The inhibition effect of Chlorpromazine against theβ-lactam resistance of MRSA
Ryong Kong, Ok-Hwa Kang, Yun-Soo Seo, Su-Hyun Mun, Tian Zhou, Dong-Won Shin, Dong-Yeul Kwon*BK Plus Team, Professional Graduate School of Oriental Medicine, Wonkwang University, Iksan, Jeonbuk 570-749, KoreaDepartment of Oriental Pharmacy, College of Pharmacy, Wonkwang Oriental Medicines Research Institute, Institute of Biotechnology, Wonkwang UDnei vp ea rrstimt ye, nItk so a f n O, rJi ee on n tab lu Mk 5 ed7 i0c-in7 e4 R9,e s Ko ou rreca es, College of Bio Industry Science, Sunchon National University, Sunchon, Jeonnam 540-74, Korea
The inhibition effect of Chlorpromazine against theβ-lactam resistance of MRSA
Ryong Kong1, Ok-Hwa Kang2, Yun-Soo Seo2, Su-Hyun Mun1, Tian Zhou2, Dong-Won Shin3, Dong-Yeul Kwon2*1BK21 Plus Team, Professional Graduate School of Oriental Medicine, Wonkwang University, Iksan, Jeonbuk 570-749, Korea
2Department of Oriental Pharmacy, College of Pharmacy, Wonkwang Oriental Medicines Research Institute, Institute of Biotechnology, Wonkwang U
3Dnei vp ea rrstimt ye, nItk so a f n O, rJi ee on n tab lu Mk 5 ed7 i0c-in7 e4 R9,e s Ko ou rreca es, College of Bio Industry Science, Sunchon National University, Sunchon, Jeonnam 540-742, Korea
ARTICLE INFO ABSTRACT
Article history:
in revised form 16 March 2016 Accepted 15 April 2016
Available online 20 June 2016
Chlorpromazine PBP2a
β-lactamase
mecA
blaZ
MRSA
Objective: To investigate the gene related to β-lactam resistance and to confirm the mechanism about a synergy effect between CPZ and β-lactam antibiotics. Methods: To measure antibacterial activity, we performed a minimum inhibitory concentration (MIC) and synergy test. Transmission electron microscopy (TEM) was used in morphological analysis. To analyze gene expression, we conducted reverse transcriptase polymerase chain reaction (PCR). Results: We confirmed a synergy effect between CPZ and β-lactam antibiotics. Furthermore,we observed that CPZ affect the cell envelope of MRSA by using TEM. At the gene level, CPZ reduced the expression of resistance genes. Conclusions: Through this result, we hypothesize that a decrease of resistance factor expressions was caused by CPZ because it disrupts the activity of a sensor protein located in the cell membrane.
1. Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a significant cause of infections worldwide [1]. This pathogenic organism has been implicated in soft or cutaneous tissue infections, septic arthritis,necrotizing pneumonia, osteomyelitis, and bacteremia [2]. Moreover,MRSA can develop various mutations that confer antibiotic resistance. Penicillin binding proteins (PBPs) are enzymes that catalyze a pentapeptide cross-linking reaction[3, 4]. PBPs have a high affinity for β-lactam antibiotics, particularly since these drugs bind the active site of PBPs that are involved in peptidoglycan linking [5]. MRSA produces PBP2a enzymes (encoded by mecA) when exposed to β-lactam antibiotics. PBP2a is eventually substituted for PBP in the synthesis of the bacterial cell wall, and it possesses low binding affinities for drugs with β-lactam rings[6]. MRSA also produces a β-lactamase (encoded by blaZ) that decreases the activity of β -lactam antibiotics [7].
Several studies have shown that several non-antibiotics exhibit an effect against multidrug resistance [8, 9]. Among the non-antibiotics,several phenothiazines were known to be an efficient compound against multidrug resistance. Chlorpromazine (CPZ), which is one of the phenothiazines with an antibacterial effect, was reported to have a synergy effect against MRSA by using it in combination with β-lactam antibiotics[10, 11].
The object of this study is to investigate the gene related to β -lactam resistance. Furthermore, we will endeavor to confirm themechanism about a synergy effect between CPZ and β-lactam antibiotics.
