1A·g-1.
(1) Zhou,X.W.;Chen,Q.S.;Zhou,Z.Y.;Sun,S.G.J.Nanosci. Nanotechnol.2009,9,2392.doi:10.1166/jnn.2009.SE34
(2)Gao,Y.Y.;Cao,D.X.;Wang,G.L.;Yin,C.L.ActaPhys.-Chim. Sin.2010,26,29.[高胤义,曹殿学,王贵领,尹翠蕾.物理化学学报,2010,26,29.]doi:10.3866/PKU.WHXB20100102
(3) Fried,T.;Shemer,G.;Markovich,G.Adv.Mater.2001,13, 1158.
(4) Jolley,C.;Pool,V.;Idzerda,Y.;Douglas,T.Chem.Mater.2011, 23,2392.
(5)Xu,Z.C.;Hou,Y.L.;Sun,S.H.J.Am.Chem.Soc.2007,129, 8698.
(6) Zhong,J.Y.;Cao,C.B.Sens.Actuators B 2010,145,651.doi: 10.1016/j.snb.2010.01.016
(7) Chaubal,N.S.;Joshi,V.Y.J.Porous.Mater.2011,18,177.doi: 10.1007/s10934-010-9368-2
(8) Liu,X.H.;Wang,J.Q.;Zhang,J.Y.;Yang,S.G.Mater.Sci. Eng.A 2006,430,248.doi:10.1016/j.msea.2006.05.059
(9) Lina,G.;Meng,G.Y.Mater.Res.Bull.2008,43,1555.doi: 10.1016/j.materresbull.2007.06.027
(10) Mandzhukova,T.;Khrussanova,M.;Grigorova,E.;Stefanov,P.; Khristov,M.;Peshev,P.J.Alloy.Compd.2008,457,472.doi: 10.1016/j.jallcom.2007.03.006
(11) Keng,C.S.;Tsin,L.K.;Zulkarnain,Z.Electrochim.Acta 2012, 67,67.doi:10.1016/j.electacta.2012.02.014
(12) Verma,S.;Joshi,H.M.;Jagadale,T.;Chawla,A.;Chandra,R.; Ogale,S.J.Phys.Chem.C 2008,112,15106.doi:10.1021/ jp804923t
(13) Li,Q.F.;Zeng,L.X.;Wang,J.C.;Tang,D.P.;Liu,B.Q.; Chen,G.N.;Wei,M.D.ACS Appl.Mater.Interfaces 2011,3, 1366.doi:10.1021/am200228k
(14) Tavares,A.C.;Cartaxo,M.A.M.;Pereira,M.I.D.S.;Costa,F. M.J.Electroanal.Chem.1999,464,187.doi:10.1016/S0022-0728(99)00018-2
(15) Sangeeta,N.K.;Anil,D.J.;Seema,V.Nanomed-Nanotechnol. 2012,8,452.doi:10.1016/j.nano.2011.07.010
(16) Cabo,M.;Pellicer,E.;Rossinyol,E.;Estrader,M.;Ortega,A. L.;Nogues,J.;Castell,O.;Surinach,S.;Baro,M.D.J.Mater. Chem.2010,20,7021.doi:10.1039/c0jm00406e
(17) Bao,J.Z.;Wang,S.L.Acta Phys.-Chim.Sin.2011,27,2849. [鲍晋珍,王森林.物理化学学报,2011,27,2849.]doi:10.3866/ PKU.WHXB20112849
(18)Guan,H.Y.;Shao,C.;Liu,Y.C.;Yu,N.;Yang,X.H.Solid State Electrochem.2004,131,107.