2. Materials and methods
2.1. Bacterial strains and growth condition
Among the four strains used in this research, ATCC 33591(MRSA) and ATCC25923 (methicillin-susceptible Staphylococcus aureus, MSSA) were purchased from ATCC (American Type Culture Collection, Manassas, VA). The rest strains are clinical MRSA isolates (DPS-1, DPS-2) obtained from two different patients atWonkwang University Hospital, Iksan City, Jeollabukdo, South Korea. The strains were routinely grown at 37 ℃ with aeration in Mueller-Hinton agar (MHA) and Mueller-Hinton agar(MHB). Methicillin resistance factors (mecA and β-lactamase) were confirmed by using the polymerase chain reaction (Table 1).
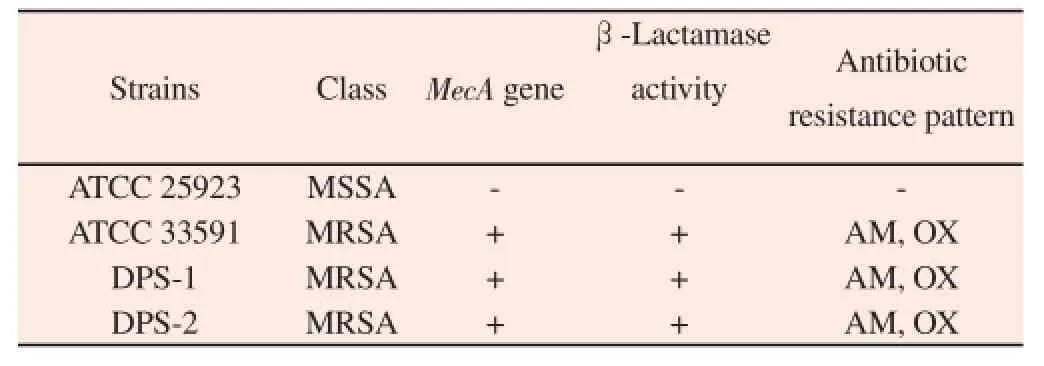
Table 1Determination of the mecA gene of the Staphylococcus aureus strains used in the experiment.
2.2. Minimum inhibitory concentration (MIC) and synergy effect test
The MIC was determined using the broth microdilution method,in accordance with the Clinical and Laboratory Standard Institute guidelines (CLSI) [12]. Serial two-fold dilutions of CPZ and 10 μL antibiotics in 100 μL MHB were prepared in 96-well microplates. The microplates were injected with MRSA and adjusted to 0.5 McFarland standards (approximately 10 μL of 1.5×108CFU/ mL, which was 1.5×107CFU/mL). The MIC was the lowest CPZ concentration in which Staphylococcus aureus growth was suppressed after 24 h incubation at 37 ℃. The checkerboard method was used to measure the synergistic effect of CPZ and antibiotics [13]. This assay was executed with CPZ in combination with ampicillin (AM)and oxacillin (OX). The antibiotics were mixed in MHB and serially diluted with CPZ. The MRSA concentration was adjusted to 1.5× 107CFU/mL. Growth suppression was checked after 24 h incubation at 37 ℃. Each experiment was repeated three times. The interactions between CPZ and antibiotics were determined using the fractional inhibitory concentration index (FICI).
2.3. Transmission electron microscopy (TEM)
The experiments with mechanism were performed by using only MRSA (ATCC 33591) because the strain was a reference strain and was inhibited by CPZ combined with antibiotics. The MRSA of a logarithmic phase were prepared by diluting overnight cultures into MHB and continuing growth at 37 ℃ until the cultures reached the mid-logarithmic phase of growth. The MRSA of an MHB-grown logarithmic phase were treated with 1/2 MIC and MIC of CPZ for 30 min. Following the treatment, 2 mL of the culture was collected by centrifugation at 13 000 rpm for 10 min. After removing the supernatant, pellets were fixed by immersion in modified Karnovsky fixative solution containing 2% glutaraldehyde and 2% paraformaldehyde in a 0.05 M (pH 7.2) sodium cacodylate buffer[14]. For TEM analysis, the specimens were fixed by a postfixed solution containing 1% osmium tetroxide in a 0.05 M (pH 7.2)sodium cacodylate buffer for 1.5 h at 4 ℃. After post-fixation, the samples were dehydrated in an increasing series of ethanol. And, the mixture of propylene oxide and embed 812 resin were infiltrated into a dehydrated samples, in accordance with Mun et al. [15] Ultrathin sections were obtained in an ultramicrotome with a diamond knife,and collected on 200-mesh grids with Formvar film. Grids were counterstained with uranyl acetate and lead citrate, and examined with an energy-filtering transmission electron microscope at 120 kV (LIBRA 120; Carl Zeiss, Oberkochen, Germany) The electronic signals transmitted were recorded using a 4 k • 4 k slow-scan chargecoupled device camera (Ultrascan 4000 SP; Gatan, Pleasanton, CA),which was attached to the electron microscope.
2.4. Reverse transcriptase polymerase chain reaction (PCR)
OX was used to confirm the resistance gene expression degree of MRSA because it was used to test the β-lactam resistance profile of MRSA. The MRSA (ATCC 33591) samples were cultured in MHB(OD600 0.35-0.45) and treated with CPZ of various concentrations and OX. The MRSA samples treated with CPZ or OX were incubated for 20 minutes at 37 ℃. The RNA was extracted using easy-REDTMBYF Total RNA Extraction Kit, in conformity with the manufacturer’s instructions. Single-strand cDNA synthesis was performed using Quantitact Reverse Transcription Kit, in accordancewith the manufacturer’s instructions. A PCR was performed using a 20 μL mixture of LugenTM Sensi 2X PCR Premix (Lugen Sci Co.,Ltd., ROK) primer, cDNA sample, and sterilized distilled water. The primer sequences of mecA, mecI, mecR1, blaZ, blaI, blaR1,and 16s rRNA are represented in Table 2. After the PCR, equal amounts of PCR products (12 μL) were subjected to 1.5% agarose gel electrophoresis. The single bands were visualized by ultraviolet(UV) light.
2.5. Statistical analysis
Data from the experiments are presented as the mean ± SEM. The level of statistical analysis was performed by Student’s t-test (SPSS. ver. 22) for multiple comparisons. The P-values < 0.05, < 0.005 were considered significant.
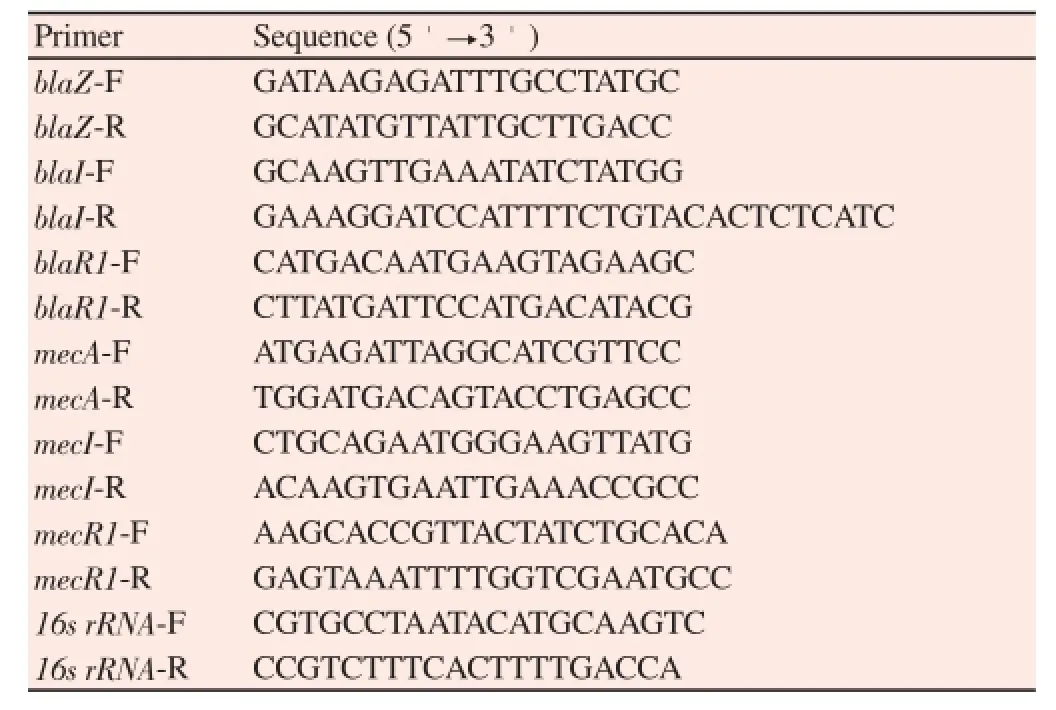
Table 2Primer used in this experiment.