(19) Salunkhe,R.R.;Jang,K.H.;Yu,H.;Yu,S.;Ganesh,T.;Han,S. H.;Ahn,H.J.Alloy.Compd.2011,509,6677.doi:10.1016/ j.jallcom.2011.03.136
(20)Hu,L.F.;Wu,L.M.;Liao,M.Y.;Hu,X.H.;Fang,X.S.Adv. Funct.Mater.2012,22,998.doi:10.1002/adfm.v22.5
(21)Yu,L.Q.;Chen,S.L.;Chang,S.;Li,Y.H.;Gao,Y.Y.;Wang, G.L.;Cao,D.X.Acta Phys.-Chim.Sin.2011,27,615.[于丽秋,陈书礼,常 莎,李云虎,高胤义,王贵领,曹殿学.物理化学学报,2011,27,615.]doi:10.3866/PKU.WHXB20110317
(22) Caruso,R.A.;Schauka,J.H.;Greiner,A.Adv.Mater.2001,13, 1577.doi:10.1002/1521-4095(200110)13:20<1577::AIDADMA1577>3.0.CO;2-S
(23) Ochanda,F.;Jones,W.E.Langmuir 2005,21,10791.doi: 10.1021/la050911s
(24)Kim,G.M.;Lee,S.M.;Michjler,G.H.;Roggendorf,H.; Güsele,U.;Knez,M.Chem.Mater.2008,20,3085.doi: 10.1021/cm703398b
(25) Loscertales,I.G.;Banero,A.;Márquez,M.;Spretz,R.; Velardeortiz,R.;Larsen,G.J.Am.Chem.Soc.2004,126,5376. doi:10.1021/ja049443j
(26) Zhan,S.H.;Chen,D.R.;Jiao,X.L.;Tao,C.H.J.Phys.Chem. B 2006,110,11199.doi:10.1021/jp057372k
(27) Li,D.;Xia,Y.N.Nano Lett.2004,4,933.doi:10.1021/ nl049590f
(28)Bazilevsky,A.V.;Yarm,A.L.;Meganridis,C.M.Langmuir 2007,23,2311.doi:10.1021/la063194q
(29) Sanders,E.H.;Kloefkorn,R.;Bowlin,G.L.;Simpson,D.G.; Wnek,G.F.Macromolecules 2003,36,3803.doi:10.1021/ ma021771l
(30)Lang,J.W.;Kong,L.B.;Liu,M.;Luo,Y.C.;Kang,L. J.Electrochem.Soc.2010,157,1341.doi:10.1149/1.3497298
(31) Cheng,Y.L.;Zou,B.L.;Yang,J.L.;Wang,C.J.;Liu,Y.J.; Fan,X.Z.;Zhu,L.;Wang,Y.;Ma,H.M.;Cao,X.Q. CrystEngComm 2011,13,2268.doi:10.1039/c0ce00802h
(32)Zhang,Z.Y.;Li,X.H.;Wang,C.H.;Wei,L.M.;Liu,Y.C.; Shao,C.L.J.Phys.Chem.C 2009,113,1939.doi:10.1021/ jp806088m
(33) Pankhurst,O.A.;Polllard,R.J.Phys.Rev.Lett.1991,67,248. doi:10.1103/PhysRevLett.67.248
(34) Sun,G.H.;Li,K.X.;Sun,C.G.Microporous Mesoporous Mat.2010,128,56.doi:10.1016/j.micromeso.2009.07.027
(35) Xu,M.W.;Zhao,D.D.;Bao,S.J.;Li,H.L.J.Solid State Electrochem.2007,11,1101.doi:10.1007/s10008-006-0246-4
(36) Zhang,S.S.;Xu,K.;Jow,T.R.Electrochim.Acta 2004,49, 1057.doi:10.1016/j.electacta.2003.10.016
July 2,2012;Revised:September 6,2012;Published on Web:September 6,2012.
Magnetic and Electrochemical Properties of NiCo2O4Microbelts Fabricated by Electrospinning
ZHU Fu-Liang1,*ZHAO Jing-Xin1,2CHENG Yong-Liang2LI Hai-Bao1YAN Xing-Bin2,*
(1School of Material Science and Engineering,Lanzhou University of Technology,Lanzhou 730050,P.R.China;2Laboratory of Clean Energy Chemistry and Materials,Lanzhou Institute of Chemical Physics,
Chinese Academy of Sciences,Lanzhou 730000,P.R.China)
Cobaltate nickel(NiCo2O4)microbelts were fabricated by direct calcination of electrospun precursor samples with an appropriate heating rate.The crystal structure,morphology,magnetic properties,and electrochemical properties of the NiCo2O4microbelts were investigated by X-ray diffraction, scanning electron microscopy,vibrating sample magnetometry,and electrochemical analysis.The results showed that a heating rate of 1°C·min-1resulted in the formation of cubic spinel NiCo2O4microbelts.After calcination at high temperatures,the microbelts retained their one-dimensional structure.Magnetization results indicated that the NiCo2O4microbelts were superparamagnetic and their magnetization value at 10 kOe was 6.35 emu·g-1.Moreover,the electrochemical results suggest that the capacitance of the NiCo2O4microbelts is typical pseudocapacitive capacitance,and the value of the specific capacitance gradually decreases with increasing discharge current density.
NiCo2O4;Microbelt; Magnetic property;Electrochemical property;Rate capability
10.3866/PKU.WHXB201209063
*Corresponding authors.ZHU Fu-Liang,Email:chzfl@lut.cn;Tel:+86-931-9491509;Fax:+86-931-2976578.YAN Xing-Bin, Email:xbyan@licp.cas.cn;Tel/Fax:+86-931-4968055.
The project was supported by the Natural Science Foundation of Gansu Province,China(3ZS042-B25-029)and National Natural Science Foundation of China(51005225).
甘肃省自然科学基金(3ZS042-B25-029)和国家自然科学基金(51005225)资助项目
O646