3. Results
3.1. Measurement of MIC and the synergy effect
MICs of CPZ and antibiotics against MRSA and MSSA strains were measured in the broth microdilution method. The MICs of CPZ and antibiotics are represented in Table 3. The growth of the strains was inhibited in concentration of 62.5 μg/mL CPZ. The synergistic effects of CPZ and antibiotics against MRSA and MSSA strains were measured using checkerboard assay. The synergy effects of the CPZ combination with β-lactam antibiotics are represented in Table 3. The results show that CPZ combined with antibiotics inhibited growth of the strains except for one strain, respectively.
3.2. Observation of cell by TEM
The TEM image of MRSA showed a change of the cytoplasmic membrane and cell wall following exposure to CPZ. The untreated MRSA strains were observed to be intact. Whereas, MRSA strains exposed to 31.25 μg/mL CPZ were observed to change the cell wall. Furthermore, MRSA cells treated with 62.5 μg/mL CPZ were showed to break down the cytoplasmic membrane and cell wall(Figure 1).
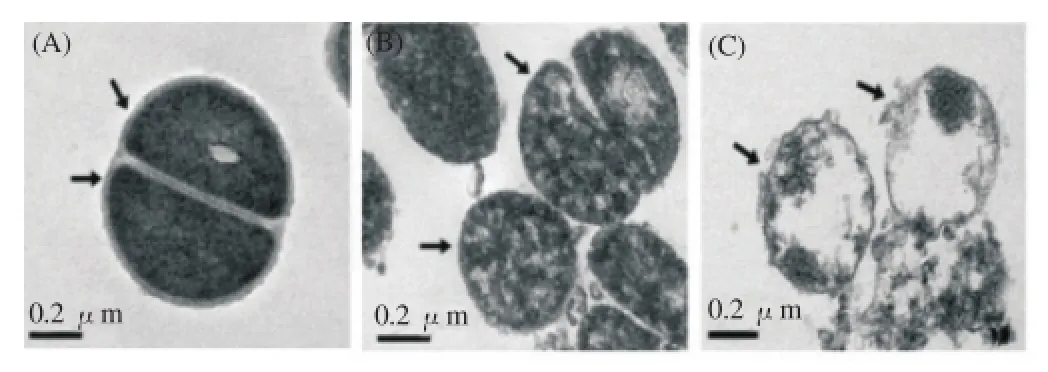
Figure 1. TEM images of MRSA (A, B, C) after 24 h CPZ treatment.(A) Untreated control MRSA. (B) MRSA treatment with 31.25 ug/mL CPZ.(C) MRSA treatment with 62.5 ug/mL CPZ.
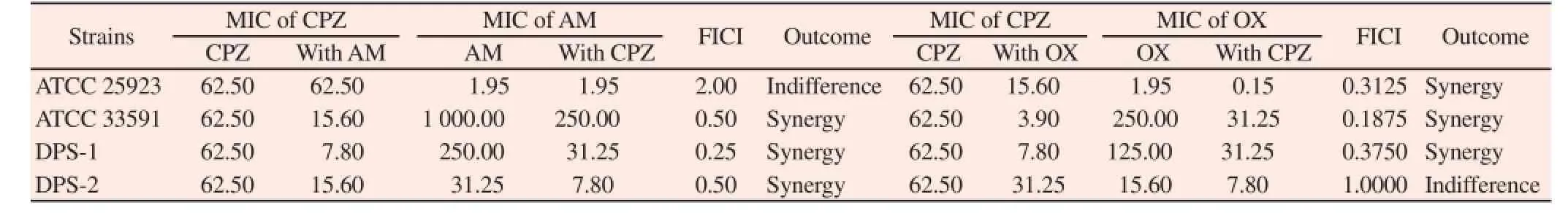
Table 3Synergistic effects of Chlorpromazine (CPZ) combined with β-lactam antibiotics against four Staphylococcus aureus strains.
3.3. Confirmation of mRNA expression by reverse transcription PCR
At the mRNA level, β-lactam resistance gene expression degree was confirmed by a reverse transcription PCR. In bacteria treated with only oxacillin, resistance gene expression was activated by the antibiotic. But, in bacteria treated with CPZ and oxacillin together, the gene expression mostly displayed a decreased tendency. Especially, when the strain was treated with CPZ, the expression of mecA and blaZ gene, which were involved in encoding of PBP2aand β-lactamase, showed a concentration dependent reduction. Furthermore, the expression of regulator gene was reduced by CPZ(Figure 2, 3).
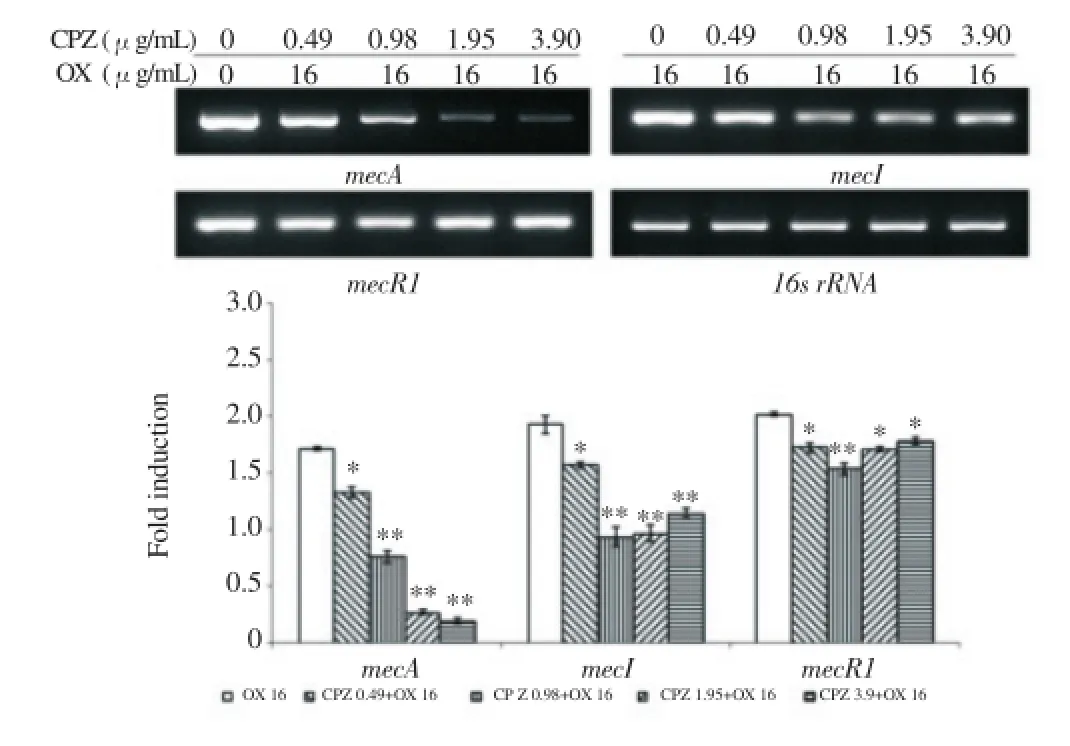
Figure 2. The gene expression related to PBP2a. PCR showing the expression of mecA, mecI and mecR1 of MRSA exposed to CPZ of various concentrations in combination with 16 ug/mL oxacillin.16s rRNA was used as loading control. Expression levels were normalized to 16s rRNA level. The data are mean±S.D. for triple-independent experiments.*P<0.05,**P<0.005 as compared to OX alone, were determined.
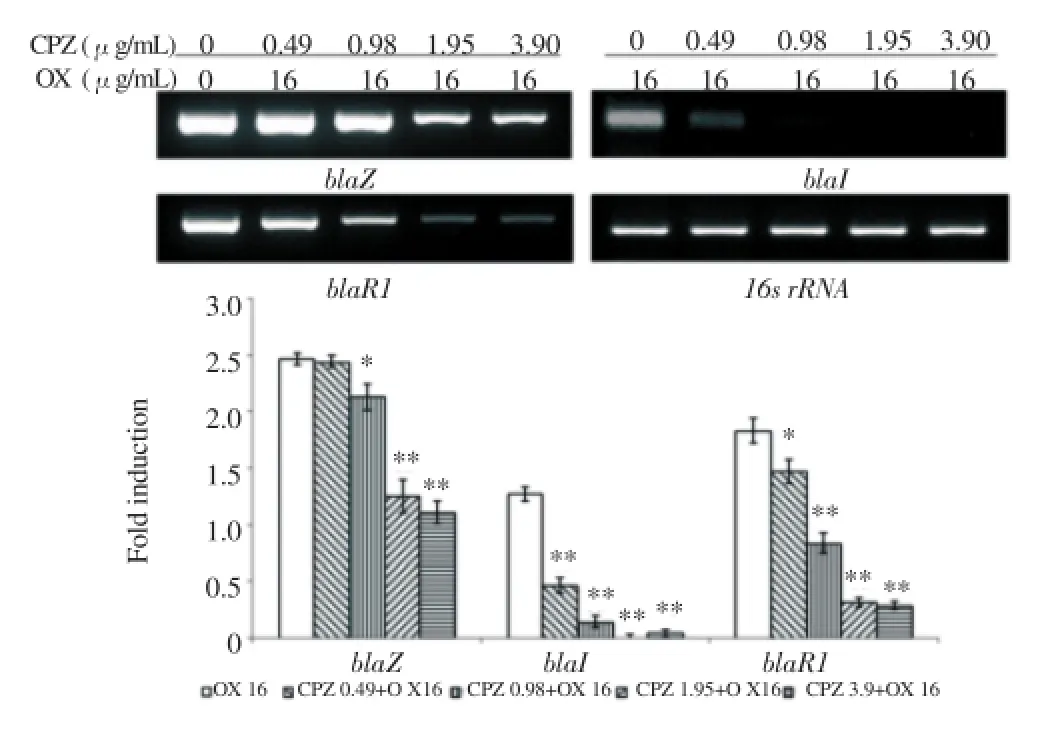
Figure 3. The gene expression related to β-lactamase. PCR showing the expression of blaZ, blaI and blaR1 of MRSA exposed to CPZ of various concentrations in combination with 16 ug/mL oxacillin.16s rRNA was used as loading control. Expression levels were normalized to 16s rRNA level. The data are mean±S.D. for triple-independent experiments. * P<0.05, ** P<0.005 as compared to OX alone, were determined.
4. Discussion
Infectious diseases by MRSA are a growing problem worldwide. Recently, infections by MRSA pose an important menace to patients[16]. Infections associated with multidrug-resistant bacteria are increasingly difficult to treat because of the decrease of antibiotic efficacy by increased resistance [17]. To solve this problem, the studies on several phenothiazines were conducted by researchers [8,9]. We performed the study on CPZ among phenothiazine that was known to possess antibacterial activity against MRSA [11].
生:(B组4)(更来劲了)补充:我也不喜欢。我想,斑羚群在有组织的逃亡过程中。表现出了难得的“舍己救人”的崇高觉悟,表现了难得的“以老救少”的集体主义精神,这可能吗?
PBP2a and β-lactamase are a major resistance factor against β -lactam antibiotics, such as penicillin and ceftaroline [18, 19]. PBP2a and β-lactamase were encoded by mecA and blaZ, respectively. The transcription of mecA and blaZ progresses by following a series of processes. When the MRSA was exposed to β-lactam antibiotic,MecR1 (or BlaR1), a sensor-inducer protein located in cell membrane that was encoded by mecR1 (or blaR1), sense the antibiotic and induce cleavage of MecI (or BlaI), which was a repressor protein. MecI (or BlaI), encoded by mecI (or blaI), represses transcription of mecA (or blaA) because MecI (or BlaI) binds an operator site upstream of mecA (or blaZ). Therefore, the transcription of mecA (or blaZ) was initiated by the cleavage of MecI (or BlaI). At the same time, the transcription of mecI (or bla I) and mecR1 (or blaR1) is located in the opposite side to mecA (or blaZ) start [20- 24].
We confirmed that CPZ have an antibacterial activity against MRSA through the measurement of MIC. Furthermore, we verified a synergy effect between CPZ and antibiotics by a checkerboard method. Through this result, we hypothesized that the CPZ affect the susceptibility of β-lactam antibiotics.
To demonstrate a cause about the reduction of β-lactam resistance,we performed morphological analysis, gene analysis, and western blotting analysis. In a TEM image, MRSA, treated with CPZ of 1/2 MIC, was observed to alter a cell envelope and MRSA, treated with MIC, was observed to be undergoing necrosis. This result showed that the CPZ affect a cell envelope of MRSA. We performed gene analysis to confirm how the activity of CPZ on a cell envelope affects the β-lactam resistance factor. In the strains treated with CPZ, the expression of bla and mec genes showed a decreasing tendency. The bla and mec genes transcription were important to produce resistance factors such as PBP2a and β-lactamase. Furthermore, this process was initiated by the signal transmission of a sensor-inducer protein located in the cell membrane [20]. The result showed that CPZ affect cell membrane of bacteria. Moreover, we confirmed that gene expression was reduced by CPZ. The data suggests that CPZ affects a sensor-inducer protein by perturbation of the cell membrane of MRSA. Consequently, this action of CPZ increases the susceptibility of β-lactam antibiotics against MRSA.
Conflict of interest statement
The authors declare there have been no involvements that might raise the question of bias in the work reported or in the conclusions,implications, or opinions stated.
Acknowledgement
This study was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education (NRF-2013R1A1A2064673), NRF-2013R1A1A2060380, the Korea government (MSIP) (2008-0062484), Republic of Korea.
[1] Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary,epidemiologic, and therapeutic odyssey. Clin Infect Dis 2005; 40: 562-573.
[2] Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol 2010; 64: 143-162.
[3] Fishovitz J, Hermoso JA, Chang M, Mobashery S. Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life 2014; 66: 572-577.
[4] Scheffers DJ, Pinho MG. Bacterial cell wall synthesis: new insights from localization studies. Microbiol Mol Biol Rev 2005; 69: 585-607.
[5] Chambers HF. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev 1997; 10: 781-791.
[6] Berger-Bächi B, Rohrer S. Factors influencing methicillin resistance in staphylococci. Arch Microbiol 2002; 178: 165-171.
[7] Brown DF, Reynolds PE. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett 1980; 122: 275-278.
[8] Amaral L, Viveiros M, Kristiansen JE. "Non-Antibiotics": alternative therapy for the management of MDRTB and MRSA in economically disadvantaged countries. Curr Drug Targets 2006; 7(7): 887-891.
[9] Kristiansen JE, Hendricks O, Delvin T, Butterworth TS, Aagaard L,Christensen JB,. Reversal of resistance in microorganisms by help of nonantibiotics. J Antimicrob Chemother 2007; 59(6): 1271-1279.
[10] Kaatz GW, Moudgal VV, Seo SM, Kristiansen JE. Phenothiazines and thioxanthenes inhibit multidrug efflux pump activity in Staphylococcus aureus. Antimicrob Agents Chemother 2003; 47: 719-726.
[11] Kristiansen MM, Leandro C, Ordway D, Martins M, Viveiros M,Pacheco T, et al. Phenothiazines alter resistance of methicillin-resistant strains of Staphylococcus aureus (MRSA) to oxacillin in vitro. Int J Antimicrob Agents 2003; 22: 250-253.
[12] Clinical and Laboratory Standards Institute. M11-A8. Methods for antimicrobial susceptibility testing of anaerobic bacteria that grow aerobically. 8th ed. Wayne, Pa: Clinical and Laboratory Standards Institute ;2009.
[13] Mun SH, Kang OH, Joung DK, Kim SB, Seo YS, Choi JG, et al. Combination therapy of sophoraflavanone B against MRSA: In vitro synergy testing. Evid Based Complement Alternat Med 2013;2013:823794. Doi: 10.1155/2013/823794.
[14] Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 1965; 27:137-8A.
[15] Mun SH, Joung DK, Kim SB, Park SJ, Seo YS, Gong R, et al. The mechanism of antimicrobial activity of sophoraflavanone B against methicillin-resistant Staphylococcus aureus. Foodborne Pathog Dis 2014;11(3): 234-239.
[16] Figueroa M, Jarmusch AK, Raja HA, El-Elimat T, Kavanaugh JS,Horswill AR, et al. Polyhydroxyanthraquinones as quorum sensing inhibitors from the guttates of Penicillium restrictum and their analysis by desorption electrospray ionization mass spectrometry. J Nat Prod 2014; 77: 1351-1358.
[17] Li M, Du X, Villaruz AE, Diep BA, Wang D, Song Y, et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med 2012; 18: 816-819.
[18] Otero LH, Rojas-Altuve A, Llarrull L, Carrasco-López C, Kumarasiri M, Lastochkin E, et al. How allosteric control of Staphylococcus aureus penicillin binding protein 2a enables methicillin resistance and physiological function. Proc Natl Acad Sci USA 2013; 110: 16808-16813.
[19] Dubée V, Soroka D, Cortes M, Lefebvre AL, Gutmann L, Hugonnet JE,et al. Impact of β-lactamase inhibition on the activity of ceftaroline against Mycobacterium tuberculosis and Mycobacterium abscessus. Antimicrob Agents Chemother 2015; 59(5): 2938-2941.
[20] Archer GL, Bosilevac JM. Signaling antibiotic resistance in staphylococci. Science 2001; 291: 1915-1916.
[21] Hackbarth CJ, Chambers HF. blaI and blaR1 regulate beta-lactamase and PBP 2a production in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 1993; 37: 1144-1149.
[22] Zhang HZ, Hackbarth CJ, Chansky KM, Chambers HF. A proteolytic transmembrane signaling pathway and resistance to beta-lactams in staphylococci. Science 2001; 291: 1962-1965.
[23] Sharma VK, Hackbarth CJ, Dickinson TM, Archer GL. Interaction of native and mutant MecI repressors with sequences that regulate mecA,the gene encoding penicillin binding protein 2a in methicillin-resistant staphylococci. J Bacteriol 1998; 180: 2160-2166.
[24] Hiramatsu K, Ito T, Tsubakishita S, Sasaki T, Takeuchi F, Morimoto Y,et al. Genomic basis for methicillin resistance in Staphylococcus aureus. Infect Chemother 2013; 45(2): 117-136.
Document heading 10.1016/j.apjtm.2016.04.008
15 February 2016
*Corresponding author: Yeul Kwon, Department of Oriental Pharmacy, College of Pharmacy, Wonkwang Oriental Medicines Research Institute, Institute of Biotechnology, Wonkwang University, Iksan, Jeonbuk 570-749, Korea.
Tel./Fax: +82-063-850-6802
E-mail: sssimi@wku.ac.kr
Foundation project: This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2012-0004337).
 Asian Pacific Journal of Tropical Medicine2016年6期
Asian Pacific Journal of Tropical Medicine2016年6期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Experiment research of cisplatin implants inhibiting transplantation tumor growth and regulating the expression of KLK7 and E-cad of tumor-bearing mice with gastric cancer
- Correlation study of biological characteristics of non-small cell lung cancer A549 cells after transfecting plasmid by microbubble ultrasound contrast agent
- Expression and significance of angiostatin, vascular endothelial growth factor and matrix metalloproteinase-9 in brain tissue of diabetic rats with ischemia reperfusion
- Change of the peripheral blood immune pattern and its correlation with prognosis in patients with liver cancer treated by sorafenib
- Comparative study of the chitooligosaccharides effect on the proliferation inhibition and radiosensitization of three types of human gastric cancer cell line
- Are efforts up to the mark? A cirrhotic state and knowledge about HCV prevalence in general population of Pakistan
